Abstract
Threats of nuclear and other radiologic exposures have been increasing but no countermeasure for acute radiation syndrome has been approved by regulatory authorities. In prior publications we have demonstrated the efficacy of tocopherol succinate (TS) as a promising radiation countermeasure with the potential to protect against lethal doses of ionizing radiation exposure. The aim of this study was to gain further insight regarding how TS protects mice against a lethal dose of radiation. CD2F1 mice were injected subcutaneously with 400 mg/kg of TS, and 24 h later exposed to 60Co γ–radiation. Intestinal tissues or spleen/thymus were harvested after irradiation and analyzed for CD68-positive inflammatory cells and apoptotic cells by immunostaining of jejunal cross-sections. Comet assay was used to analyze DNA damage in various tissues. Phospho-histone H3(pH3) and the proliferating cell nuclear antigen (PCNA) were used as mitotic markers for immunostaining jejunal cross-sections. We observed that injecting TS significantly decreased the number of CD68-positive cells, DNA damage and apoptotic cells (BAX, caspase 3 and cleaved poly(ADP-ribose) polymerase-positive cells) as judged by various apoptotic pathway markers. TS treatment also increased proliferating cells in irradiated mice. Results of this study further support our contention that TS protects mice against lethal doses of ionizing radiation by inhibiting radiation-induced apoptosis and DNA damage while enhancing cell proliferation.
Keywords: Apoptosis, mice, radiation, tocopherol succinate
INTRODUCTION
Nuclear accidents and terrorism present a serious threat for causing mass-casualty scenarios [1]. The risk of exposure to ionizing radiation due to terrorist activities is widely thought to be increasing [2]. A terrorist attack could result in the exposure of thousands of people, resulting in death and/or severe illness from acute radiation syndrome (ARS). Use of radiation countermeasure would be aimed at reducing near-term mortality as well as limiting radiation damage that causes long-term adverse health effects [3, 4]. Further, large numbers of patients are routinely exposed to anti-cancer radiotherapy and radiation countermeasures could be useful in ameliorating the negative side effects of the therapy and enabling safe application of higher doses of radiation [5].
The biological effects of radiation on mammalian organisms are strongly dependent upon the dose of radiation received [6, 4]. ARS developing from whole- or partial-body irradiation can involve hematopoietic, gastrointestinal and cerebrovascular components [7]. Cerebrovascular damage invariably leads to death within several days. In contrast, mortality from hematopoietic and gastrointestinal syndromes occurs with lower frequency and more slowly (over weeks rather than days), and is more likely to be amenable to radiation countermeasures.
Although efforts to find suitable radiation countermeasures were initiated more than 50 years ago, no safe and effective radiation countermeasure for ARS has been approved by the United States Food and Drug Administration (FDA). Thus, there is a pressing need for radiation countermeasures to address ARS [8]. Radio-protectors or radio-protectants are prophylactic agents that are administered before exposure to prevent radiation-induced cellular and molecular damage [9]. Radiation mitigators are drugs administered shortly after irradiation that accelerate recovery or repair of radiation injury. Radiation therapeutics or treatments are agents given after overt symptoms appear, to stimulate repair or regeneration. Numerous candidate radiation countermeasures have been identified and investigated. The major themes of countermeasure development have been free radical scavengers and stimulation of hematopoietic progenitors. Other therapeutic avenues are also being explored, such as enhancing DNA repair or blocking cell death pathways.
Some naturally occurring tocol isoforms have been demonstrated as promising radiation countermeasures. The term ‘vitamin E’ refers to a family of tocols. These tocols exist as eight isomers (four tocopherols: α, β, δ and γ with a saturated side chain and four tocotrieniols: α, β, δ and γ with an unsaturated side chain). These isomers commonly occur in foods such as oil (wheat, sunflower, safflower, nuts), rockfish, avocados, mangoes, olives and papayas. Of the tocols, α-tocopherol has a long safety record and is used in foods as a preservative and nutritional supplement [10–12].
We recently demonstrated that α-tocopherol succinate (TS), when administered 24 h before irradiation, protects mice against radiation-induced hematopoietic as well as gastrointestinal syndromes, improves radiation-induced cytopenia, and has a dose reduction factor of 1.28 for 30-day survival [13, 14]. TS-modulated antioxidant enzymes and oncogene expression leads to hematopoietic recovery [15], and inhibited gut bacterial translocation to the heart, spleen and liver in irradiated mice [14]. Our objective in this study was to further investigate the radio-protective efficacy of TS in mice exposed to higher doses of 60Co γ-radiation that induced the gastrointestinal injury. We demonstrate that TS treatment inhibits CD68+ cells in jejunum villi, inhibits DNA damage and apoptotic cells, and also increases mitotic cells in irradiated mice.
MATERIALS AND METHODS
Mice
Male 6–8 week-old CD2F1 mice were purchased from Harlan (Indianapolis, IN, USA) and housed in an air-conditioned facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All mice were kept in rooms with a 12-h light/dark cycle. The mice holding room was maintained at 21 ± 2°C with 10–15 h cycles of fresh air and a relative humidity of 50 ± 10%. Upon arrival, the mice were held in quarantine for 10 days. A microbiological examination of representative samples ensured the absence of Pseudomonas aeruginosa[16]. Mice were provided with certified rodent rations (Harlan Teklad Rodent Diet, Harlan Teklad, WI, USA) and acidified water (HCl, pH = 2.5–2.8) ad libitum. All animal procedures were performed according to a protocol approved by the Armed Forces Radiobiology Research Institute (AFRRI) Institutional Animal Care and Use Committee. Research was conducted according to the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources, National Research Council, US National Academy of Sciences [17].
Drug preparation and administration
TS (Sigma-Aldrich, St. Louis, MO, USA) was administered as a suspension, as described earlier [18]. For a 400-mg/kg dose (10 mg for a 25-g mouse), 1000 mg of TS was dispersed in 8.35 ml polyethylene glycol (PEG)-400 and 0.5 ml Tween-80 for a total volume of 10 ml. For vehicle control, the same composition of Tween-80 and PEG-400 was used. TS was administered subcutaneously (sc) in a volume of 0.1 ml using a 23-gauge needle.
