Abstract
Intensity-modulated radiation therapy, when used in the clinic, prolongs fraction delivery time. Here we investigated both the in vivoand in vitroradiobiological effects on the A549 cell line, including the effect of different delivery times with the same dose on A549 tumor growth in nude mice. The in vitroeffects were studied with clonogenic assays, using linear-quadratic and incomplete repair models to fit the dose-survival curves. Fractionated irradiation of different doses was given at one fraction per day, simulating a clinical dose-time-fractionation pattern. The longer the interval between the exposures, the more cells survived. To investigate the in vivoeffect, we used sixty-four nude mice implanted with A549 cells in the back legs, randomly assigned into eight groups. A 15 Gy radiation dose was divided into different subfractions. The maximum and minimum tumor diameters were recorded to determine tumor growth. Tumor growth was delayed for groups with prolonged delivery time (40 min) compared to the group receiving a single dose of 15 Gy (P< 0.05), and tumors with a 20 min delivery time had delayed growth compared to those with a 40 min delivery time [20′ (7.5 Gy × 2 F) vs 40′ (7.5 Gy × 2 F), P= 0.035; 20′ (3 Gy × 5 F) vs 40′ (3 Gy × 5 F); P= 0.054; 20′ (1.67 Gy × 9 F) vs 40′ (1.67 Gy × 9 F), P= 0.028]. A prolonged delivery time decreased the radiobiological effects, so we strongly recommend keeping the delivery time as short as possible.
Keywords: radiobiological effect, A549 cell, in vitro assay, nude mice
INTRODUCTION
The current popularity of intensity-modulated radiation therapy (IMRT), an irradiation technique developed to restrict the target dose and spare normal tissue, has improved treatment results [1–3]. This new technique generally employs multiple arc or fixed beams from a linear accelerator. Therefore, considerable time is required to setup each dose, including positioning of the gantry and couch [4–6]. Whereas conventional external beam radiotherapy takes about 2–5 min to deliver a dose fraction, IMRT with static delivery typically requires 15–45 min to deliver the same dose. In the 1960s, Elkind and coworkers [7–9] reported that, after radiation, cultured mammalian cell survival increased when there were intervals between the two radiation doses. This phenomenon is known as repair of sublethal damage (SLDR). The purpose of this study is to investigate the radiobiological effects of prolonged radiation exposure on A549 cells, and the effect of different delivery times with the same dose on tumor growth in nude mice implanted with A549 cells.
MATERIALS AND METHODS
Reagents
The A549 lung cancer cell line was purchased from the Shanghai Cell Bank of the Chinese Academy of Medical Science (acquired from the American Type Culture Collection, Shanghai, China). 1640 culture medium was obtained from GIBCO (Shanghai, China). Fetal calf serum (FCS) was purchased from Hyclone (Shanghai, China). Penicillin and streptomycin were provided by Shanghai Sanda Jinyi Tech Info (Shanghai, China). Hematoxylin and 95% ethanol were purchased from Shanghai Chemical Reagent Factory No. 2 (Shanghai, China).
Cell culture, radiation and radiation schedule
The A549 cells were maintained in 1640 medium containing 20% FCS with 100 U/ml penicillin and 100 µg/ml streptomycin in a humidified atmosphere supplemented with 5% CO2at 37°C. Exponentially growing cells were used for experiments. Irradiation was carried out at room temperature using a 180 kV X-ray machine [X.S.S 250(F2); Guiyang Medical Equipment, Guizhou, China]. Doses of 0, 2, 4, 6 or 8 Gy were given as a single dose at 102.3 cGy/min. A549 cells were given fractionated radiation in doses of 2 Gy, 2 Gy × 2, 2 Gy × 3, or 2 Gy × 4 at one treatment per day to simulate clinical dose fractionation patterns. Each fraction dose was given in two equal subfractions, with an interval between subfractions of 2 min, 20 min or 40 min per fraction. Three parallel samples were treated at each radiation dose with varying schedules.
