Abstract
This study sought to evaluate the differential effects of bladder distention on point A-based (AICBT) and three-dimensional conformal intracavitary brachytherapy (3D-ICBT) planning for cervical cancer. Two sets of CT scans were obtained for ten patients to evaluate the effect of bladder distention. After the first CT scan, with an empty bladder, a second set of CT scans was obtained with the bladder filled. The clinical target volume (CTV), bladder, rectum, and small bowel were delineated on each image set. The AICBT and 3D-ICBT plans were generated, and we compared the different planning techniques with respect to the dose characteristics of CTV and organs at risk. As a result of bladder distention, the mean dose (D50) was decreased significantly and geometrical variations were observed in the bladder and small bowel, with acceptable minor changes in the CTV and rectum. The average D2 cm3and D1 cm3showed a significant change in the bladder and small bowel with AICBT; however, no change was detected with the 3D-ICBT planning. No significant dose change in the CTV or rectum was observed with either the AICBT or the 3D-ICBT plan. The effect of bladder distention on dosimetrical change in 3D-ICBT planning appears to be minimal, in comparison with AICBT planning.
Keywords: intracavitary brachytherapy, bladder distention, 3D-conformal intracavitary brachytherapy, cervical cancer
INTRODUCTION
Radiation therapy has played an important role in the treatment of cervical cancer; however, the delivery of high-dose radiation to a target surrounded by organs at risk (OARs), in particular the bladder, rectum and small bowel, is difficult without the occurrence of normal tissue complications. Radiation therapy has traditionally been combined with consecutive external beam radiotherapy (EBRT) and intracavitary brachytherapy (ICBT) in the treatment of cervical cancer. ICBT involves the insertion of a radioactive source for local delivery of a high dose of radiation to the tumor with a rapid dose falloff in the surrounding normal tissue. This is very important for conformal boost treatment without the occurrence of complications in normal tissue. However, despite this advantage, because ICBT is usually started after EBRT, the OARs near the target are not completely free from the dose during treatment planning. Therefore, brachytherapy planning should be performed within a limited permissible dose for the OARs, and reduction of the dose to the OARs during ICBT planning is very important. With respect to minimizing the normal tissue dose during treatment planning for ICBT, some reports have noted that the size and position of the bladder changes according to the filling status, and this geometrical change can contribute to a reduction in the normal tissue dose near the target volume [1–3].
However, they employed the point A-based conventional ICBT (AICBT) planning technique with 3D dose calculation using CT images. In addition, a traditional dose distribution without dose optimization was applied to the target volume for conformal ICBT. In recent years, the technique of ICBT planning for cervical cancer has shown rapid advancement, from orthogonal plane X-ray image-based 2D planning to multi-modality 3D image-based 3D-conformal planning [4, 5]. Based on these recent changes, the use of multimodality 3D image data is recommended for accurate target delineation and customized 3D-conformal ICBT (3D-ICBT) planning based on the target volume, rather than traditional planning, which applies a uniform pear-shaped dose pattern by point A in ICBT for cervical cancer. In addition, for accurate dose evaluation and specific reports of planning results, cumulative dose volume histograms (DVH) are recommended, particularly in terms of D90, D100, doses encompassing 90% and 100% of the CTV, V150,or V200, which is enclosed by 150% or 200% of the prescribed dose, respectively, for the target, and D0.1 cm3, D1 cm3, and D2 cm3, which are the minimum dose in the most irradiated tissue volume adjacent to the applicator (0.1, 1 and 2 cm3), for the OAR, instead of the point dose, as proposed by the International Commission on Radiation Units and Measurements Report 38 (ICRU 38) [6]. Differences in the dose distribution between the AICBT and 3D-ICBT planning were reported in a previous study [7, 8]. A re-evaluation of the bladder distention effect according to the new guidelines is needed. The effects of bladder distention are expected to differ between the two treatment methods. The aim of this study was to evaluate the effects of bladder distention on geometrical and dosimetrical variation between AICBT and 3D-ICBT planning for cervical cancer with recent new dose evaluation guidelines.