Irradiation
Mice were placed in well-ventilated Plexiglas boxes compartmentalized to accommodate eight mice per box, and exposed to bilateral irradiation in the AFRRI 60Cofacility at a dose rate of 0.6 Gy/min. After irradiation, mice were returned to their cages and monitored. Sham-irradiated mice were treated in the same way as irradiated animals except that the 60Corods were not raised from the pool of shielding water. Radiation dosimetry was based on the alanine/electron paramagnetic resonance (EPR) system [19, 20], currently accepted as one of the most accurate methods and used for intercomparison among national metrology institutions. The calibration curves used in dose measurements at the Armed Forces Radiobiology Research Institute (EPR spectrometer e-Scan, Burker Biospin, Inc., Madison, WI, USA) are based on standard alanine calibration sets purchased from the United States National Institute of Standards and Technology (Gaithersburg, MD, USA). The accuracy of the constructed calibration curve was verified additionally by intercomparison with the United Kingdom National Physical Laboratory (Teddington, UK). Dose rates were measured using small tissue-equivalent alanine pellets (FarWest Technology, Inc., Goleta, CA, USA) in the cores of the mice phantoms positioned in the compartments of the mouse rack. Traditional ionization chambers, and a new generation of Gafchromic film dosimetry were used as supporting and quality assurance methods.
Histopathology of jejunum
To evaluate the effect of TS on radiation-induced gastrointestinal damage, two groups of CD2F1 mice, one treated with TS (one dose, 400 mg/kg) and another receiving only vehicle (PEG and Tween-80), were irradiated (11 Gy) 24 h later. Jejuna were collected from euthanized mice at different times after irradiation. Samples were gently perfused with formalin, placed in a cassette and submerged in formalin for at least 12 h. Jejuna were immersion-fixed in a 20:1 volume of fixative (Z-FIX®, Anatech Ltd., Battle Creek, MI, USA) to tissue for at least 24 h and up to 7 days [15]. Paraffin sections were used for immunohistochemistry examination. Cross-sections of jejunum were cut with a manual rotary microtome at 4 μm [21].
Jejunal cross-sections were used to evaluate the expression of BAX, caspase 3, cleaved poly(ADP-ribose) polymerase-positive cells (cPARP), phospho-histone H3 (pH3) and proliferating cell nuclear antigen (PCNA). The sections were deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA, USA), dehydrated in 100% ethanol and rehydrated by sequentially immersing the slides through graded ethanol washes (95%, 90%, 75% and 50%) for 3 min each at room temperature. Slides were washed in distilled water before performing heat-induced antigen retrieval (HIAR). Respective retrieval solutions for each marker evaluated were used, and slides heated for 40 min at 95°C in in coplin jars placed in a steamer (IHC World, LLC), then cooled to room temperature. Specific immunostaining procedures were then followed for evaluation of each marker.
Evaluation of CD68 expression in mice jejunum
Response of the small intestine to radiation-induced inflammation was evaluated by scoring CD68 positive cells per villus. TS- and vehicle-treated mice were euthanized at 1, 2 and 4 h after irradiation (11 Gy at 0.6 Gy/min) and jejunum samples were collected for immunohistochemistry as described above. The jejunum sections were deparaffinized, dehydrated in 100% ethanol and rehydrated and washed in phosphate buffered saline (PBS). Endogenous peroxidase was quenched with 3% hydrogen peroxide in PBS for 10 min and washed in PBS (three times). The antigen was retrieved in citrate buffer (Thermo Scientific, Pittsburgh, PA, USA) for 20 min, and then cooled to room temperature for 20 min. After washing in PBS, sections were blocked in serum solution (PBS with 0.1% Tween-20 with 3% rabbit serum) for 45 min at room temperature. The excess serum solution was blotted off from the tissue sections. The sections were incubated for 45 min at room temperature in CD68 primary antibody (AbD Serotec, Raleigh, NC, USA) diluted in serum solution (1:75) and washed in PBS. The sections were treated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA), diluted in serum solution (1:25) for 45 min at room temperature and washed. The tissue sections then were incubated with VECTASTAIN® Elite ABC Reagent (Vector Laboratories) for 30 min at room temperature. After one wash, sections were exposed to peroxidase substrate solution for 2–3 min and rinsed in deionized water. The sections were counterstained with hematoxylin for 1 min, rinsed in water and mounted in Histomount™ (National Diagnostics, Atlanta, GA, USA). CD68-positive cells per villus were scored under a light microscope (Nikon Eclipse TS100 microscope, Melville, NY, USA) equipped with the Retiga 2000R Q imaging camera (Surrey, BC, Canada).
Comet assay
An alkaline comet assay was used to determine the DNA damage caused by radiation (9.2 Gy, 0.6 Gy/min) in mice [22]. Blood, thymus and spleen were collected 30 min and 4 h after irradiation. Thymus and spleen were minced using sterile frosted slides in a Petri dish, and cell suspensions were collected. Peripheral blood mononuclear cells were separated using histopaque 1083 solution (Sigma-Aldrich). All cell samples were washed three times and re-suspended in 1 × 105cells/ml using ice-cold PBS. The manufacturer's protocol of alkaline comet assay kit (Trevigen, Gaithersburg, MD, USA) was followed. We mixed cell suspensions with molten LM (low melting) agarose (1:10) and immediately pipetted onto a comet slide. The slides were kept at 4°C in the dark for at least 10 min and immersed in pre-chilled lysis solution (Trevigen) for 45 min at 4°C. Slides were electrophoresed in alkaline buffer (pH > 13) for 15 min (1 V/cm and 300 mA) and immersed twice in distilled water for 10 min each to remove the alkali. The electrophoresed slides were treated with 70% ethanol for 5 min and dried at room temperature. DNA of a comet cell was stained with SYBR®green (Trevigen) for 5 min at 4°C. The comet cells were captured under a Zeiss Axio Observer D1 fluorescence microscope with Axiovision 4.7 software (Göttingen, Germany) and analyzed using comet score (TriTek, Sumerduck, VA, USA). We analyzed for average comet tail length (µm) and percent DNA in a comet tail. At least 100 cells were scored for each mouse and eight mice were used to generate each data point.