Clonogenic assays
Cells plated in culture flasks were incubated undisturbed for 10 d. Following radiation exposure, cells were fixed with 95% ethanol and stained with hematoxylin. Colony counts (a colony was defined as 50 or more cells) were performed by visual inspection. Colony plating efficiency was calculated as the number of viable cells expressed as a percentage of cells plated. Cell survival at each dose of each irradiation protocol was determined by dividing the plating efficiency of the irradiated cells by that of the untreated control. Dose-survival curves for each experiment were constructed by plotting the mean surviving fractions semi-logarithmically as a function of irradiation dose. The data were analyzed, and survival curves were plotted following the linear quadratic (LQ) model 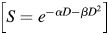 [10, 11] using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). The value of α and β from the best fit survival curves were used to calculate cell survival at 2 Gy (SF2). Differences between the survival under different irradiation protocols were studied with a paired t-test.
[10, 11] using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). The value of α and β from the best fit survival curves were used to calculate cell survival at 2 Gy (SF2). Differences between the survival under different irradiation protocols were studied with a paired t-test.
Xenograft model radiation and radiation schedule
A549 cells subcultured the day before transplantation were inoculated subcutaneously into the right hind legs (100 000 cells per leg) of male 6–8 week old nude mice. The Ethics Committee of Fudan University Shanghai Cancer Center assessed the procedures and protocols of this manuscript carefully, and confirmed that the animal experiments in this paper have complied fully with the requirements of their Ethics Code of Research involving animals for scientific purposes.
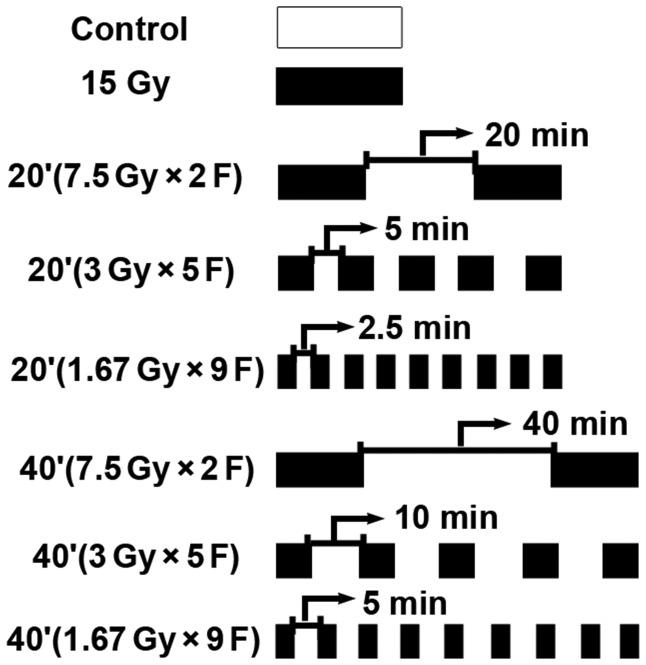
Experiments were performed when the tumor reached 0.8 to 1 cm in diameter. Sixty-four nude mice were randomized into eight groups: control; a single fraction at 15 Gy; two subfractions, with a 20 min interval; five subfractions, with a 5 min interval; nine subfractions, with a 2.5 min interval; two subfractions, with a 40 min interval; five subfractions, with a 10 min interval; and nine subfractions, with a 5 min interval (Fig. 1). Unanesthetized tumor-bearing mice were immobilized in a jig with customized modules and irradiated using a 6 MV X-ray machine (GK-06-100; Shanghai Hi-Tech Radiotherapy Equipment Co., Shanghai, China) at 105.7 cGy/min at a depth of 1 cm. All mice were shielded with a specially designed lead apparatus to allow irradiation only to the right hind limb. Mice were kept under these conditions until all irradiation was finished.
Fig. 1.
Schematic diagram of radiation schedule.
