Abstract
Placental extracts have been reported to have anti-oxidative and anti-inflammatory activities. Because there is increasing evidence that ionizing radiation induces the release of reactive oxygen species (ROS) and inflammatory cytokines, we examined the protective effects of a placental extract against radiation injury. C57BL/6 mice were exposed to 1 Gy of γ-ray radiation every day for 5 days, and placental extract (1 mg/day) was administrated orally soon after each exposure. At 2 days after the last irradiation, mice were euthanized to examine the numbers, colony-forming capacity, and DNA damage of stem/progenitor cells in the peripheral blood and bone marrow. To understand the related mechanisms, we also measured the levels of intracellular and mitochondrial ROS, and 8-OHdG in the plasma and urine, and IL-6 and TNF-α in the plasma. Compared with the placebo treatment, oral administration of placental extract significantly increased the number and colony-forming capacity, but decreased the DNA damage of bone marrow stem/progenitor cells. However, neither the levels of intracellular and mitochondrial ROS in bone marrow cells, nor the levels of 8-OHdG in the urine and plasma significantly differed between groups. Interestingly, in comparison with the placebo treatment, placental extract significantly decreased the levels of the inflammatory cytokines IL-6 and TNF-α in the plasma. Placental extract significantly attenuated the acute radiation injury to bone marrow-derived stem/progenitor cells, and this protection is likely to be related to the anti-inflammatory activity of the placental extract.
Keywords: placental extract, radiation injury, bone marrow stem/progenitor cell, inflammatory, oxidative stress
INTRODUCTION
The placenta, an essential organ for fetal growth and development, contains a wide range of bioactive peptides and proteins. The authors of past studies have found that placental extracts contain uracil, tyrosine, phenylalanine, and tryptophan, which demonstrate strong anti-oxidative and anti-inflammatory activities [1–4]. Therefore, placental extracts prepared through various methods have been commercially used as cosmetics, health supplements, and wound-healing salves [5, 6], particularly in many Asian countries. Furthermore, placental extracts have been experimentally and clinically investigated for the treatment of inflammatory diseases and other disorders, including cartilage degradation, chronic pain, ischemic brain injury, and liver damage [7–13].
Ionizing radiation, such as γ-rays, can directly induce DNA double-strand breaks and cause injury to cells [14]. Additionally, ionizing radiation is known to ionize water molecules, which results primarily in the generation of the hydroxyl radical, the most toxic form of reactive oxygen species (ROS). These ROS can oxidize biomolecules, including proteins, lipids and DNA, which indirectly contribute to radiation injury [15, 16]. Previous studies have demonstrated that radiation injury can be attenuated by the administration of antioxidants [17–20]. Amifostine, an organic thiophosphate prodrug that potently scavenges free radicals, has been approved as a cytoprotective adjuvant for patients receiving radiotherapy [21]. Considering the anti-oxidative and anti-inflammatory activities of placental extracts, it is plausible that placental extracts can also effectively protect from radiation injury. In fact, it has been previously demonstrated that the oral administration of placental extracts significantly improved the survival of mice exposed to high-dose irradiation [22]. Furthermore, placental extracts have also been reported to effectively attenuate radiation-induced oral mucositis and acute radiodermatitis in patients [23, 24]. However, the efficacy of placental extracts against radiation injury has been poorly documented, and the relevant mechanism of action has not been fully understood.
In this study, we orally administered a placental extract to mice after exposure to γ-ray radiation and then examined the protective effect and relevant mechanisms of action of the placental extract against radiation injury with a focus on the bone marrow-derived stem/progenitor cells.
MATERIALS AND METHODS
Animals, γ-ray radiation, and oral administration of placental extracts
We used 12-week-old male C57BL/6 mice (SLC, Japan) for this study. All of the experiments were approved by the Institutional Animal Care and Use Committee of Nagasaki University, and the animal procedures were performed in accordance with institutional and national guidelines.
Animals were exposed to 1 Gy γ-ray with a 137Cs source at a dose rate of 0.86 Gy/min with a PS-3100SB γ-ray irradiation system (Pony Industry Co. Ltd, Osaka, Japan) every day for 5 days. Porcine placental extract (kindly provided by Snowden Co. Ltd, Tokyo, Japan) was given orally soon after each exposure (1 mg in 0.2 ml dH2O/mouse/day; Placenta group, n= 6). Mice that received oral dH2O alone were used as a placebo control (Control group, n= 6). Mice were euthanized 2 days after the end of treatments for the following experiments.