Evaluation of Bcl-associated X protein (BAX) expression in the intestine of mice
Overexpression of Bcl-associated X protein (BAX) accelerates apoptotic cell death. We evaluated BAX expression in the intestine of mice injected with TS or vehicle 24 h before irradiation (11 Gy). The sections were prepared based on the procedure described above, and IHC-Tek™ epitope retrieval solution (IHC world, LLC, Woodstock, MD) was used for performing heat-induced antigen retrieval. The endogenous peroxidase was inhibited by adding a peroxidase quench (SuperPicTure™ 3rd Gen IHC Detection Kit, by Invitrogen Life Technologies, Grand Island, NY, USA) and rinsed in PBS. Slides were incubated in an anti-BAX antibody (1:50) within an antibody diluent (IHC world, LLC) overnight at 4°C. Sections were washed and treated with rabbit horseradish peroxidase (HRP; Invitrogen Life Technologies) and incubated in a moist chamber for 60 min at room temperature. BAX-positive cells were detected by the application of 3,3'-diaminobenzidine (DAB) substrate solution. Sections were counterstained with hematoxylin, dehydrated, mounted (liquid glycerol gelatin mounting medium) and scored under a light microscope (Nikon Eclipse 80i) equipped with a Nikon DS Fi1 imaging camera. The data were presented as the number of BAX-positive cells per crypt.
Identification of cysteine-aspartic proteases 3 (caspase 3) in mice intestine
We selected active caspase 3 as a marker of apoptosis, as its activation plays a pivotal role in the apoptotic process. Prepared slides were placed in coplin jars containing IHC-Tek™ epitope retrieval solution (IHC world, LLC, Woodstock, MD, USA) to perform heat-induced antigen retrieval. The endogenous peroxidase was inhibited by adding a peroxidase quench (Reagent A), SuperPicture™ 3rd Gen IHC Detection Kit (Invitrogen Life Technologies, Grand Island, NY, USA) and rinsed in PBS. Slides were incubated in anti-active caspase 3 antibody overnight at 4°C. Sections were washed and treated with rabbit HRP polymer (Invitrogen Life Technologies)before incubating in a moist chamber for 60 min at room temperature. To detect caspase 3-positive cells, we applied DAB substrate solution. The sections were counterstained with hematoxylin, dehydrated, mounted and scored under a light microscope (Nikon Eclipse 80i) equipped with a Nikon DS Fi1 imaging camera. The data were presented as the number of active caspase 3-positive cells per HPF.
Identification of cleaved poly(ADP-ribose) polymerase (cPARP) in mouse jejunum
Paraffin-embedded jejunum sections were used to detect cPARP following the manufacturer's protocol (Eton Bioscience Inc., San Diego, CA, USA). We deployed a heat-induced antigen-retrieval method using a citrate buffer (0.01 M, pH 6.0) to retrieve cPARP antigen on prepared slides. To minimize non-specific binding, slides were blocked in blocking buffer (Eton Bioscience Inc.) and rinsed in PBS. Slides were incubated in anti-cPARP antibody overnight at 4°C. Sections were washed and covered with rabbit horseradish peroxidase polymer and incubated in a moist chamber for 60 min at room temperature. The cPARP-positive cells were detected by applying DAB substrate solution. The sections were counterstained with hematoxylin, dehydrated and scored after mounting under a light microscope (Nikon Eclipse 80i) equipped with a Nikon DS Fi1 imaging camera. Data are presented as the number of cPARP-positive cells per crypt.
Evaluation of phospho-histone H3 (pH3) protein expression as a mitotic marker
We evaluated pH3 protein expression in mice intestine as a mitotic marker by using paraffin-embedded jejunum sections following the manufacturer's protocol (Eton Bioscience Inc.). As described above in the section on ‘Histopathology of jejunum,’ sections were deparaffinized briefly in Histo-Clear (National Diagnostics, Atlanta, GA, USA), dehydrated and rehydrated by sequentially immersing the slides through graded ethanol washes (95%, 75% and 50%). Slides were heated with citrate buffer (0.01 M, pH 6.0) for 40 min at 95°C to retrieve the pH3 antigen. Slides were blocked in blocking buffer (Eton Bioscience Inc.), rinsed twice (PBS) and incubated in anti-pH3 antibody overnight at 4°C. We incubated each section with rabbit HRPpolymer in a moist chamber for 60 min at room temperature, washed twice (PBS), and pH3-positive cells were detected by applying DAB substrate solution. Sections were counterstained with hematoxylin and mounted using liquid glycerol gelatin mounting medium. We scored the pH3-positive cells per crypt under a light microscope (Nikon Eclipse 80i) equipped with a Nikon DS Fi1 imaging camera.
Proliferating cell nuclear antigen (PCNA) expression in mice intestine
The paraffin-embedded jejunum sections were used to detect the PCNA-positive cells following the manufacturer's protocol (Life Technologies, Grand Island, NY, USA) with slight modifications [23]. After preparing the slides based on the procedure described above, slides were immersed in IHC-Tek™ epitope retrieval solution (IHC World, Woodstock, MD, USA) and heated for 40 min at 95°C in a steamer (IHC World). The endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min and incubated with biotinylated mouse anti-PCNA primary antibody in a moist chamber for 60 min at room temperature. Following one wash in PBS, slides were incubated with streptavidin-peroxidase, and PCNA-positive cells were detected by covering each section with DAB chromogen. The sections were washed in deionized (DI) water and counterstained with hematoxylin. After dehydrating the slides in graded ethanol and mounting them with HistomountTM(Life Technologies), PCNA-positive cells were scored under a light microscope (Nikon Eclipse 80i) equipped with a Nikon DS Fi1 imaging camera. Data are presented as numbers of PCNA-positive cells per crypt.
Statistical analysis
Caspase 3 and cPARP were scored by identifying eight HPFs per section. BAX, pH3 and PCNA were scored in 10 to 12 crypts in each section. All histological studies used eight mice per treatment group and six sections per mouse. Statistical mean with standard error was reported if applicable. Analysis of variance (ANOVA) was used to detect significant differences among groups. If significant, pair-wise comparison by Tukey–Kramer was used to identify which group was different from the others. A significance level was set at 5% for each test. All statistical tests were two-sided. Statistical software, SPSS v.19, was used for statistical analyses.