In vivostudy
Tumor dimensions were measured with Vernier calipers once every three days. Tumor volumes were calculated using the Steel formula: a*b2/2. (a= length, b= width of the tumor). The tumor growth time (TGT) was defined as the time required for the initial tumor size to quadruple after the first day of treatment. The tumor growth delay time was defined as the TGT in each treated animal minus the mean TGT in the control group.
Statistical analysis
SPSS v. 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data. All data are presented as the mean ± standard deviation (SD) from three independent experiments. A Pvalue of less than 0.05 was considered significant.
RESULTS
The effect of total interfraction interval time on A549 cells in vitro
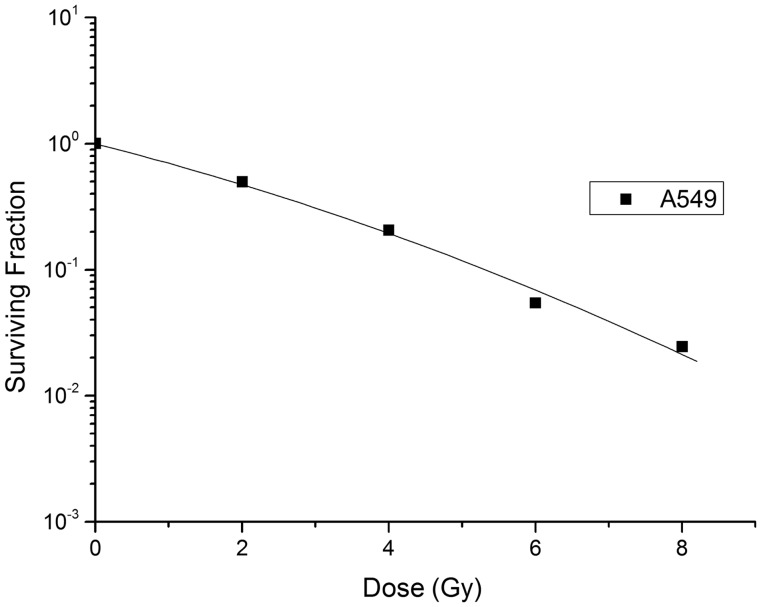
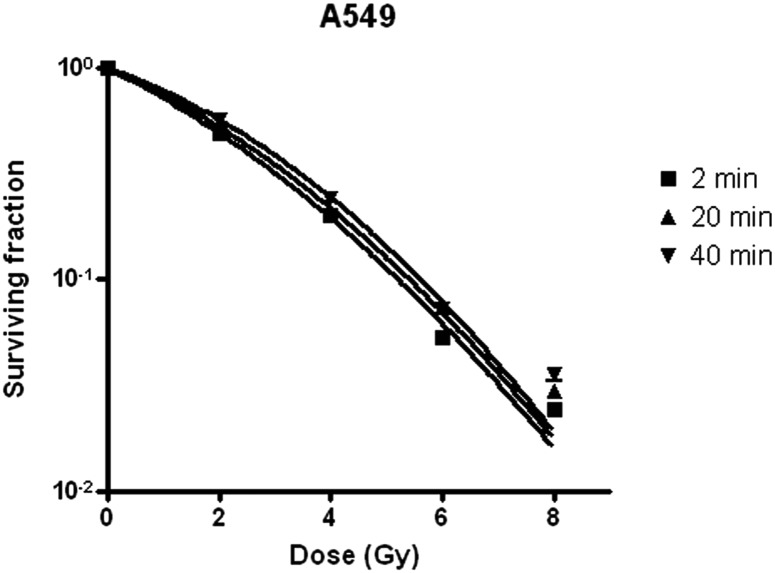
A standard dose-survival curve of A549 cells, fit to the standard LQ model, is shown in Fig. 2. The radiobiological characteristics of the A549 cell line, described with the parameters of the mathematical models, are listed in Table 1. A549 cells survived irradiation for a total fraction delivery time of 2, 20 or 40 min in a manner proportional to the interfraction interval (Table 2). Statistical analysis confirmed that longer delivery time is the main reason for higher SF2value (Table 3). The dose-survival curve of irradiated A549 cells was fitted to an LQ model (Fig. 2). Effects of fractionated irradiation on A549 cell survival are shown in Fig. 3.