MATERIALS AND METHODS
Patient selection and CT scanning
With approval from the Institutional Review Board at Samsung Cancer Center, 10 patients with cervical cancer, FIGO Stages IIB to IIIB, who underwent PET/CT-guided high dose rate (HDR) ICBT with a dose schedule of 4 Gy/fraction for 6 fractions after EBRT, were included in this study. All patients had squamous cell carcinoma with a bulky size of 4 cm or greater. The patient characteristics were summarized in Table 1. We obtained two sets of CT images, with and without bladder distention, for each patient to evaluate geometrical variation and the resulting dosimetrical effects associated with bladder distention. CT scans (PET/CT, discovery STe, GE Healthcare, Milwaukee, WI, USA) were performed for all patients soon after insertion of a standard Fletcher type applicator without shielding. The first CT scan was performed with an empty bladder (EB), which was drained intentionally by insertion of a Foley catheter. After the first CT scan, the bladder was gradually filled as much as possible, to median 367 cm3(range, 215–597), by intravenous administration of saline, without causing discomfort to the patient or changing patient position. Using the same scan conditions, a second set of full bladder (FB) CT scans was performed. For consistency, all CT scans were performed with 2.5 mm-slice thickness up to the intervertebral disk space (L4–5). Following all the CT scans, additional PET images were obtained for PET/CT image-guided target delineation.
Table 1.
Patient characteristics
| Characteristics | Number of patients |
|---|---|
| Age | Median 55 years (range, 44 to 78 years) |
| Tumor size | Median 5.2 cm (range, 4 to 6 cm) |
| FIGO stage | |
| IIB | 9 |
| IIIB | 1 |
| Pelvic lymph node | |
| positive | 9 |
| negative | 1 |
| Paraaortic lymph node | |
| positive | 3 |
| negative | 7 |
FIGO = the International Federation of Gynecology and Obstetrics.
Analysis of geometrical variation due to bladder distention
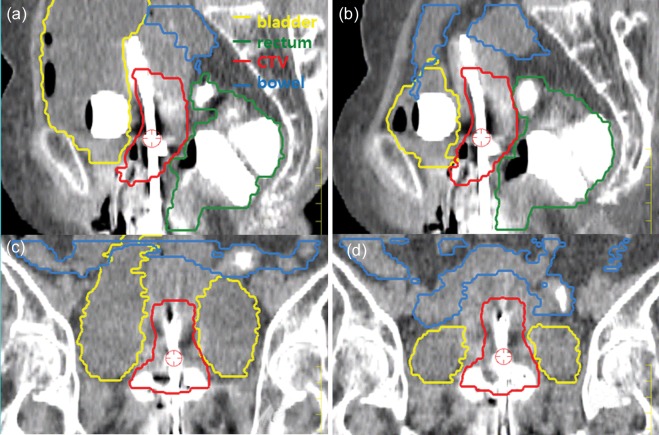
The two sets of CT images were transferred into the treatment planning system (TPS, Pinnacle3, Philips Medical System, Holland), because more detailed and easy contouring and calculating of volume statistics could be obtained using TPS for EBRT than dedicated TPS for brachytherapy (PLATO, version 14.3, Nucletron, the Netherlands) and co-registered, based on applicator position using fusion software. The external contours of the clinical target volume (CTV), bladder, rectum and small bowel were delineated on both fused image sets (Fig. 1). The outer wall of the rectum was defined from the anus to the recto-sigmoid flexure. The small bowel was contoured up to the CT slice, and the uterine tip was visible. The gross tumor volume (GTV) was determined by adjusting the window and level of PET/CT to a reasonable value and outlining the edge of the enhancing area, which was done in consultation with a nuclear medicine physician. A PET/CT guided volume-based plan was designed to cover the CTV, which included the GTV with 1 cm expansion toward the soft tissue mass boundaries of the uterus, cervix and vagina on the axial and sagittal images [9]. The entire cervix was included in all patients. In order to eliminate inter-observer variation, delineation of all CTV and OARs was performed at the same time by an attending radiation oncologist with sufficient experience in ICBT, using fusion software. The positions of the centers of mass and volume of both CTV and OARs were measured in order to verify the geometrical variation with bladder distention.