Measurement of nucleated cells and stem/progenitor cells in peripheral blood
We collected peripheral blood with heparinization. The numbers of nucleated cells in peripheral blood were counted with a Nucleocounter cell counter (Chemotetec A/S, Denmark). Plasma was collected and stored at –80°C for the measurement of 8-OHdG, IL-6 and TNF-α.
To measure the c-kit-positive (c-kit+) and CD34-positive (CD34+) stem/progenitor cells in peripheral blood, we isolated the nucleated cells by density gradient centrifugation and then labeled cells with PE-conjugated anti-mouse c-kit antibody (eBioscience) or FITC-conjugated anti-mouse CD34 antibody (BD Bioscience) for 45 min. After washing, quantitative flow cytometry analysis was performed using a FACSCalibur (Becton Dickinson) [25].
Measurement of mononuclear cells and stem/progenitor cells in bone marrow
Bone marrow cells were collected from the femur and tibia, and mononuclear cells were isolated by density gradient centrifugation [25]. The total cells collected from each mouse were counted with the Nucleocounter cell counter.
To measure the c-kit+and CD34+stem/progenitor cells, the isolated mononuclear cells were labeled with PE-conjugated anti-mouse c-kit antibody or FITC-conjugated anti-mouse CD34 antibody (BD Bioscience) for 45 min. After washing, quantitative flow cytometry analysis was performed using a FACSCalibur [25].
Colony forming assay
The colony forming capacity of stem/progenitor cells from peripheral blood and bone marrow was estimated in mouse methylcellulose complete media, according to the manufacturer's instructions (R&D System). Briefly, 1 × 105peripheral nucleated cells or 3 × 104bone marrow mononuclear cells were mixed well in 1 ml media, plated in 3 cm diameter culture dishes, and then incubated at 37°C in a 5% CO2incubator. After 9 days (for bone marrow mononuclear cells) or 12 days (for peripheral blood nucleated cells), the formation of colonies was observed by microscopy, and the total number of colonies in each dish was counted. The mean numbers of colonies in duplicate assays were used for statistical analysis.
Immunocytochemistry
To detect DNA damage, isolated bone marrow mononuclear cells were seeded on 4-well chamber culture slides (Nalge Nunc International) coated with 10 µg/ml fibronectin (Invitrogen) at a density of 2 × 106cells/ml in IMDM 1640 medium supplemented with 10% fetal bovine serum (HyClone), 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco), and incubated at 37°C in 5% CO2. The cells were fixed in 1% formaldehyde for 10 min after 7 days of culture. After blocking with 2% bovine serum albumin, the cells were reacted with anti-mouse 53BP1 antibody (Abcam) and then with a FITC-conjugated secondary antibody. The nuclei were stained with Hoechst 33258. The numbers of cells with or without the formation of 53BP1-foci were counted under fluorescence microscopy with 200-fold magnification. At least 200 cells were counted, and the percentages of cells with the formation of 53BP1-foci were used for the statistical analysis.
Detection of intracellular and mitochondrial ROS
To better understand the relevant mechanisms of radiation damage and protection, we estimated the intracellular ROS levels based on the oxidation of H2DCFDA to form the fluorescent compound 2',7'-dichlorofluorescein (DCF), as described previously [26]. Briefly, freshly isolated bone marrow mononuclear cells were incubated with 10 µM H2DCFDA at 37°C for 30 min. After the cells were washed with PBS, the fluorescence intensity in the cells was estimated with a FACSCalibur.
Similarly, the mitochondrial ROS was analyzed with the MitoSOX Red mitochondrial superoxide indicator, as described previously [27]. Briefly, freshly isolated bone marrow cells were incubated with 5 µM MitoSOX Red at 37°C for 30 min. After washing, the fluorescence intensity in the cells was estimated by using a FACSCalibur.
Measurements of 8-OHdG, TNF-α, and IL-6 levels in plasma and urine
We measured the concentration of 8-OHdG (8-oxo-2'-deoxyguanosine) in the urine and plasma using an ELISA kit (Nikken SEIL Corporation, Shizuoka, Japan) according to the manufacturer's instructions. The concentrations of TNF-α and IL-6 in the plasma were measured with ELISA kits (R&D Systems) as described previously [28]. The mean values of duplicate assays of each experiment were used for statistical analysis.
Statistical analysis
All results are presented as the mean ± SD. The statistical significance of a difference between two groups was determined using the 2-tailed unpaired t-test (Dr. SPSS II, Chicago, IL). Differences were considered significant when P< 0.05.