RESULTS
Effect of TS treatment on radiation-induced CD68 expression
To evaluate the role of TS in the radiation-induced inflammatory response, CD68 expression in intestinal villi was scored. Mice were treated with TS (400 mg/kg) or vehicle 24 h before 60Co γ-radiation (11 Gy at 0.6 Gy/min). Jejuna of both groups of mice were harvested 1, 2 and 4 h after radiation exposure, immunostained as described under materials and methods. CD68-positive cells were scored in six villi per section, six sections per mouse, and eight mice in each group. TS-treated mice displayed a significantly lower number of CD68-positive cells per villus 2 and 4 h after irradiation compared with vehicle-treated control mice (P< 0.05) (Figure 1). Two sham irradiated groups of mice were used as controls: one was injected with TS and harvested 28 h after injection, and the other was untreated (bottom panel).
Fig. 1.
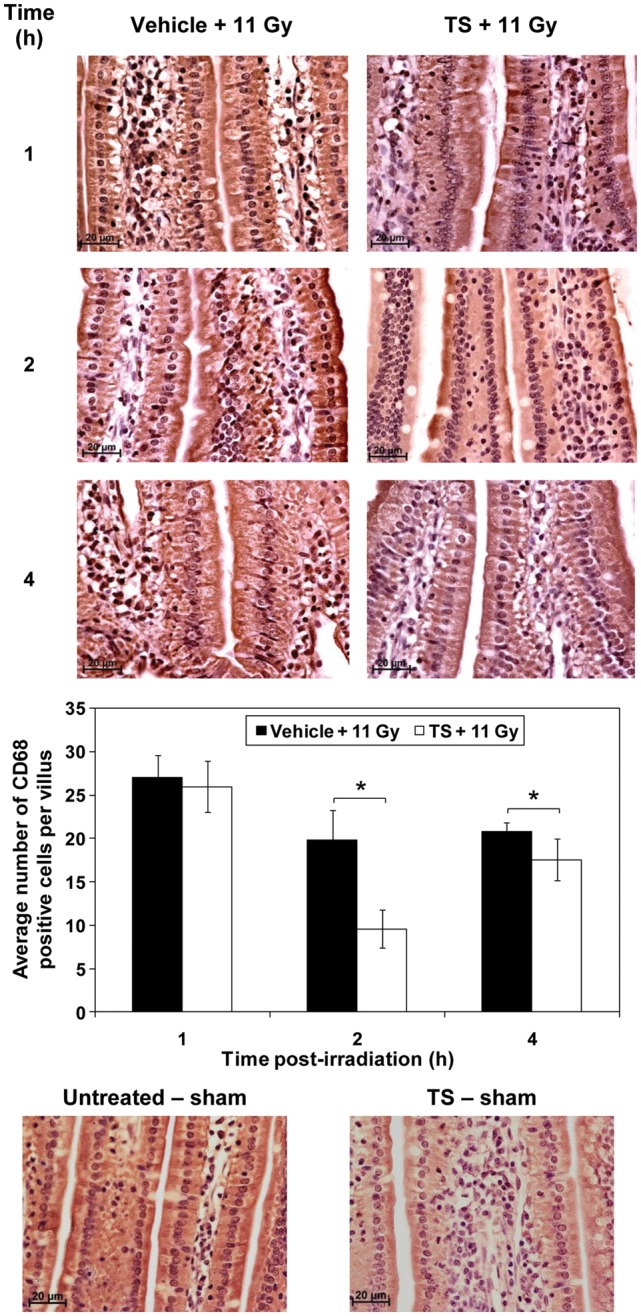
Effect of TS treatment on CD68 cells in mice receiving 60Co γ-radiation. Jejunum samples from TS- or vehicle-treated and irradiated (11 Gy) mice were collected 1, 2 and 4 h after irradiation. After fixation and processing, radiation-induced inflammatory response was evaluated by scoring cross-sections of jejunum for CD68-positive cells per villus by immunochemistry. Representative areas are shown (×600 magnification) in photomicrographs. TS-treated mice had significantly fewer CD68-positive cells 2 and 4 h after irradiation compared with vehicle-treated mice. *Denotes statistically significant difference between indicated groups. Two sham irradiated groups were used as controls: one was untreated and other was injected with TS.
The effect of TS administration on radiation-induced DNA damage as assessed by alkaline comet assay
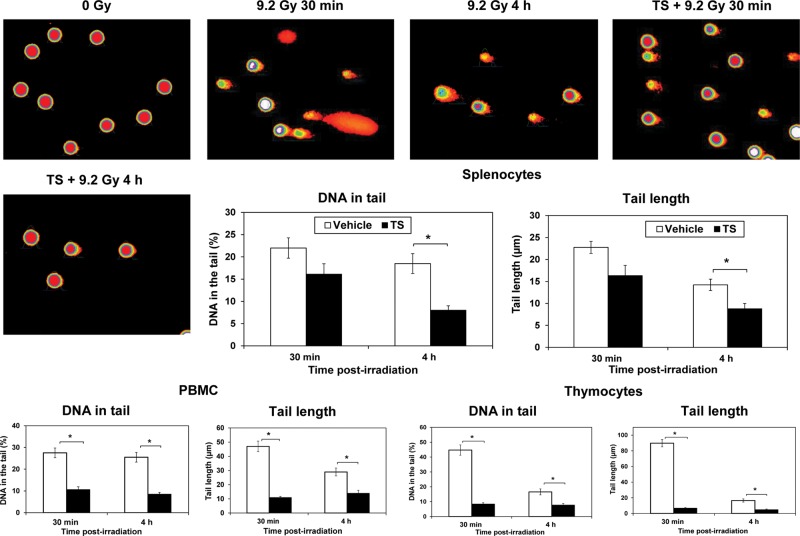
We used the alkaline comet assay to determine the protective effect of TS treatment against DNA damage caused by ionizing radiation in peripheral blood mononuclear cells, splenocytes and thymocytes. As previously mentioned, mice were treated with vehicle or TS (400 mg/kg), and exposed to 9.2 Gy 60Co γ-radiation 24 h after drug injection. Peripheral blood, spleen and thymus were collected 30 min and 4 h after irradiation, and used for alkaline comet assay. Unirradiated mice were used as controls. At both 30 min and 4 h, the administration of TS significantly inhibited DNA damage in peripheral blood cells and thymocytes compared with vehicle-treated mice (P< 0.001), evidenced by the shorter tail length and smaller percentage of DNA in the comet tail. Similar results were observed in splenocytes from TS-treated mice at 4 h post-irradiation (P< 0.05) (Figure 2). Photomicrographs of spleen cell comets are presented in the figure.