Fig. 1.
Two sets of CT images for a patient [with (aand c) and without (band d) bladder distention] were registered based on the applicator position, and reconstructed in the sagittal (aand b) and coronal (cand d) plan. Figures show a significant geometrical change of the bladder and small bowel as the result of bladder distention, while clinically acceptable minor changes were detected in the CTV and rectum. The distended bladder pushed the small bowel upward (aand c).
Treatment planning and analysis of dosimetrical effect of bladder distention
The two sets of CT images obtained with and without bladder distention, and all contour data obtained from the image sets for each patient, were sent to TPS for brachytherapy through the DICOMRT protocol. For comparison of the differences in dose effects between AICBT and 3D-ICBT planning with respect to bladder distention, four sets of treatment plannings were performed for each patient, using both AICBT and 3D-ICBT planning with two sets of CT images. For AICBT planning, point A was defined as 2.0 cm above and lateral to the cervical os, and 4 Gy of treatment dose at this point was prescribed (Fig. 2a). The source positions were defined by referring to the tip end of both a tandem and two colpostats on CT images for each patient. The traditional dwell point and time of standard low-dose rate (LDR) ICBT were applied without dose optimization to the target volume.
Fig. 2.
Pictures showing an example of the difference in dose distribution between AICBT (a)and 3D-ICBT (b). The AICBT plan, which has a uniform pear-shaped dose pattern based on point A, produces a relatively large high-dose volume compared with the 3D-ICBT plan. The 3D-ICBT plan showed better dose conformity by dose optimization to the target volume.
For comparison of the effects of bladder distention on dose according to different planning techniques, a 3D-ICBT plan was generated using two sets of CT images for each patient. Using a graphical dose optimization tool, which can adjust isodose line with the click of a mouse on the screen, supplied by TPS for brachytherapy, the plan was optimized to reference dose points on the surface of the CTV by iteration of manual trial, followed by manual fine-tuning of the dwell point and its weights while monitoring the DVH data for CTV and OARs (Fig. 2b and 3b). We prescribed 4 Gy to reference dose points on the surface of the CTV, while limiting the dose for OARs (D2cc) to less than the prescribed dose level and as low as possible. For dose comparison, we obtained dose characteristics for doses encompassing 90% and 100% (D90and D100) of the CTV and the maximum dose, 1 cm3and 2 cm3, to the most irradiated normal tissue volume (D1 cm3and D2 cm3) for OARs based on the GEC ESTRO guidelines [5] from 4 sets of planning for each patient, a total of 40 sets. In addition, in order to understand patterns of difference in dose distribution, we measured and compared the volume received over the prescribed dose (V4 Gy) in the body for both AICBT and 3D-ICBT.
We conducted nonparametric Wilcoxon signed-rank tests (PASW statistics ver. 18, IBM, USA) for the quantitative analysis of geometrical and dosimetrical variation for CTV and OAR due to bladder distention, with statistical significance set at 5%.