RESULTS
Placental extract attenuated the radiation-induced decrease of peripheral blood nucleated cells and stem/progenitor cells
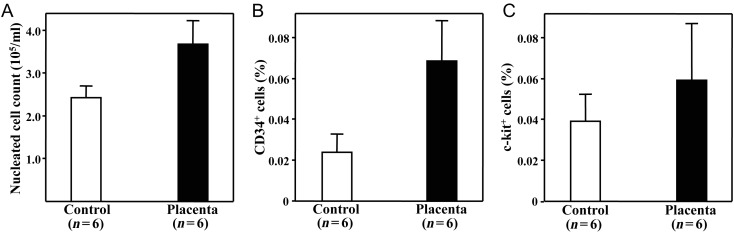
The number of nucleated cells in the peripheral blood decreased dramatically in the mice of the Control group (2.4 ± 0.7) × 105/ml after irradiation to approximately 15% of the non-irradiated healthy mice (16.4 ± 4.2) × 105/ml. The administration of placental extracts partially attenuated the radiation-induced decrease of nucleated cells in the peripheral blood (3.7 ± 1.3) × 105/ml, P= 0.069 vsControl (Fig. 1A), although the nucleated cell count of the Placenta group was still very low in comparison with healthy mice. Furthermore, the administration of placental extract caused a trend toward an increase in the percentages of c-kit+and CD34+stem/progenitor cells in the peripheral blood after irradiation (P= 0.067 and 0.093 vsControl, respectively; Fig. 1B and C).
Fig. 1.
Numbers of nucleated cells and stem/progenitor cells in the peripheral blood of mice after treatments. Mice were exposed daily to 1 Gy of γ-ray ionizing radiation and were then given placental extract (Placenta group) or placebo treatment (Control group) for 5 days. The mice were euthanized 2 days after the end of treatments. The nucleated cells in the peripheral blood (A)were directly counted, and the CD34+(B)and c-kit+stem/progenitor cells (C)were measured by flow cytometry. All of these parameters showed a trend toward higher values in the Placenta group compared with the Control group. The data are presented as the mean ± SD for six mice from each group.
Placental extract attenuated radiation-induced damage of bone marrow stem/progenitor cells
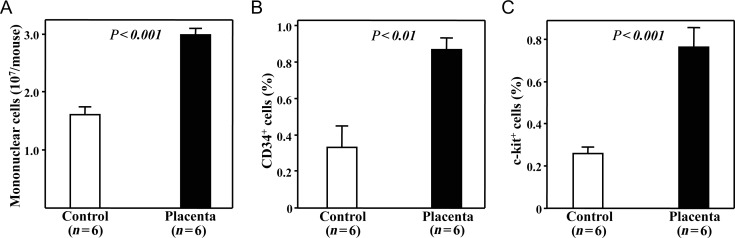
Although all of the procedures of cell collection and isolation were performed in the same way, significantly fewer bone marrow mononuclear cells were collected from the mice that received placebo treatment than from the mice given placental extract (P< 0.001, Fig. 2A). Furthermore, the percentages of CD34+and c-kit+stem/progenitor cells among freshly collected bone marrow mononuclear cells were also significantly lower in the mice of the Control group (0.33 ± 0.12% and 0.26 ± 0.03%, respectively) than in those of the Placenta group (0.87 ± 0.07% and 0.77 ± 0.09%; P< 0.01 and 0.001, respectively; Fig. 2B and C). This finding indicated that the oral administration of placental extract significantly attenuated the radiation-induced decrease of stem/progenitor cells in the bone marrow. However, the percentage of c-kit+and CD34+stem/progenitor cells in the mice that received placental extract were still much lower than that of non-irradiated healthy mice (2.72 ± 0.38% and 4.28 ± 0.12%, respectively).
Fig. 2.
Numbers of mononuclear cells and stem/progenitor cells in bone marrow of mice after treatments. (A)Significantly fewer bone marrow mononuclear cells were collected and separated from mice that received placebo treatment than from those given placental extract. Compared to the Control group, the percentages of CD34+(B)and c-kit+(C)stem/progenitor cells among bone marrow mononuclear cells were significantly higher in the Placenta group as assessed by flow cytometry.The data represent the mean ± SD for six mice from each group.
By colony-forming assay, we found that the number of colonies formed from bone marrow mononuclear cells was significantly greater in the Placenta group than in the Control group (37.1 ± 3.3 vs20.3 ± 2.6, P< 0.01; Fig. 3), although the number of colonies in both groups was approximately one-third that of the non-irradiated healthy mice (92.0 ± 8.6). Furthermore, we could not detect any colony formation within 12 days by seeding 1 × 105peripheral nucleated cells from mice of the placebo- and placental extract-treated mice; however, approximately 15 colonies were formed from the same number of cells from the non-irradiated healthy mice.