Fig. 2.
Efficacy of TS treatment in preventing DNA damage induced by radiation in splenocytes, peripheral blood cells and thymocytes as assessed with alkaline comet assay. One group of mice (n= 8) received TS treatment (400 mg/kg) 24 h before irradiation and another group (n= 8) received vehicle. Mice were exposed to 9.2 Gy of 60Co γ-radiation (LD90/30dose) and samples were collected 30 min and 4 h after irradiation. Sham irradiated mice (n= 8) were used as controls. Representative areas are shown (×200 magnification) in photomicrographs. A significantly smaller percentage of DNA was found in TS-treated mice compared with vehicle-treated mice. TS-treated mice also had a shorter tail length compared with vehicle-treated mice. *Denotes statistically significant difference between vehicle- and TS-treated groups.
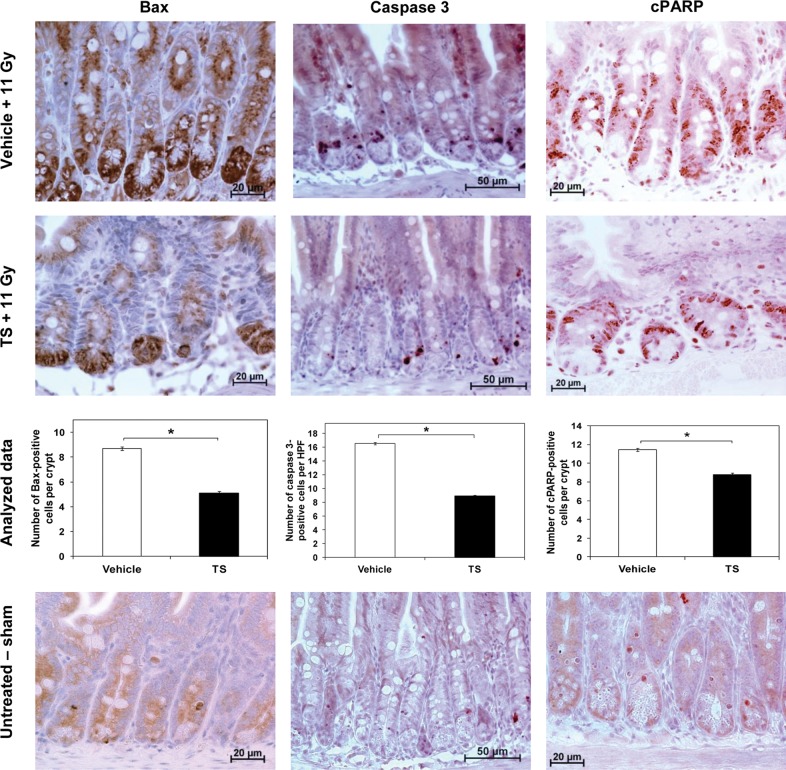
Effect of TS on radiation-induced apoptosis in jejunum
To evaluate the molecular mechanism of TS-mediated intestinal protection Bax, caspase 3 and poly (ADP-ribose) polymerase (PARP) were explored. The jejuna of irradiated mice (11 Gy) injected with TS or vehicle were harvested 4 h after irradiation and jejunal cross-sections were processed as described in previous sections. Sham irradiated mice were used as control. A significantly lower number of BAX-positive cells per crypt (P< 0.0001) was observed in TS-treated mice compared with vehicle-treated mice, indicating that TS inhibits radiation-induced apoptosis by inhibiting the expression of BAX (Figure 3, left panel).
Fig. 3.
The effect of TS administration on the number of BAX-, caspase 3 and cPARP-positive cells in mice exposed to 60Co γ-radiation. Mice treated with TS or vehicle 24 h before exposure to 11-Gy radiation were harvested for jejuna samples 4 h post-irradiation. After fixation and processing, cross-sections of jejunum were analyzed for BAX (left panel), caspase 3 (middle panel) and cPARP (right panel) expression by immunohistochemistry. Representative areas are shown (×600 magnification for BAX and cPARP, and X400 for caspase 3) in photomicrographs. *Denotes statistically significant difference between TS- and vehicle-treated groups. Sham irradiated mice were used as controls (bottom panel).
Cross-sections of jejunum were also evaluated for expression of caspase 3, a member of the cysteine-aspartic acid protease family, which plays a central role in the execution phase of cell apoptosis. Jejunal sections were scored for active caspase 3-positive cells in at least six HPF per section and six sections per mouse. TS-treated mice showed a significantly lower number of caspase 3-positive cells per HPF (P< 0.0001) compared with their vehicle-treated counterparts (Fig. 3, middle panel). This suggests an inhibition of apoptosis by TS treatment, and significant protection of cells from radiation-induced damage.
PARP is a family of proteins that is inactivated through caspase 3 cleavage, usually occurring where DNA damage is extensive. The extent of apoptotic damage can therefore be determined through the levels of cleaved-PARP (cPARP) in cells. After fixation and processing, cross-sections of jejunum of two groups of mice were analyzed for cPARP expression by immunohistochemistry, as described above. Significantly lower numbers of cPARP-positive cells per crypt were observed in TS-treated mice compared with vehicle-treated mice (P< 0.0001), indicating a lower level of DNA fragmentation, leading to increased protection from radiation damage in mice treated with TS (Figure 3, right panel). Sham irradiated control mice photomicrographs for each test are presented in the bottom panel.