RESULTS
Analysis of geometrical variation due to bladder distention
The average volume of an empty bladder (EB) was 124.4 ± 32.7 cm3. The volume significantly increased to 470.1 ± 120.7 cm3with bladder distention (full bladder, FB) through intravenous saline injection. Significant changes in the volume and position of the bladder were detected in our statistical analysis (P< 0.05) (Table 2). Distention of the bladder pushed the small bowel superiorly in the patient (Fig. 1). Due to this geometrical change, the volume of the small bowel was significantly decreased (EB–FB), with a median volume of 92.5 cm3(range, –16.2 to 132.5 cm3; P< 0.001). The average position of the center of mass significantly changed in the longitudinal direction (Y) for the small bowel (median, –0.94 cm; range, 2.81 to –0.15 cm; P= 0.003) and bladder (median, –2.39 cm; range, –3.72 to –1.46 cm; P< 0.001). At the same time, the position of the center of mass of the bladder changed in the vertical direction (Z), while there was no observable change in the position of the small bowel. Statistical analysis showed that there was no significant change in the position of the center of mass in the lateral direction (X) in either the bladder or small bowel (P> 0.05).
Table 2.
Differences (EB–FB) in position of center of mass and volume for CTV and OARs due to bladder distention
| CTV |
Rectum |
Bladder |
Bowel |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position differences of center of mass (cm) |
Volume difference (cm3) | Position differences of center of mass (cm) |
Volume difference (cm3) | Position differences of center of mass (cm) |
Volume difference (cm3) | Position differences of center of mass (cm) |
Volume difference (cm3) | |||||||||
| X | Y | Z | X | Y | Z | X | Y | Z | X | Y | Z | |||||
| Median (EB–FB) | –0.01 | 0.00 | –0.04 | 0.65 | 0.00 | 0.00 | 0.03 | –3.7 | 0.06 | –2.39 | 1.09 | –367.3 | –0.08 | –0.94 | 0.34 | 92.5 |
| Range | –0.02 to 0.03 | –0.03 to 0.06 | –0.19 to 0.04 | –0.53 to 1.90 | –0.16 to 0.45 | –0.22 to 0.27 | –0.07 to 0.38 | –15.3 to 15.2 | –0.84 to 1.13 | –3.72 to –1.46 | 0.60 to 4.33 | –597.6 to –215.8 | –0.43 to 0.90 | 2.81 to –0.14 | –3.58 to 1.59 | –16.2 to 132.5 |
| Pvalue | 0.209 | 0.182 | 0.021 | 0.026 | 0.450 | 0.505 | 0.044 | 0.412 | 0.915 | <0.001 | 0.002 | <0.001 | 0.810 | 0.003 | 0.805 | <0.001 |
X = lateral, Y = longitudinal, Z = vertical, EB = empty bladder, FB = full bladder.
A clinically acceptable small volume difference was detected in the CTV (median, 0.65 cm3; range –0.53 to 1.90 cm3; P= 0.026), however, there was no significant volume difference in the rectum as a result of bladder distention (P= 0.412). The average position of the center of mass of the CTV (difference, median –0.04 cm; range –0.19 to 0.04 cm; P= 0.021) and rectum (difference, median 0.03 cm; range –0.07 to 0.38 cm; P= 0.021) were moved slightly downward in the Z direction due to bladder distention, however, the change in position was clinically acceptable.
Analysis of dosimetrical effect of bladder distention on different planning techniques
Major differences in dose distribution were observed between AICBT and 3D-ICBT planning. The high-dose region increased over a wide area in AICBT planning compared with 3D-ICBT planning (Fig. 2). This finding resulted in a significant difference in the volume received over the prescribed dose (V4 Gy) [123.70 ± 14.47 cm3(AICBT) vs 58.43 ± 15.50 cm3(3D-ICBT), P= 0.005]. However, due to irregularity in the CTV, this dose expansion, which has a uniform pattern (a pear-shape based on point A), did not result in improved dose conformity for CTV, when compared with the 3D-ICBT plan (Fig. 2).