Fig. 3.
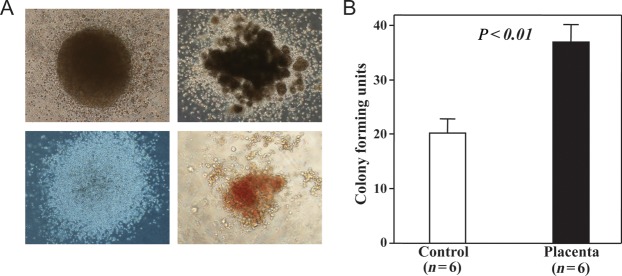
Colony forming assay. Freshly isolated bone marrow mononuclear cells were cultured in methylcellulose complete medium, and colonies of different types (A)were observed under microscopy at 9 days after incubation. (B)Significantly more total colonies (>50 cells) were formed from the cells of mice given placental extract than from those that received placebo treatment. The data represent the mean ± SD for six separate experiments with duplicated assays.
Placental extract reduced the DNA damage of bone marrow stem/progenitor cells
We estimated the DNA damage of bone marrow stem/progenitor cells by detecting the formation of 53BP1 foci in the nuclei of cells with immunostaining. Quantitative analysis showed that the percentage of cells with nuclear 53BP1 foci was significantly lower in the Placenta group than in the Control group (25.8 ± 4.1 vs37.7 ± 4.9, P< 0.05, Fig. 4). However, the percentage of cells with 53BP1 foci in the mice that received placental extract was much higher than that of the non-irradiated healthy mice (12.5 ± 5.1%).
Fig. 4.
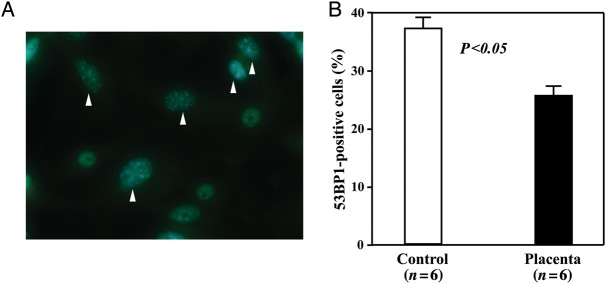
DNA damage in bone marrow-derived stem/progenitor cells. Bone marrow cells from mice were cultured for 7 days, and the DNA damage in cells was estimated by immunostaining with anti-53BP1 antibody. (A)Representative image showing the formation of 53BP1 foci in the nuclei of some cells (arrowheads). (B)Quantitative analysis showing that the percentage of cells with 53BP1 foci was significantly lower in the Placenta group than in the Control group. The data represent the mean ± SD for six separate experiments.
Placental extract did not significantly change the levels of intracellular and mitochondrial ROS
To further investigate the relevant mechanisms of placental extract radioprotection, we measured the levels of intracellular and mitochondrial ROS in bone marrow cells. As shown in Fig. 5, the intracellular ROS level was higher in cells from mice given placental extract than in those that received placebo treatment (395.7 ± 53.5 vs326.7 ± 71.6, P= 0.09, Fig. 5A). This finding could indicate that the cells from the mice given placental extract had less damage because the intracellular ROS level was observed to be even higher in the cells from the non-irradiated healthy mice (421.8 ± 53.5). Furthermore, the mitochondrial ROS levels in cells from mice given placental extract did not differ from those that received placebo treatment (407.6 ± 63.25 vs378.3 ± 38.5, P= 0.36, Fig. 5B), and these levels were even higher in the cells from the non-irradiated healthy mice (441.4 ± 51.3). Although we did not examine the ROS levels immediately after irradiation, the slight increase of intracellular ROS and the comparable mitochondrial ROS levels in the cells of mice given placental extract indicated that the placental extract protected the bone marrow stem/progenitor cells against radiation injury, which was likely to be through mechanisms other than its well-known anti-oxidative bioactivity.
Fig. 5.