Fig. 2.
The A549 cell dose-survival curve fitted by the linear-quadratic model.
Table 1.
A549 cell parameters fit to the LQ model
| Cell line | SF2READ | SF2EST | α (Gy−1) | β (Gy−2) | α/β (Gy) |
|---|---|---|---|---|---|
| A549 | 0.49 | 0.50 | 0.30 | 0.02 | 12.4 |
SF2READ= surviving fractions of 2 Gy (read), SF2ESTimate= surviving fractions of 2 Gy (estimated).
Table 2.
Surviving fraction of A549 cell irradiated with a total fraction delivery time of 2, 20 or 40 min
| DOSE (Gy) | SF ± SD (2 min) | SF ± SD (20 min) | SF ± SD (40 min) |
|---|---|---|---|
| 0 | 1 | 1 | 1 |
| 2 | 0.49 ± 0.27 | 0.53 ± 0.04 | 0.57 ± 0.02 |
| 4 | 0.20 ± 0.01 | 0.21 ± 0.02 | 0.24 ± 0.01 |
| 6 | 0.05 ± 0.00 | 0.07 ± 0.01 | 0.07 ± 0.00 |
| 8 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 |
Table 3.
Results of t-tests for cell survival after exposure to 8 Gy using different fraction delivery time protocols
| Fraction delivery time time protocols | T | P |
|---|---|---|
| Irradiate for 2 min vs 20 min | 2.07 | 0.05 |
| Irradiate for 2 min vs 40 min | 2.15 | 0.05 |
| Irradiate for 20 min vs 40 min | 1.80 | 0.07 |
Fig. 3.
Effects of fractionated irradiation on A549 cell survival. Cells received fractionated irradiation of 2 Gy, 2 Gy × 2, 2 Gy × 3, and 2 Gy × 4, given with one fraction per day simulating clinical dose-time-fractionation pattern. Each fraction dose was given in two equal sub-fractions, with a total fraction delivery time of 2min, 20 min, and 40 min per fraction.
The effect of total interfraction interval time on the growth of tumor tissues
The results of the growth delay experiment are shown in Fig. 4, with TGT listed in Table 4. The effects of 15 Gy given in 40 min were much lower than that of a single 15 Gy dose, but there were no substantial differences between the 20 min group and the single 15 Gy dose group. A 15 Gy dose given as 2, 5 or 9 subfractions in 20 min or in 40 min did not make a difference (P> 0.05; Table 5). However, 15 Gy given in 40 min had significantly less effect than 20 min [20′ (7.5 Gy × 2 F) vs40′ (7.5 Gy × 2F), P= 0.035; 20′ (3 Gy × 5 F) vs40′ (3 Gy × 5 F); P= 0.054; 20′ (1.67 Gy × 9 F) vs40′ (1.67 Gy × 9 F), P= 0.028; Table 6].
Fig. 4.