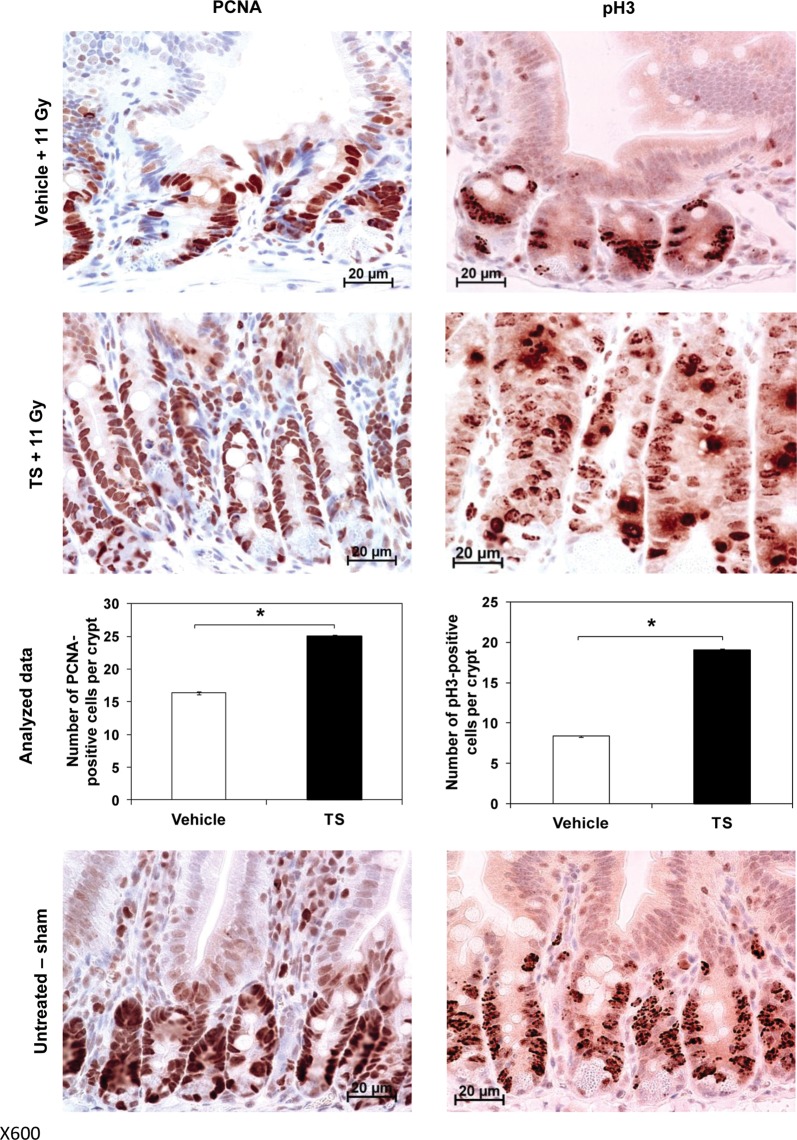
Effect of TS administration on cell proliferation in jejunum of irradiated mice
In order to determine crypt cell regeneration potential in TS-treated mice, we evaluated pH3 ( a marker for mitosis) and PCNA (a nuclear protein and a co-factor of DNA polymerase δ). TS-, vehicle-treated and irradiated mice (11 Gy) were harvested for jejuna 4 h post-irradiation, and analyzed for pH3 and PCNA expression. A group of sham irradiated mice served as control. Figure 4(left panel) shows that TS-treated mice displayed a significantly higher number of PCNA-positive cells per crypt compared with vehicle-treated mice (P< 0.0001).
Fig. 4.
The effect of TS treatment on cell proliferation in mice exposed to 60Co γ-radiation. Two groups of mice, irradiated with 11 Gy and treated with TS or vehicle 24 h before irradiation, were harvested for jejuna samples 4 h post-irradiation. A group of sham irradiated mice was used as control. Cross-sections of jejunum were analyzed for PCNA (left panel) and pH3 (right panel) expression by immunohistochemistry. Representative areas are shown (×600 magnification) in photomicrographs. Significantly higher levels of PCNA and pH3 were detected in TS-treated mice compared with vehicle-treated mice, indicating a higher level of cell growth. *Denotes statistically significant difference between indicated groups.
We also evaluated PCNA, a nuclear protein and a co-factor of DNA polymerase δ, which is involved in the control of eukaryotic DNA replication and repair. As described above, two groups of mice were exposed to 11 Gy radiation and injected with TS or vehicle 24 h prior to exposure. Jejuna were collected 4 h post-radiation, and cross-sections were immunostained for PCNA expression. Figure 4right panel indicates a significantly greater number of pH3-positive cells per crypt (P< 0.0001) in TS-treated mice compared with vehicle-treated mice, showing a greater level of cell proliferation and radiation protection in TS-treated mice. Sham irradiated control mice photomicrographs for both tests are presented in the bottom panel.
DISCUSSION
Terrorist threats and recent accidents in nuclear installations emphasize the urgent need to develop radiation countermeasures [3]. We reported recently that TS is a promising radiation countermeasure that protects mice against hematopoietic as well as GI doses of irradiation when administered 24 h before radiation exposure [13–15]. Administration of TS modulated the expression of antioxidant enzymes and inhibited expression of oncogenes in irradiated mice. It also increased colony-forming unit-spleen numbers and bone marrow cellularity in irradiated mice. We were interested to explore the mechanism of radio-protective efficacy provided by TS. In this study, we demonstrate that TS treatment inhibits CD68-positive cells, radiation-induced DNA damage and apoptosis, and increases mitosis in irradiated mice.
CD68 is particularly useful as a marker for the various cells of the macrophage lineage including monocytes, histocytes, giant cells, Kupffer cells and osteoclasts. There are a large number of studies using CD68 markers to identify inflammatory cells [24, 25]. We observed lower numbers of CD68-positive cells 2 and 4 h after irradiation in the jejunum of mice receiving TS treatment compared with vehicle control, suggesting that TS treatment is involved in suppressing inflammation in response to irradiation.