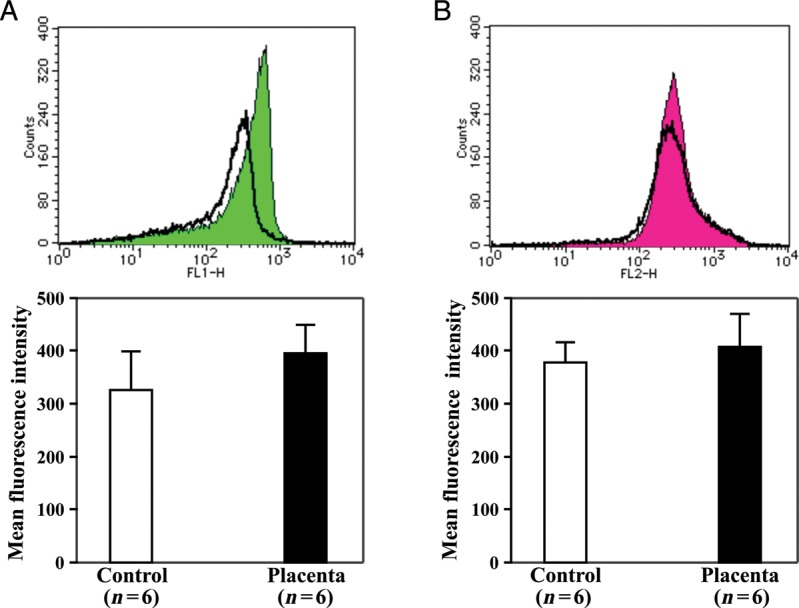
Intracellular and mitochondrial ROS in bone marrow cells. Bone marrow mononuclear cells were loaded with 10 µM H2DCFDA or 5 µM MitoSOX Red at 37°C for 30 min, and the intracellular ROS (A)and mitochondrial ROS (B)were detected by flow cytometry as the fluorescence intensity of cells. Representative histograms are shown in the upper panel, and the mean fluorescence intensity was quantitatively measured for statistical analysis (lower bar graphs). The data represent the mean ± SD for six separate experiments.
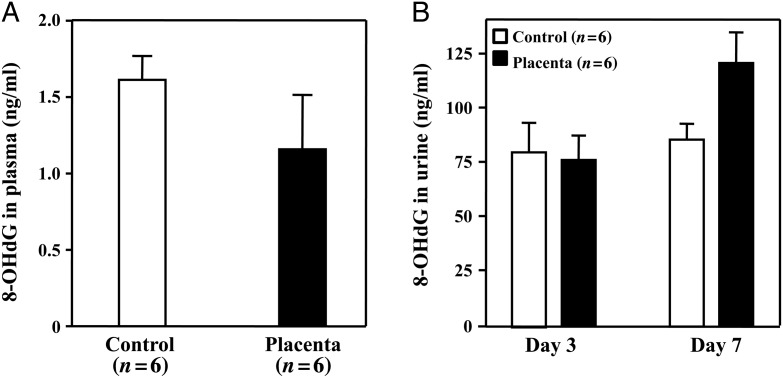
Placental extract did not decrease the plasma or urinary 8-OHdG levels
The plasma 8-OHdG levels were very low in mice of both the Control and Placenta groups, without a significant difference (P= 0.15, Fig. 6A). The levels of urinary 8-OHdG did not differ between groups at 3 days, but was somewhat higher at 7 days after radiation in the mice given placental extract (P= 0.092, Fig. 6B). The reason for the high urinary 8-OHdG levels in the mice given placental extract was unknown. It could be related to differential damage of the kidneys between groups, and values of 8-OHdG adjusted for urinary creatinine are needed.
Fig. 6.
The levels of 8-OHdG in the plasma and urine. The plasma and urine were collected, and 8-OHdG levels were measured using an ELISA kit. (A)The 8-OHdG levels in plasma were very low and did not significantly differ between groups. (B)The urinary 8-OHdG levels did not differ between groups at 3 days, but showed a trend toward a higher value in the Placenta group than in the Control group at 7 days after the initiation of radiation and treatments. The data represent the mean ± SD for six separate experiments with duplicated assays.
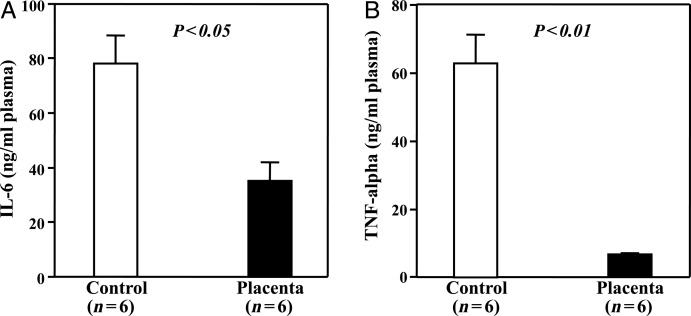
Placental extract decreased inflammatory cytokines in the plasma
To further elucidate the protective mechanism of placental extract against radiation injury, we measured the levels of the inflammatory cytokines IL-6 and TNF-α in the plasma. We found that the levels of IL-6 and TNF-α in the plasma were both significantly decreased in the mice given placental extract in comparison with the mice that received placebo treatment (P< 0.05 and 0.01, respectively, Fig. 7), thereby demonstrating the anti-inflammatory activity of the placental extract.
