Summary
Background
The overlap of dyspepsia and gastroesophageal reflux (GER) is known to be frequent but whether the overlap group is a distinct entity or not remains unclear.
Aims
To evaluate whether the overlap of dyspepsia and GER (dyspepsia-GER overlap) occurs more than expected due to chance alone, and evaluate the risk factors for dyspepsia-GER overlap.
Methods
In 2008 and 2009, a validated Bowel Disease Questionnaire was mailed to a total of 8006 community sample from Olmsted County, MN. Overall, 3831 of the 8006 subjects returned surveys (response rate 48%). Dyspepsia was defined by symptom criteria of Rome III; GER was defined by weekly or more frequent heartburn and/or acid regurgitation.
Results
Dyspepsia and GER occurred together more commonly than expected by chance. The somatic symptom checklist score was significantly associated with dyspepsia-GER overlap vs. GER alone or dyspepsia alone (OR=1.9 [1.4, 2.5), and 1.6 [1.2, 2.1), respectively). Insomnia was also significantly associated with dyspepsia-GER overlap vs. GER alone or dyspepsia alone (OR=1.4 [1.1, 1.7], OR=1.3 [1.1, 1.6], respectively). Moreover, proton pump inhibitor use was significantly associated with dyspepsia-GER overlap vs. dyspepsia alone (OR=2.4 [1.5, 3.8]).
Conclusions
Dyspepsia-GER overlap is common in the population and is greater than expected by chance.
Keywords: Population-based study, dyspepsia, gastroesophageal reflux
Background
Functional dyspepsia and gastroesophageal reflux (GER) are among the most prevalent upper gastrointestinal disorders.1-3 Functional dyspepsia, which is characterized by persistent or recurrent pain or discomfort centered in the upper abdomen based on Rome criteria, is reported to be approximately 20% in the general population.1, 2 Gastroesophageal reflux, which is defined by the presence of frequent heartburn or acid regurgitation, also affects 20% of the population.4 Both dyspepsia and GER are frequently chronic, often need long-term treatment, and cause significant impairment of quality of life.5-7 Currently the pathogenesis and overlap of GER and dyspepsia is poorly understood, even though it is recognized these conditions frequently overlap.
Studies have reported that a subgroup of patients with functional dyspepsia have pathological acid reflux based on abnormal 24-hour pH esophageal monitoring.8-10 Tack, et al.9 showed that 23% of dyspepsia patients with a negative upper gastrointestinal endoscopy had abnormal 24-hour esophageal acid exposure (>5% of the time). Moreover, several studies investigating the prevalence of upper GI symptoms have reported a higher prevalence of dyspeptic symptoms in patients with GER.11, 12
However, little population-based data are available to confirm these predominantly outpatient observations. Thus, the overlap of GER with functional dyspepsia is frequent but whether the overlap group is a distinct entity or not remains unclear, as is the magnitude of the overlap in the community. Furthermore, risk factors that may account for the overlap between dyspepsia and reflux symptoms have not been defined.
Thus, we aimed to determine if the overlap of dyspepsia and GER (dyspepsia-GER overlap) occurs over and above that due to chance, and evaluate if there are specific risk factors for dyspepsia-GER overlap.
Methods
This is a population-based cohort study of subjects who were sent a GI symptom survey (including detailed dyspepsia and reflux symptom questions) in 2008 and 2009. This study was approved by the institutional review boards of Mayo Clinic and the Olmsted Medical Center.
Subjects
The Olmsted County, Minnesota, population comprises approximately 120,000 persons, of which 89% are white; sociodemographically, this community resembles the U.S. white population. Mayo Clinic is the major provider of medical care.13 During any given four-year period, over 95% of local residents will have had at least one local health care contact.13 Pertinent clinical data are accessible because the Mayo Clinic has maintained, since 1910, extensive indices based on clinical and histologic diagnoses and surgical procedures.14 The system was further developed by the Rochester Epidemiology Project, which created similar indices for the records of the other providers of medical care to Olmsted County residents. The Rochester Epidemiology Project records linkage system, therefore, provides what is essentially an enumeration of the population from which samples can be drawn.13 These data resources have been utilized in a series of investigations into the epidemiology of functional GI disorders.15-17 As approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center, we used this system previously to draw a series of random samples of Olmsted County residents stratified by age (5-year intervals between 20 and 94 years) and sex (equal numbers of men and women), and to draw a new random sample of Olmsted County residents stratified by age (25-65 years) and sex.