Ionizing radiation has been shown to induce apoptotic pathways. Several caspases play essential roles in the execution of apoptosis [26]. Caspase 3, an effector caspase, is responsible for the proteolytic cleavage of several key proteins including the nuclear enzyme poly(ADP-ribose) polymerase (PARP) [27]. TS inhibited the expression of caspase 3 in irradiated mice, which further explains the role of TS in the inhibition of radiation-induced apoptosis. Our observation of lower numbers of caspase 3-positive cells in TS-treated mice compared with vehicle-treated mice also explains the lower number of cleaved PARP-positive cells in TS-treated mice.
To further evaluate the molecular mechanism of the inhibition of caspase 3 in the TS-treated mice, we evaluated the expression of BAX protein. BAX is a pro-apoptotic protein of the Bcl2 family containing BH1, BH2 and BH3 domains. Expression of BAX is known to accelerate apoptotic cell death by releasing cytochrome c oxidase from mitochondrion to cytoplasm [28]. Released cytochrome c from mitochondria facilitates the formation of the apoptosome-containing Apaf-1 and caspase 9, which converts the pro-caspase 3 to active caspase 3 [29, 30]. TS inhibited the expression of BAX in the jejunal crypts of irradiated mice, which supports our earlier finding of regeneration of crypts in TS-treated and irradiated mice [14]. Lower numbers of BAX-positive cells in TS-treated and irradiated mice are in correlation with lower numbers of active caspase 3-positive cells in TS-treated mice. Park et al.have also shown the eckol-mediated inhibition of BAX in the crypt of irradiated mice, supporting our current observations [31].
The comet assay, also called single-cell gel electrophoresis (SCGE), is a sensitive and rapid technique for quantifying and analyzing DNA damage in individual cells. This is a standard technique for evaluation of DNA damage/repair. Several radio-protective agents such as eckol and dieckol have been shown to reduce radiation-induced DNA damage using comet assays [22, 32]. Our comet assay results support earlier findings that radio-protective agents can reduce radiation-induced DNA damage/repair. We found similar results in comet assay with samples obtained from spleen, thymus and peripheral blood cells of TS-treated and irradiated mice. We have used a 9.2-Gy radiation dose for experiments presented in Figure 2since 9.2 Gy is the LD90/30dose that causes hematopoietic injury and this dose was suitable for analyzing DNA damage in cells of spleen, thymus and peripheral blood.
The effect on mitosis, which is crucial for the regular replacement of intestinal epithelial lining, may be an important mechanism for the protection of gastrointestinal tract, as observed in mice injected with TS. Octamers of histone proteins (two each of H2A, H2B, H3 and H4 histone proteins) function as spools to package eukaryotic DNA into repeating nucleosome units. During the prophase of mitosis, chromosomal condensation starts with phosphorylation of histone H3, which occurs in a step-wise and orderly manner [33, 34]. Phosphorylation of histone 3 has been considered a hallmark of mitosis [35]. We evaluated the expression of phospho-histone H3 (pH3) expression as a mitotic marker of intestinal epithelial cells. As expected, we observed significantly higher numbers of pH3-positive cells in mice treated with TS and exposed to 60Co γ-radiation, compared with vehicle control. Results presented here further confirm our earlier findings with bromodeoxyuridine and Ki-67 markers in tocopherol succinate-treated and irradiated mice [14, 21]. The phosphorylation of histone H3 at Ser10 (pH3) has been considered an important marker of mitosis [35], since it only occurs in cells undergoing mitosis. We observed significantly higher numbers of pH3-positive cells per crypt in TS-treated mice exposed to 60Co γ-radiation compared with vehicle-treated mice. PCNA is a nuclear protein and a co-factor of DNA polymerase δ, which is involved in the control of eukaryotic DNA replication and repair. In response to DNA damage, this protein is ubiquitinated and is involved in the RAD6-dependent DNA repair pathway. Our results show higher numbers of PCNA-positive cells per crypt in TS-injected mice compared with vehicle control. Overall these results demonstrate an increased proliferation of jejunum cells of lethally irradiated mice treated with TS. The effect on mitosis, which is crucial for the regular replacement of intestinal epithelial lining, may be an important mechanism for the protection of the gastrointestinal tract observed in mice treated with TS. Park et al.have demonstrated a similar radio-protective mechanism with eckol [22, 31]. They have demonstrated that eckol's protective effects include an improvement in hematopoietic recovery, the repair of damaged DNA in immune cells and an enhancement of their proliferation, which had been severely suppressed by ionizing radiation.
Our results demonstrate that TS has the potential to protect tissue from radiation injury beyond the hematopoietic system by improving structural integrity, inhibiting apoptosis and enhancing cell proliferation in vital gastrointestinal tissue in mice exposed to high doses of 60Co γ-radiation. TS did not protect mice when administered as a mitigator after irradiation [14] and it can be used only as a radio-protector. TS will be of use to the military where they are aware of potential incidences of exposure, such as missions where a significant possibility of exposure to radiation exists. Furthermore, TS could also be used in limited situations where the potential for exposure will continue long after a nuclear accident and new workers might be brought in who may benefit from pretreatment with such an agent. TS has been used at a dose of 400 mg/kg to be consistent with recent published results [14, 15]. The lowest effective dose of TS is 200 mg/kg [36]. Furthermore, different species require different drug doses based on body surface area [37, 38]. Protection provided by TS is of great significance because the radiation dose used in this study is equivalent to highly lethal doses for humans. TS appears to be an attractive radiation countermeasure candidate with no known toxicity. It is a candidate for further development as a radiation countermeasure in large animals such as canines, minipigs or nonhuman primates for the ultimate use in humans.
ACKNOWLEDGEMENTS
The authors are thankful to Dr Christopher R. Lissner, AFRRI Scientific Director, for helpful discussions, and to Dr Mark H. Whitnall, for critical review of this manuscript. We gratefully acknowledge AFRRI's Veterinary Sciences Department and Cobalt Radiation Facility for their support. This study was supported by the intramural awards RAB2CZ and RBB2GQ to VKS. PKS is supported by a fellowship from the National Research Council, Washington, DC.