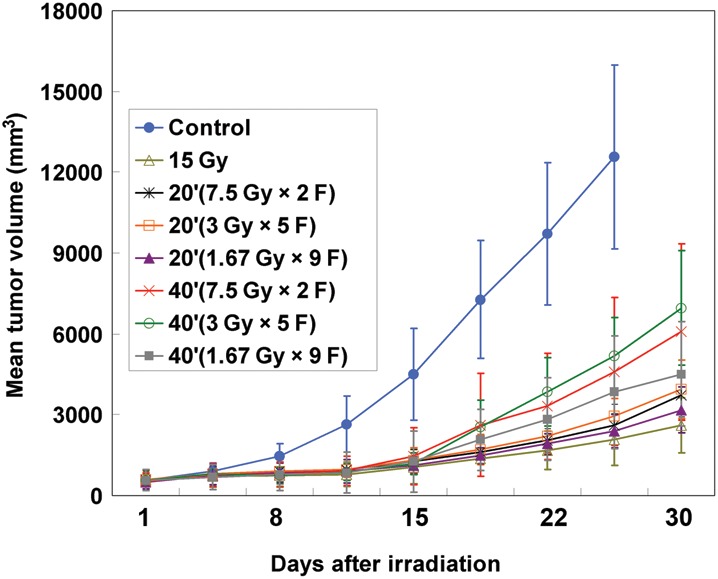
Radiation decreased A549-derived tumor volumes. Growth of A549-derived tumors in nude mice was monitored as described in the Materials and Methods section. Tumor volume was decreased after a single dose of 15 Gy, or 15 Gy given as 2, 5, or 9 subfractions for 20 min or 40 min. Bars represent SD for eight mice.
Table 4.
Tumor growth time (TGT) to reach four times the initial volume
| Group | TGT (days) |
|
|---|---|---|
| Mean | SE | |
| 15 Gy as a single dose | 9.2 | 1.8 |
| 20′ (7.5 Gy × 2 F) | 6.7 | 3.9 |
| 20′ (3 Gy × 5 F) | 7.0 | 2.2 |
| 20′ (1.67 Gy × 9 F) | 8.2 | 3.9 |
| 40′ (7.5 Gy × 2 F) | 3.9 | 2.6 |
| 40′ (3 Gy × 5 F) | 4.4 | 2.4 |
| 40′ (1.67 Gy × 9 F) | 5.2 | 2.5 |
Table 5.
Results of t-tests for TGT for the 20 min or 40 min groups with different subfractions
| Group vs Group | P |
|---|---|
| 20′ (7.5 Gy × 2 F) vs 20′ (3 Gy × 5 F) | 0.85 |
| 20′ (7.5 Gy × 2 F) vs 20′ (1.67 Gy × 9 F) | 0.27 |
| 20′ (3 Gy × 5 F) vs 20′ (1.67 Gy × 9 F) | 0.35 |
| 40′ (7.5 Gy × 2 F) vs 40′ (3 Gy × 5 F) | 0.71 |
| 40′ (7.5 Gy × 2 F) vs 40′ (1.67 Gy × 9 F) | 0.31 |
| 40′ (3 Gy × 5 F) vs 40′ (1.67 Gy × 9 F) | 0.51 |
Table 6.
Results of t-tests for TGT for the 20 min and 40 min groups with the same subfractions
| Group vs Group | P |
|---|---|
| 20′ (7.5 Gy × 2 F) vs 40′ (7.5 Gy × 2 F) | 0.035 |
| 20′ (3 Gy × 5 F) vs 40′ (3 Gy × 5 F) | 0.054 |
| 20′ (1.67 Gy × 9 F) vs 40′ (1.67 Gy × 9 F) | 0.028 |
DISCUSSION
The LQ model was used to examine the relationship between prolonged fraction delivery time and the radiobiological effects. While much work has been done to evaluate the effects of radiation on tumor cells, a few radiobiological issues remain unresolved regarding the evaluation of radiation doses employed in the treatment modalities. Here, we investigated the association between delivery time and the radiobiological effects using both in vitroand in vivomodels.