Survey Methods
For the current study, a previously assembled cohort (n=4451)15-17 and a new age- and gender-stratified random sample (n=3555) of the Olmsted County, MN, residents (total mailed=8006),18 were mailed validated self-report gastrointestinal symptom questionnaires (Talley Bowel Disease Questionnaire [BDQ]) in 2008 and 2009. The original Talley BDQ17 was designed to measure GI symptoms experienced over the previous year and to collect the past medical history with somatic symptom checklist (SSC), and has been modified over time. The original BDQ and modified versions have been shown to be a reliable and valid measure of GI symptoms.19, 20 Finally, the current modified version of BDQ, which consists of 27 gastrointestinal symptoms including dyspepsia and reflux symptoms, also has been shown to be a reliable and valid measure of bowel symptoms.18 The SSC has been validated and in this version of the BDQ consisted of questions about six relevant non-gastrointestinal symptoms and illnesses (e.g. headaches, backaches, insomnia, general stiffness, dizziness, and weakness), and subjects are instructed to indicate how often each occurred (0=not a problem to 4=occurs daily) and how bothersome each was (0=not a problem to 4=extremely bothersome when occurs) during the past year, using separate 5-point scales.21, 22.
The study questionnaire and an explanatory letter were mailed to a total of 8006 residents of Olmsted County. Reminder letters were mailed at week 3 to 6 after the initial mailing to non-responders. Subjects who indicated at any point that they did not wish to complete the survey were not contacted further. Otherwise, non-responders were contacted by telephone at week 10 to request their participation and verify their residence within the county. A completed questionnaire was returned by 3831 subjects, giving a response rate of 48%. A total of 50% of females responded and 45% of males, with the mean (±SD) age of respondents being 61 (±16) and non-respondents, 53 (±18) years. Using a logistic regression model for response (no/yes), females and older subjects had greater odds for response (OR [95%CI], females relative to males=1.20[1.10, 1.31], OR[95%CI] per 10 years of age=1.30 [1.26, 1.33]).
Definition of GER and Dyspepsia
Dyspepsia was defined by symptom criteria of Rome III,1 with one or more of following (for more than 6 months); 1) unable to finish a regular size meal more than once a week, 2) feels uncomfortably full after regular size meal more than once a week, and/or 3) pain or burning in the middle of abdomen at least once a week, but above the umbilicus.
Postprandial distress syndrome (Rome III criteria), all of the following: 1) bothersome postprandial fullness, more than 1 day/week and 2) early satiation, more than 1 day/week
Epigastric pain syndrome (Rome III criteria) was defined as epigastric pain or burning, at least once a week
Gastroesophageal reflux (GER) was defined by one or more of the following4: 1) heartburn, at least once a week 1 day/week in the last year and/or 2) acid regurgitation, at least 1 day/week in the last year
Irritable bowel syndrome (IBS) was defined by Rome III criteria;23 namely, pain or discomfort at least 2 or 3 days per month and 2 or more of the following at least sometimes: 1) pain relieved by bowel movements (BMs), 2) change in frequency (more or fewer), 3) change in consistency (looser or harder).
Potential Risk Factors
The potential risk factors in this study were selected from items contained in the questionnaire and were based on the a priori expectation that they could be associated with reporting dyspepsia and GER status data, including demographics, physician visits, medications, somatic symptom checklist (SSC) items, and IBS status.
Statistical Analyses
Using responses from the bowel disease questionnaire, demographic and clinical characteristics of subjects with GER, dyspepsia, or dyspepsia-GER overlap were summarized. Comparison of observed and expected proportions of subjects for pairs of overlapping symptom complexes was based on the exact binomial test for proportions, the expected number computed assuming the two symptom complexes were independent. The associations of sociodemographic features, SSC scores, medication use, IBS, constipation, and diarrhea with dyspepsia/GER subgroups (defined as dyspepsia alone, GER alone, or dyspepsia-GER overlap) were evaluated using polychotomous logistic regression models (i.e. the dyspepsia/GER subgroup was the three category response variable). The odds ratios (OR) for overlap (and 95% confidence intervals) were estimated from the coefficients (and their standard errors) obtained in the logistic regression models. A p-value of <0.05 was considered statistically significant. The results of an analysis incorporating “weights” to reflect the fact that females and older age were associated with increased odds for response to the current survey was also examined. Specifically, age and sex specific weights were generated that resulted in an age (10 year groupings) and sex frequency distribution of the respondents that exactly matched the age and sex distribution of all the subjects that were mailed to. All analyses utilized the SAS® statistical analysis package (SAS Institute Inc., Cary, NC).