REFERENCES
- 1.Allison G. Nuclear disorder: surveying atomic threats. Foreign Affairs. 2010;89:74–89. [Google Scholar]
- 2.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. International Journal of Radiation Biology. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 3.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 4.Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Greenberger JS. Radioprotection. In Vivo. 2009;23:323–36. [PMC free article] [PubMed] [Google Scholar]
- 6.Singh VK, Seed TM. Radiation Effects. In: Roy MJ, editor. Physician's Guide to Terrorist Attack. Totowa: Humana Press; 2003. pp. 339–362. [Google Scholar]
- 7.Koenig KL, Goans RE, Hatchett RJ, et al. Medical treatment of radiological casualties: current concepts. Annals of Emergency Medicine. 2005;45:643–52. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.United States Department of Defense. Department of Defense Chemical and Biological Defense Program: Annual Report to Congress. Washington, DC: Department of Defense.; 2010. http://www.jpeocbd.osd.mil/packs/DocHandler.ashx?DocID=11104 . [Google Scholar]
- 9.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Radiat Res. 2003;162:711–28. doi: 10.1667/rr3276. Report of an NCI Workshop, 3–4 December. [DOI] [PubMed] [Google Scholar]
- 10.Papas A. Antioxidant Status, Diet, Nutrition, and Health. Boca Raton: CRC Press; 1999. Vitamin E: tocopherols and tocotrienols. In: Papas AM; pp. 189–210. [Google Scholar]
- 11.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB Journal. 1999;13:1145–55. [PubMed] [Google Scholar]
- 12.Traber MG, Packer L. Vitamin E: beyond antioxidant function. American Journal of Clinical Nutrition. 1995;62:1501S–9S. doi: 10.1093/ajcn/62.6.1501S. [DOI] [PubMed] [Google Scholar]
- 13.Singh VK, Brown DS, Kao TC. Tocopherol succinate: a promising radiation countermeasure. International Immunopharmacology. 2009;9:1423–30. doi: 10.1016/j.intimp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Singh PK, Wise SY, Ducey EJ, et al. Alpha-tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat Res. 2012;177:133–45. doi: 10.1667/rr2627.1. [DOI] [PubMed] [Google Scholar]
- 15.Singh VK, Parekh VI, Brown DS, et al. Tocopherol succinate: modulation of antioxidant enzymes and oncogene expression, and hematopoietic recovery. Int J Radiat Oncol Biol Phys. 2011;79:571–8. doi: 10.1016/j.ijrobp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Singh VK, Christensen J, Fatanmi OO, et al. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res. 2012;177:781–91. doi: 10.1667/rr2894.1. [DOI] [PubMed] [Google Scholar]
- 17.National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 18.Singh VK, Brown DS, Kao TC, et al. Preclinical development of a bridging therapy for radiation casualties. Experimental Hematology. 2010;38:61–70. doi: 10.1016/j.exphem.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 19.ISO-ASTM Standard practice for use of an alanine dosimetry. ISO/ASTM International Standard. 2004:51607. [Google Scholar]
- 20.Nagy V. Accuracy considerations in EPR dosimetry. Appl Radiat Isot. 2000;52:1039–50. doi: 10.1016/s0969-8043(00)00052-x. [DOI] [PubMed] [Google Scholar]
- 21.Singh PK, Wise SY, Ducey EJ, et al. Radioprotective efficacy of tocopherol succinate is mediated through granulocyte-colony stimulating factor. Cytokine. 2011;56:411–21. doi: 10.1016/j.cyto.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Park E, Ahn GN, Lee NH, et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008;582:925–30. doi: 10.1016/j.febslet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Zeymer U, Fishbein MC, Forrester JS, et al. Proliferating cell nuclear antigen immunohistochemistry in rat aorta after balloon denudation. Comparison with thymidine and bromodeoxyuridine labeling. Am J Pathol. 1992;141:685–90. [PMC free article] [PubMed] [Google Scholar]
- 24.Erkin G, Ugur Y, Gurer CK, et al. Effect of PUVA, narrow-band UVB and cyclosporin on inflammatory cells of the psoriatic plaque. Journal of Cutaneous Pathology. 2007;34:213–19. doi: 10.1111/j.1600-0560.2006.00591.x. [DOI] [PubMed] [Google Scholar]
- 25.Haas M, Buttner M, Rau TT, et al. Inflammation in gastric adenocarcinoma of the cardia: how do EBV infection, Her2 amplification and cancer progression influence tumor-infiltrating lymphocytes? Virchows Archiv: An International Journal of Pathology. 2011;458:403–11. doi: 10.1007/s00428-011-1058-1. [DOI] [PubMed] [Google Scholar]
- 26.Cryns V, Yuan J. Proteases to die for. Genes & Development. 1998;12:1551–70. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH, Desnoyers S, Ottaviano Y, et al. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85. [PubMed] [Google Scholar]
- 28.Jurgensmeier JM, Xie Z, Deveraux Q, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Critical Reviews in Toxicology. 2000;30:609–27. doi: 10.1080/10408440008951122. [DOI] [PubMed] [Google Scholar]
- 31.Park E, Lee NH, Joo HG, et al. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochemical and Biophysical Research Communications. 2008;372:792–7. doi: 10.1016/j.bbrc.2008.05.140. [DOI] [PubMed] [Google Scholar]
- 32.Park E, Ahn G, Yun JS, et al. Dieckol rescues mice from lethal irradiation by accelerating hemopoiesis and curtailing immunosuppression. Int J Radiat Biol. 2010;86:848–59. doi: 10.3109/09553002.2010.487011. [DOI] [PubMed] [Google Scholar]
- 33.Goto H, Tomono Y, Ajiro K, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 34.Sauve DM, Anderson HJ, Ray JM, et al. Phosphorylation-induced rearrangement of the histone H3 NH2-terminal domain during mitotic chromosome condensation. J Cell Biol. 1999;145:225–35. doi: 10.1083/jcb.145.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hans F, Dimitrov S. Histone H 3 phosphorylation, cell division. Oncogene. 2001;20:3021–7. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 36.Singh VK, Brown DS, Kao TC. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol. 2010;86:12–21. doi: 10.3109/09553000903264515. [DOI] [PubMed] [Google Scholar]
- 37.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 38.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, Maryland: US Food and Drug Administration; 2002. [Google Scholar]