This difference in dose distribution showed different dosimetrical effects of bladder distention between AICBT and 3D-ICBT planning (Table 3and Fig. 3). No significant dose differences due to bladder distention were observed in terms of D100and D90for CTV and D2 cm3and D1 cm3for the rectum in either planning type (P> 0.05). However, the average D2 cm3and D1 cm3for the bladder showed a significant increase, from 4.38 and 4.62 Gy to 5.31 and 5.55 Gy, respectively, with AICBT (P< 0.05). However, we did not observe any significant dose differences in D2 cm3and D1 cm3for the bladder with 3D-ICBT planning due to bladder distention (P> 0.05). In contrast, the average D2 cm3and D1 cm3for the small bowel were significantly decreased with AICBT (P< 0.05), while no significant dose differences in D2 cm3and D1 cm3were observed in 3D-ICBT due to bladder distention (P> 0.05). However, the mean doses (D50) of the bladder and small bowel were significantly decreased by bladder distention with both the AICBT and 3D-ICBT plans (P< 0.05).
Table 3.
Different dose effects of the bladder distention for AICBT and 3D-ICBT planning (Gy)
| CTV |
Rectum |
Bladder |
Small bowel |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D90 | D100 | D50% | D2 cm3 | D1 cm3 | D50% | D2 cm3 | D1 cm3 | D50% | D2 cm3 | D1 cm3 | ||
| AICBT | EB | 5.27 ± 0.71 | 3.49 ± 0.82 | 1.42 ± 0.36 | 3.72 ± 0.69 | 4.05 ± 0.79 | 1.61 ± 0.34 | 4.38 ± 0.80 | 4.62 ± 0.82 | 1.02 ± 0.24 | 4.32 ± 1.16 | 4.69 ± 1.22 |
| FB | 4.89 ± 1.11 | 3.23 ± 0.97 | 1.46 ± 0.38 | 3.76 ± 0.80 | 4.08 ± 0.85 | 1.01 ± 0.22 | 5.31 ± 1.09 | 5.55 ± 1.10 | 0.70 ± 0.31 | 3.23 ± 0.91 | 3.75 ± 1.02 | |
| Pvalue | 0.314 | 0.594 | 0.154 | 0.203 | 0.241 | 0.005 | 0.007 | 0.007 | 0.005 | 0.005 | 0.005 | |
| 3D-ICBT | EB | 4.66 ± 0.51 | 2.91 ± 0.51 | 0.99 ± 0.33 | 3.23 ± 1.12 | 3.48 ± 1.26 | 0.96 ± 0.25 | 3.12 ± 0.85 | 3.43 ± 0.91 | 0.55 ± 0.12 | 2.74 ± 0.86 | 3.20 ± 1.10 |
| FB | 4.61 ± 0.47 | 2.89 ± 0.56 | 1.00 ± 0.34 | 3.30 ± 1.24 | 3.60 ± 1.31 | 0.59 ± 0.13 | 3.18 ± 1.13 | 3.48 ± 1.09 | 0.40 ± 0.16 | 2.33 ± 0.90 | 2.74 ± 1.16 | |
| Pvalue | 0.445 | 0.635 | 0.553 | 0.515 | 0.475 | 0.005 | 0.878 | 0.859 | 0.005 | 0.074 | 0.093 | |
AICBT = point A-based intracavitary brachytherapy, 3D-ICBT = 3D conformal intracavitary brachytherapy, EB = empty bladder, FB = full bladder.
Fig. 3.

Comparison of the dose volume histograms for bladder, rectum and bowel, and the CTV of a patient between the AICBT (a)and the 3D-ICBT (b)plan.