Results
Clinical features of dyspepsia-GER overlap
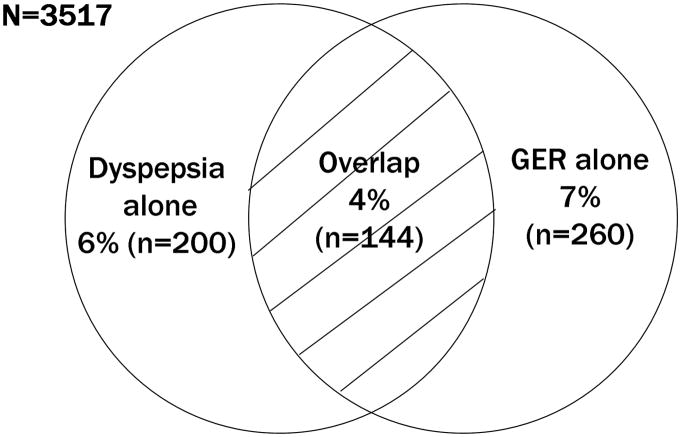
Among a total of 3,831 subjects responding to the questionnaire, 3,517 subjects who completed both dyspepsia and GER symptom questions were eligible for the current study (123 subjects had incomplete questionnaires, 163 had filled out a preliminary version of the questionnaire not containing the requisite items, and 28 were not eligible for other reasons). Among the 3,517 subjects, the mean age was 61 years (±15 [SD]) and 52% were female. Figure 1 shows the proportions of subjects reporting dyspepsia alone, GER alone, and dyspepsia-GER overlap. The proportion with dyspepsia alone was 6% (200/3517, 95% CI: 4.9, 6.5), while the proportion with GER alone was 7% (260/3517, 95% CI: 6.6, 8.3), and the proportion with dyspepsia-GER overlap was 4% (144/3517, 95% CI: 3.5, 4.8).
Figure 1. Proportions of subjects reporting dyspepsia alone, gastroesophageal reflux (GER) alone, and dyspepsia-GER overlap.
The demographic characteristics of subjects with GER alone, dyspepsia alone, or dyspepsia-GER overlap are summarized in Table 1. There were no significant associations of subject status (dyspepsia alone, GER alone, or dyspepsia-GER overlap) with age, gender, frequent physician visits, or medication use, except proton pump inhibitor (PPI) use. (The proportion reporting use of PPIs was higher in the dyspepsia-GER overlap subgroup compared to others.) However, SSC scores were associated with subject symptom status (p<0.001) with scores in the dyspepsia-GER overlap subgroup higher than that in the dyspepsia alone subgroup or GER alone subgroup (Table 1). Specifically, the mean score of all the SSC items in the dyspepsia-GER overlap subgroup was greater than the others. IBS was more common in subjects with dyspepsia-GER overlap or dyspepsia alone subgroup than the GER alone subgroup.
Table 1. Demographic Characteristics in subjects with gastroesophageal reflux (GER) alone, dyspepsia alone, or dyspepsia-GER overlap.