Fig. 7.
Inflammatory cytokines in plasma. The levels of the inflammatory cytokines IL-6 (A)and TNF-α (B)in the plasma were measured by ELISA. Both IL-6 and TNF-α were significantly decreased in mice given placental extract when compared with mice that received placebo treatment. The data represent the mean ± SD for six separate experiments with duplicated assays.
DISCUSSION
In this study, we demonstrated that the oral administration of a placental extract to mice after high-dose ionizing radiation exposures significantly increased the numbers and colony-forming capacity but decreased the DNA damage of bone marrow-derived stem/progenitor cells. However, the mechanism of the protective effect for acute radiation injury is likely to be related to anti-inflammatory activity rather than the well-known anti-oxidative effect of placental extracts.
Beyond the direct induction of DNA double-strand breaks, increasing evidence indicates that exposure to ionizing radiation could trigger the release of ROS and inflammatory cytokines [15, 16, 29], which indirectly contribute to cell death and injuries through damaging signals. Placental extracts are known to consist of various components with anti-inflammatory and anti-oxidative properties, and the intake of placental extracts has been reported to be beneficial for many pathological conditions related to inflammatory and oxidative stress [7–13, 30, 31]. Although previous studies have demonstrated that the administration of antioxidants can attenuate radiation injury during both the acute and chronic phases, the protective effect and relevant mechanisms of placental extracts for radiation injury are not well documented.
By exposing mice to1 Gy of γ-rays daily for 5 days, we confirmed that the intake of placental extract immediately after each exposure significantly improved the number and colony-forming capacity of bone marrow-derived stem/progenitor cells, suggesting a protective effect of placental extract for radiation injury. However, the administration of placental extract did not decrease the levels of intracellular or mitochondrial ROS in bone marrow cells. Furthermore, the oxidative stress marker 8-OHdG did not significantly decrease in the plasma or urine of mice given placental extract when compared with those that received placebo treatment. Conversely, placental extract caused a non-significant increase in the ROS and urinary 8-OHdG levels at 7 days after the initiation of radiation and treatment (i.e. 2 days after the end of treatment). Although data at earlier time points and further in-depth analyses are needed, the mechanism of action of placental extracts for radiation injury seems to be more complex than the result of simple anti-oxidative activity.
Interestingly, the intake of placental extract significantly decreased the levels of two major inflammatory cytokines, IL-6 and TNF-α, in the plasma of mice exposed to radiation, suggesting that the protective effects of placental extracts for radiation injury may be associated with anti-inflammatory activity. In fact, the cytokine response to radiation exposure has been widely recognized since the first report by Talas et al.[32]. A multitude of cytokines respond within a few hours of exposure to radiation, even at a minimally toxic dose [29, 32–37]. Furthermore, cytokine responses induced by ionizing radiation might vary with the dose of radiation and the types of cells/tissues [33, 34]. However, the details of systemic and local (i.e.bone marrow) cytokine responses were not investigated in this study.
Placental extracts are prepared by various methods from human, bovine and porcine placenta as the raw material sources. Therefore, the components in placental extracts can be very complex and may differ widely among products. Previous studies have indicated that placental extracts consist of N-acetylneuraminic acid, glucosamine, omega-3 fatty acids, diverse fatty acids, and many other amino acids and nucleotides [1, 2] that exhibit anti-oxidative or anti-inflammatory properties [3, 4, 7, 10, 38]. We speculated that our product of porcine placental extract might also contain most of these components. However, we did not confirm the key factors and signaling pathways relevant to the inhibition of the inflammatory response after radiation in the present study. Although it is likely to be extremely difficult to identify the key molecular mechanisms of the protective effects of placental extracts for radiation injury, further studies are required to understand how placental extracts can change the levels and patterns of cytokines released after radiation.
A number of studies have reported that dietary antioxidants can effectively protect against radiation injury, including the improvement of survival, reduction of cell apoptosis, and attenuation of oxidative stress [17–20]. Antioxidants have also been found to increase the number and colony-forming capacity of hematopoietic stem cells [18, 19]. Furthermore, antioxidants have been found to decrease ROS levels in cells to mitigate radiation injury [18]. However, in the present study, the ROS in bone marrow cells were detected at a higher level in mice given placental extract than in those that received placebo treatment. We also found that placental extract decreased the levels of inflammatory cytokines in plasma, which has not been reported in previous studies using antioxidants. Although further experiments are required, the mechanisms of radiation protection might differ between antioxidants and placental extracts. Because the dose of radiation and timing of experimental assessments in the present study were completely different from the previous studies, it is impossible to compare the protective efficacy between placental extracts and antioxidants for radiation injury.