After radiation of cultured mammalian cells, cell survival increased when intervals were used between two radiation doses [7–9]; the phenomenon is known as SLDR. Wang et al. [12] calculated the influence of prolonged fraction delivery time using the generalized LQ model, and cautioned that the total time to deliver a single fraction may have a significant impact on IMRT treatment outcome for tumors with a low α/β ratio and a short repair half-time, such as prostate cancer. Mu et al. [13] showed that several radiobiological models underestimated the reduced efficiency of 10 and 20 min prolonged delivery of 2 Gy fractions to V79 Chinese hamster lung cells, while prolonging the fraction time spares tissues with rapid DNA repair. Zheng et al. [14] found that prolonged fraction delivery time modeling IMRT significantly decreased cell killing in HepG2 human hepatocellular carcinoma cells, but not in Hep3b cells. The authors proposed that SLDR was the predominant factor that decreased cell killing. In our in vitrostudy, cell survival increased when the interfraction interval was longer. These observations are consistent with a previous report that showed that cell survival increased in mouse EMT6 and SCCVII cells receiving fractionated radiation [15]. Shibamoto et al. [16] reviewed the current literature and concluded that two-fraction irradiation would yield the greatest effect among the fractionated radiation groups.
A previous in vitrostudy showed that the biological effects of radiation significantly decreased in EMT6 and SCCVII cells when doses of 2 and 8 Gy were delivered in 2, 5 or 10 fractions and the interfraction intervals were 2 min or longer [17]. The results of the in vivostudy, however, were in striking contrast with those of the in vitrostudies. In the 2-fraction experiments, the increase in cell survival with elongation of intervals was much less than that observed in the in vitrostudy [18]. In both of the 5- and 10-fraction experiments, no significant increase in cell survival resulting from fractionation was observed. Moreover, cell survival decreased at various time points when radiation exposure was interrupted. Therefore, SLDR in vivomight be counterbalanced by other phenomena, such as reoxygenation, that sensitize tumor cells to subsequent irradiation [18]. Further studies have shown that reoxygenation occurring within 5 to 15 min compensated for SLDR in SCCVII tumors. When reoxygenation was limited, the radiation effect due to SLDR decreased, indicating that reoxygenation of about one-third of hypoxic tumor cells would counter-balance SLDR in vivo[19]. In contrast, in the present study, the prolonged fraction delivery time decreased the radiobiological effects in vivo. This difference may be due to the irradiation conditions for tumor-bearing mice. Sugie et al. [18] irradiated mice without anesthesia or physical restraint, while we irradiated mice after immobilizing them in jigs. This change in irradiation conditions can produce dramatic effects in tumor oxygenation [20].
Collectively, our in vitroand in vivoresults showed that prolonged fraction delivery time substantially undermined the radiobiological effects. Therefore, we strongly recommend keeping the delivery time as short as possible, to prevent slow or insufficient reoxygenation of the tumor. However, some concerns must still be addressed. First, the effect of different delivery times in real clinical usage may differ because of the heterogeneous nature of damage repair in different cell lines. Second, the underlying mechanisms involved in the decreased biological effects with prolonged radiation treatment times are yet to be determined. Third, the effect of instantaneous dose rate, beam time and size, and the number of segments could be much more complicated than what our data provided via simplified estimation on human tumor cell and it needs further investigation.
FUNDING
This study was supported by grants from Natural Science Foundation of Shanghai, China (Grant No. 06ZR14022).
ACKNOWLEDGEMENTS
L.J. was responsible for data collection and analysis, performing experiments, interpretation of the results, and writing the manuscript. C.-S.H. and G.-P.Z. were responsible for the data analysis, in cooperation with X.-P.X. Z.-L.O. was responsible for experimental design. H.-M.Y. was responsible for experimental design, analysis and interpretation. All authors have read and approved the final manuscript.