DISCUSSION
In the past, most cervical cancer patients received ICBT with a urinary catheterization to empty the bladder for LDR brachytherapy. However, HDR brachytherapy is now more commonly used. There have been some reports regarding the advantages of bladder distension and relationship with bladder and rectal dose during HDR ICBT [1–3]. Pilepich et al.demonstrated that the OAR dose could be minimized during ICBT by using contrast material to maintain a residual vesical volume of 200–300 ml. This approach resulted in a significant reduction in radiation exposure to a large part of the bladder, while only minor displacement of the implant system was observed [1]. According to Sun et al., bladder distension results in a statistically significant decrease in the median bladder wall dose (D50) by an average of 48%, with no change in the maximum dose (D5); on the other hand, bladder distension had no adverse effects on the rectal wall dose [2]. From similar experiments, Kim et al.reported that bladder distention results in a significant reduction in bowel D2 cm3values, with an increase in bladder D2 cm3values [3]. In these studies, the conventional AICBT planning technique with 3D dose calculation was employed, but dose optimization to the target volume for conformal ICBT was not applied. We obtained similar results in our AICBT planning. However, no significant dose change was observed in either CTV or OARs in our 3D-ICBT planning. There is a possible explanation for this finding. The value of the volume that received the prescribed dose (V4 Gy) with the AICBT planning was significantly larger than that with 3D-ICBT (123.7 ± 14.4 cm3vs 58.4 ± 40.4 cm3, respectively, P= 0.005) (Fig. 2). Thus, although the posterior wall of the bladder drew slightly closer to, and the small bowel receded from, the radiation source due to bladder distention, the resulting dosimetrical effect was not significant, meaning that the dosimetrical change due to bladder distention could be minimal with 3D-ICBT planning. Bladder distention by intentional injection of sterilized water for every treatment session can be uncomfortable for patients and cumbersome to perform, while providing little benefit for the treatment. 3D-ICBT planning can eliminate this critical issue.
According to the geometrical analyses of the CTV and OARs, there were two major geometrical variations with bladder distention. First, the posterior wall of the bladder was distended slightly toward the radiation source, while other parts of the bladder wall significantly receded from the radiation source (Fig. 1). That is, the driving mean dose (D50) of the bladder was significantly reduced, while the D2 cm3and D1 cm3of the bladder increased in AICBT, which has a relatively larger high-dose volume than 3D-ICBT. However, we did not observe a significant dose change in the D1 cm3and D2 cm3of the bladder in 3D-ICBT. In addition, volumetric and positional changes of the rectum and target with bladder distention were not observed in our investigation. Applicators, which are fixed by clamps, play vital roles as rigid supporters of both the target and rectum against the downward pressure from a distended bladder. As a result, a significant dosimetrical change in the CTV and rectum due to bladder distention was not observed for either AICBT or 3D-ICBT.
Second, the distended bladder pushed the bowel in the superior direction in the patient. With AICBT, the D2cm3and D1cm3for the small bowel were 4.32 ± 0.24 Gy and 4.69 ± 1.22 Gy, respectively, under the conditions of an EB. However, under FB conditions, the value declined to 3.23 ± 0.91 and 3.75 ± 1.02 Gy, respectively, because the small bowel was pushed in the superior direction. However, the average D2cm3and D1cm3of the small bowel significantly decreased to 2.74 ± 0.86 and 3.20 ± 1.10 Gy, respectively, under an empty bladder with 3D-ICBT planning, and a significant dose reduction due to bladder distention was not detected in the statistical analysis (P> 0.05) (Fig. 3). One reason is that the radiation source has been dwelled up to the tip end of the tandem to cover the entire uterus, classically regarded as the target volume, and to make a traditional pear-shaped dose distribution in the AICBT planning. This produces an expansion of the high-dose region to up around the small bowel, which can lead to complications [10] and sensitizeation to dose variation due to bladder distention. However, this effect is not uncommon using the recent multi-modality image-guided 3D-ICBT approach, which optimizes radiation dose to image-based target volume, because, in general, tumor spread up to the uterine fundus is unusual in ICBT after EBRT for cervical cancer. Reduction of the tandem source dwell length from the tip end to fit the image-based target volume contributes to a reduction in the radiation dose near the small bowel [11].
Recently, more conformal and adequate tumor coverage was reported for large tumors with image-guided ICBT, combined with the interstitial brachytherapy technique [12, 13]. This approach has the potential to increase the target coverage with minimal increase in the dose to OARs, and further study to evaluate the effect of bladder distention when using this new technique may be necessary.