This study has a number of limitations. First, the dosage selected in this study was based on past experimental studies and the daily dosage suggested by the extract manufacturer. Therefore, the dose dependency of the protective effects of placental extracts remains unknown. Second, the benefit of placental extracts on radiation injury should be confirmed with other radiation doses and animal models. Furthermore, a head-to-head comparison of placental extracts vs other chemical compounds that have been demonstrated to protect against radiation injury (with respect to their action on antioxidants) is critical.
In summary, we demonstrated that placental extract effectively attenuated acute radiation injury of bone marrow-derived stem/progenitor cells in a mouse model. This protection was likely associated with inhibition of the inflammatory response after radiation. Although our results need to be confirmed in humans, they indicate that placental extracts may be beneficial for radiation-induced injury.
FUNDING
This study was supported in part by a Grant-in-Aid for the Global Centers of Excellence Program from the Ministry of Education, Science, Sports, Culture and Technology, Japan.
REFERENCES
- 1.Togashi S, Takahashi N, Kubo Y, et al. Purification and identification of antioxidant substances in human-placenta extracts. J Health Sci. 2000;6:17–25. [Google Scholar]
- 2.Park S-Y, Phark S, Lee M, et al. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta. 2010;31:873–9. doi: 10.1016/j.placenta.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Sur T-K, Biswas T-K, Ali L, et al. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol Sin. 2003;24:187–92. [PubMed] [Google Scholar]
- 4.Banerjee K-K, Bishayee A, Chatterjee M. Anti-inflammatory effect of human placental extract: a biochemical mechanistic approach. Riv Eur Sci Med Farmacol. 1992;14:361–6. [PubMed] [Google Scholar]
- 5.Hong J-W, Lee W-J, Hahn S-B, et al. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010;65:96–100. doi: 10.1097/SAP.0b013e3181b0bb67. [DOI] [PubMed] [Google Scholar]
- 6.De D, Chakraborty P-D, Bhattacharyya D. Regulation of trypsin activity by peptide fraction of an aqueous extract of human placenta used as wound healer. J Cell Physiol. 2011;226:2033–40. doi: 10.1002/jcp.22535. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal N, Kulshrestha V, Kriplan A. Clinical eficacy of placentrex injection in pelvic inflammatory disease. J Indian Med Assoc. 2010;108:117–8. 122. [PubMed] [Google Scholar]
- 8.Kim J-K, Kim T-H, Park S-W, et al. Protective effects of human placenta extract on cartilage degradation in experimental osteoarthritis. Biol Pharm Bull. 2010;33:1004–10. doi: 10.1248/bpb.33.1004. [DOI] [PubMed] [Google Scholar]
- 9.Marleau A-M, McDonald G, Koropatnick J, et al. Reduction of tumorigenicity by placental extracts. Anticancer Res. 2012;32:1153–61. [PubMed] [Google Scholar]
- 10.Park J-Y, Byeon J-H, Park S-W, et al. Neuroprotective effect of human placental extract on hypoxic-ischemic brain injury in neonatal rats. Brain Dev. 2012 doi: 10.1016/j.braindev.2012.01.009. Feb 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Koike K, Yamamoto Y, Suzuki N, et al. Efficacy of porcine placental extracts with hormone therapy for postmenopausal women with knee pain. Climacteric. 2012;15:30–5. doi: 10.3109/13697137.2011.590616. [DOI] [PubMed] [Google Scholar]
- 12.Liu K-X, Kato Y, Kaku T, et al. Human placental extract stimulates liver regeneration in rats. Biol Pharm Bull. 1998;21:44–9. doi: 10.1248/bpb.21.44. [DOI] [PubMed] [Google Scholar]
- 13.Jung J, Lee H-J, Lee J-M, et al. Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int Immunopharmacol. 2011;11:976–84. doi: 10.1016/j.intimp.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Snyder A-R, Morgan W-F. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–54. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 15.Tominaga H, Kodama S, Matsuda N, et al. Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. J Radiat Res. 2004;45:181–8. doi: 10.1269/jrr.45.181. [DOI] [PubMed] [Google Scholar]
- 16.Wallace S-S. Enzymatic processing of radiation-induced free radical damage in DNA. Radiat Res. 1998;150:S60–79. [PubMed] [Google Scholar]