REFERENCES
- 1.Tubiana M, Eschwege F. Conformal radiotherapy and intensity-modulated radiotherapy-clinical data. Acta Oncol. 2000;39:555–67. doi: 10.1080/028418600750013249. [DOI] [PubMed] [Google Scholar]
- 2.Brahme A. Recent advances in light ion radiation therapy. Int J Radiat Oncol Biol Phys. 2004;58:603–16. doi: 10.1016/j.ijrobp.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–54. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 4.Galvin JM, Chen XG, Smith RM. Combining multileaf fields to modulate fluence distribution. Int J Radiat Oncol Biol Phys. 1993;27:697–705. doi: 10.1016/0360-3016(93)90399-g. [DOI] [PubMed] [Google Scholar]
- 5.Bortfield TR, Kahler D, Waldron TJ, et al. X-ray field compensation with multileaf collimators. Int J Radiat Oncol Biol Phys. 1994;28:723–30. doi: 10.1016/0360-3016(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 6.Webb S. The physical basis of IMRT and inverse planning. Br J Radiol. 2003;76:678–89. doi: 10.1259/bjr/65676879. [DOI] [PubMed] [Google Scholar]
- 7.Elkind MM, Sutton H. Radiation response of mammalian cells grown in culture. I Repair of X-ray damage in surviving Chinese hamster cells. Radiat Res. 1960;13:556–93. [PubMed] [Google Scholar]
- 8.Elkind MM, Alescio T, Swain RW, et al. Recovery of hypoxic mammalian cells from sub-lethal X-ray damage. Nature. 1964;202:1190–3. doi: 10.1038/2021190a0. [DOI] [PubMed] [Google Scholar]
- 9.Elkind MM. Cell-cycle sensitivity, recovery from radiation damage and a new paradigm for risk assessment. Int J Radiat Biol. 1997;71:657–65. doi: 10.1080/095530097143662. [DOI] [PubMed] [Google Scholar]
- 10.Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515–28. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- 11.Hill AA, Skarsqard LD. Cell-age heterogeneity and deviations from the LQ model in the radiation survival responses of human tumour cells. Int J Radiat Biol. 1999;75:1409–20. doi: 10.1080/095530099139278. [DOI] [PubMed] [Google Scholar]
- 12.Wang JZ, Li XA, D'Souza WD, et al. Impact of prolonged fraction delivery times on tumor control: A note of caution for intensity-modulated radiation therapy (IMRT) Int J Radiat Oncol Biol Phys. 2003;57:543–52. doi: 10.1016/s0360-3016(03)00499-1. [DOI] [PubMed] [Google Scholar]
- 13.Mu X, Lofroth P, Karlsson M, et al. The effect of fraction time in intensity modulated radiotherapy: theoretical and experimental evaluation of an optimisation problem. Radiother Oncol. 2003;68:181–7. doi: 10.1016/s0167-8140(03)00165-8. [DOI] [PubMed] [Google Scholar]
- 14.Zheng XK, Chen LH, Yan X, et al. Impact of prolonged fraction dose-delivery time modeling intensity-modulated radiation therapy on hepatocellular carcinoma cell killing. World J Gastroenterol. 2005;11:1452–6. doi: 10.3748/wjg.v11.i10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino H, Shibamoto Y, Sugie C, et al. Biological effects of intermittent radiation in cultured tumor cells: influence of fraction number and dose per fraction. J Radiat Res. 2005;46:401–6. doi: 10.1269/jrr.46.401. [DOI] [PubMed] [Google Scholar]
- 16.Shibamoto Y, Otsuka S, Iwata H, et al. Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res. 2012;53:1–9. doi: 10.1269/jrr.11095. [DOI] [PubMed] [Google Scholar]
- 17.Shibamoto Y, Ito M, Sugie C, et al. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int J Radiat Oncol Biol Phys. 2004;59:1484–90. doi: 10.1016/j.ijrobp.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Sugie C, Shibamoto Y, Ito M, et al. Radiobiologic effect of intermittent radiation exposure in murine tumors. Int J Radiat Oncol Biol Phys. 2006;64:619–24. doi: 10.1016/j.ijrobp.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Tomita N, Shibamoto Y, Ito M, et al. Biological effect of intermittent radiation exposure in vivo: Recovery from sublethal damage versus reoxygenation. Radiother Oncol. 2008;86:369–74. doi: 10.1016/j.radonc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Shibamoto Y, Sasai K, Abe M. The radiation response of SCCVII tumor cells in C3H/He mice varies with the irradiation conditions. Radiat Res. 1987;109:352–4. [PubMed] [Google Scholar]