Although, according to our statistical analysis, geometrical variations were detected in the bladder, small bowel, rectum and CTV as a result of bladder distention. However, variations in the CTV and rectum were imperceptible and clinically acceptable. These changes caused a dosimetrical change in ICBT planning which differed for AICBT and 3D-ICBT planning. We did not observe significant dose differences in the CTV and rectum due to bladder distention for either of the planning techniques. However, the average D2 cm3and D1cm3for the bladder showed a significant increase, while the average D2cm3and D1cm3for the small bowel were significantly decreased by bladder distention with AICBT. In contrast, no significant dose changes in terms of D2cm3and D1cm3were detected in the bladder, small bowel, or rectum with 3D-ICBT. The mean doses (D50) of the bladder and small bowel were significantly decreased by bladder distention with both the AICBT and 3D-ICBT plans. Further study of the biological effect of low mean dose for bladder and small bowel will be required in the future. However, the new trend of 3D-ICBT planning may eliminate the issue of whether or not to fill the bladder for small CTV in ICBT planning for cervical cancer.
FUNDING
This work was supported by the Technology Innovation Program, 10040362, Development of an Integrated Management Solution for Radiation Therapy, funded by the Ministry of Knowledge Economy (MKE, Korea).
ACKNOWLEDGEMENTS
The authors would to thank Mr Dong Gyun Oh, RTT, Samsung Medical Center, for his technical support.
REFERENCES
- 1.Pilepich MV, Prasad S, Madoc-Jones H, et al. Effect of bladder distension on dosimetry in gynecological implants. Radiology. 1981;140:516–8. doi: 10.1148/radiology.140.2.7255731. [DOI] [PubMed] [Google Scholar]
- 2.Sun LM, Huang HY, Huang EY, et al. A prospective study to assess the bladder distension effects on dosimetry in intracavitary brachytherapy of cervical cancer via computed tomography-assisted techniques. Radiother Oncol. 2005;77:77–82. doi: 10.1016/j.radonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Kim RY, Shen S, Lin HY, et al. Effects of bladder distension on organs at risk in 3D image-based planning of intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76:485–9. doi: 10.1016/j.ijrobp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–45. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 6.ICRU. Bethesda, MD: International Commission on Radiation Units and Measurements; 1985. Dose and volume specification for reporting intracavitary therapy in gynecology (Report 38) [Google Scholar]
- 7.Tanderup K, Nielsen SK, Nyvang GB, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol. 2010;94:173–80. doi: 10.1016/j.radonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Beriwal S, Houser C, et al. Dosimetric analysis of 3D image-guided HDR brachytherapy planning for the treatment of cervical cancer: is point A-based dose prescription still valid in image-guided brachytherapy? Med Dosim. 2011;36:166–70. doi: 10.1016/j.meddos.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Nam H, Huh SJ, Ju SG, et al. 18F-fluorodeoxyglucose positron emisson tomography/computed tomography guided conformal brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2012;84:e29–34. doi: 10.1016/j.ijrobp.2012.02.055. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Huh SJ, Oh D, et al. Radiation sigmoiditis mimicking sigmoid colon cancer after radiation therapy for cervical cancer: the implications of three-dimensional image-based brachytherapy planning. J Gynecol Oncol. 2012;23:197–200. doi: 10.3802/jgo.2012.23.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara K, Nemoto K, Ohnishi K, et al. Classical tandem-source dwelling covering the entire uterus: essential in modern intracavitary radiotherapy for cervical cancer? Radiat Med. 2007;25:386–92. doi: 10.1007/s11604-007-0154-2. [DOI] [PubMed] [Google Scholar]
- 12.Kirisits C, Lang S, Dimopoulos J, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624–30. doi: 10.1016/j.ijrobp.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Jurgenliemk-Schulz IM, Tersteeg RJ, Roesink JM, et al. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–30. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]