- 17.Wambi C, Sanzari J, Wan XS, et al. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res. 2008;169:384–96. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Wang Y, Pazhanisamy SK, et al. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med. 2011;51:30–7. doi: 10.1016/j.freeradbiomed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh VK, Parekh VI, Brown DS, et al. Tocopherol succinate: modulation of antioxidant enzymes and oncogene expression, and hematopoietic recovery. Int J Radiat Oncol Biol Phys. 2011;79:571–8. doi: 10.1016/j.ijrobp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Kinoshita M, Shinomiya N, et al. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J Radiat Res. 2010;51:145–56. doi: 10.1269/jrr.09078. [DOI] [PubMed] [Google Scholar]
- 21.Bourhis J, Blanchard P, Maillard E, et al. Effect of amifostine on survival among patients treated with radiotherapy: a meta-analysis of individual patient data. J Clin Oncol. 2011;29:2590–7. doi: 10.1200/JCO.2010.33.1454. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki H, Kada T. Restorative effects of human placenta extract in X-ray-irradiated mice. J Radiat Res. 1982;23:403–10. doi: 10.1269/jrr.23.403. [DOI] [PubMed] [Google Scholar]
- 23.Kaushal V, Verma K, Manocha S, et al. Clinical evaluation of human placental extract (placentrex) in radiation-induced oral mucositis. Int J Tissue React. 2001;23:105–10. [PubMed] [Google Scholar]
- 24.Bigliardi P. Treatment of acute radiodermatitis of first and second degrees with semi-greasy placenta ointment. Int J Tissue React. 1982;4:153–4. [PubMed] [Google Scholar]
- 25.Li T-S, Ikeda S, Kubo M, et al. Diabetic impairment of C-kit bone marrow stem cells involves the disorders of inflammatory factors, cell adhesion and extracellular matrix molecules. PLoS ONE. 2011;6:e25543. doi: 10.1371/journal.pone.0025543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T-S, Marbán E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–85. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida T, Goto S, Kawakatsu M, et al. Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res. 2012;46:147–53. doi: 10.3109/10715762.2011.645207. [DOI] [PubMed] [Google Scholar]
- 28.Takemoto Y, Li T-S, Kubo M, et al. Operative injury accelerates tumor growth by inducing mobilization and recruitment of bone marrow-derived stem cells. Surgery. 2011;149:792–800. doi: 10.1016/j.surg.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Gallet P, Phulpin B, Merlin J-L, et al. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS One. 2011;6:e29399. doi: 10.1371/journal.pone.0029399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kada T, Mochizuki H. Antimutagenic activities of human placental extract on ultraviolet light- and gamma-ray-induced mutation in Escherichia coliWP 2 B/r trp. J Radiat Res. 1981;22:297–302. doi: 10.1269/jrr.22.297. [DOI] [PubMed] [Google Scholar]
- 31.Jang S-Y, Park J-W, Bu Y, et al. Protective effects of hominis placenta hydrolysates on radiation enteropathy in mice. Nat Prod Res. 2011;25:1988–92. doi: 10.1080/14786419.2010.513035. [DOI] [PubMed] [Google Scholar]
- 32.Tálas M, Szolgay E, Várterész V, et al. Influence of acute and fractional X-irradiation on induction of interferon in vivo. Arch Gesamte Virusforsch. 1972;38:143–8. doi: 10.1007/BF01249664. [DOI] [PubMed] [Google Scholar]
- 33.Ao X, Zhao L, Davis M-A, et al. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J Hematol Oncol. 2009;2:6. doi: 10.1186/1756-8722-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Yin L, Zhang K, et al. Response patterns of cytokines/chemokines in two murine strains after irradiation. Cytokine. 2012;58:169–77. doi: 10.1016/j.cyto.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Rastogi S, Coates P-J, Lorimore SA, et al. Bystander-type effects mediated by long-lived inflammatory signaling in irradiated bone marrow. Radiat Res. 2012;177:244–50. doi: 10.1667/rr2805.1. [DOI] [PubMed] [Google Scholar]
- 36.Cachaço A-S, Carvalho T, Santos A-C, et al. TNF-alpha regulates the effects of irradiation in the mouse bone marrow microenvironment. PLoS One. 2010;5:e8980. doi: 10.1371/journal.pone.0008980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ossetrova N-I, Blakely W-F. Multiple blood-proteins approach for early-response exposure assessment using an in vivomurine radiation model. Int J Radiat Biol. 2009;85:837–50. [PubMed] [Google Scholar]
- 38.Togashi S, Takahashi N, Iwama M, et al. Antioxidative collagen-derived peptides in human-placenta extract. Placenta. 2002;23:497–502. doi: 10.1053/plac.2002.0833. [DOI] [PubMed] [Google Scholar]