| Dyspepsia-GER Overlap (n=144) | GER alone (n=260) | Dyspepsia alone (n=200) | Neither GER nor Dyspepsia (n=2913) | |
|---|---|---|---|---|
| Age, mean (± SD) | 58 ± 16 | 59 ± 16 | 60 ± 17 | 61 ± 15 |
| Female (n=1909) | 103 (72%) | 149 (57%) | 141 (71%) | 1516 (52%) |
| Male gender (n=1608) | 41 (28%) | 111 (43%) | 59 (29%) | 1397 (48%) |
| SSC score, mean (SD) | 1.5 ± 0.9 | 1.0 ± 0.7 | 1.2 ± 0.7 | 0.7 ± 0.6 |
| Visiting a physician > 5 times (n=369) | 35 (24%) | 43 (17%) | 42 (21%) | 249 (9%) |
| Medication | ||||
| PPIs (n=708) | 80 (56%) | 109 (42%) | 69 (35%) | 450 (16%) |
| Calcium channel blockers (n=204) | 11 (8%) | 12 (5%) | 16 (8%) | 165 (6%) |
| Antidepressants (n=514) | 40 (29%) | 48 (19%) | 57 (29%) | 369 (13%) |
| Narcotics (n=413) | 34 (24%) | 42 (16%) | 39 (20%) | 298 (10%) |
| Antispasmodics (n=54) | 11 (8%) | 7 (3%) | 9 (5%) | 27 (1%) |
| IBS (n=683) | 81 (56%) | 82 (32%) | 102 (51%) | 418 (14%) |
| Somatic symptom checklist items | ||||
| Headaches, mean (± SD) | 1.4 ± 1.2 | 0.9 ± 1.0 | 1.0 ± 1.1 | 0.6 ± 0.8 |
| Backaches, mean (± SD) | 1.9 ± 1.3 | 1.4 ± 1.2 | 1.7 ± 1.3 | 1.0 ± 1.1 |
| Insomnia, mean (± SD) | 2.0 ± 1.3 | 1.3 ± 1.1 | 1.5 ± 1.2 | 0.9 ± 1.0 |
| General stiffness, mean (± SD) | 1.9 ± 1.4 | 1.7 ± 1.2 | 1.8 ± 1.3 | 1.1 ± 1.1 |
| Dizziness, mean (± SD) | 1.0 ± 1.2 | 0.5 ± 0.9 | 0.6 ± 0.9 | 0.3 ± 0.6 |
| Weakness, mean (± SD) | 1.0 ± 1.3 | 0.5 ± 1.0 | 0.8 ± 1.1 | 0.3 ± 0.7 |
SSC, somatic symptom checklist; PPI, proton pump inhibitors; IBS, irritable bowel syndrome
Comparison of observed vs. expected numbers
The expected overlap among dyspepsia and GER was calculated by multiplying the corresponding marginal proportions (i.e. assuming dyspepsia or dyspepsia subtype was independent of GER) and compared with the observed overlap (using the exact binomial test for proportions) (Table 2). The observed number of subjects reporting symptoms consistent with the dyspepsia-GER overlap was about 3.6 times higher than the expected number. Moreover, the observed number of subjects reporting specific subgroups of dyspepsia (namely, postprandial distress syndrome and epigastric pain syndrome) and GER was about 3 to 5 times higher than that expected by chance (Table 2).
Table 2. Comparison of overlap of dyspepsia and gastroesophageal reflux (GER) between observed and expected*.
| Observed N, (%; 95% CI) | Expected N (% of N=3517) | p-value | |
|---|---|---|---|
| Dyspepsia and GER | 144 (4.1%; 3.5, 4.8) | 40 | <0.001 |
| PDS and GER | 92 (2.6%; 2.1, 3.2) | 29 | <0.001 |
| EPS and GER | 94 (2.7%; 2.2, 3.3) | 18 | <0.001 |
, Expected numbers assuming dyspepsia, or dyspepsia subtype is independent of GER PDS= postprandial distress syndrome; EPS= epigastric pain syndrome
Potential risk factors for dyspepsia-GER overlap
The odds ratios corresponding to potential risk factors for dyspepsia-GER overlap versus the dyspepsia alone, and separately, vs. the GER alone are shown in Table 3. SSC score, PPI use, and IBS, were all associated with a greater odds for being in the dyspepsia-GER overlap compared to the GER alone, with greater SSC scores increasing the odds for overlap. In addition, insomnia among items of the SSC was significantly associated with greater odds for dyspepsia-GER overlap compared to GER alone, after adjusting age, gender, IBS, constipation, and diarrhea. However, age, gender, physician visits, and medication use aside from PPIs, were not predictors of dyspepsia-GER overlap vs. GER alone. Higher SSC scores, PPI use, and insomnia were significantly associated with greater odds for dyspepsia-GER overlap compared to dyspepsia alone, after adjusting for age, gender, IBS, constipation, and diarrhea. However, age, gender, physician visits, medication use (except PPI use), and IBS were not associated with dyspepsia-GER overlap vs. dyspepsia alone.
Table 3.
Predictors for dyspepsia-GER overlap vs. GER alone, and separately dyspepsia alone.
| Predictor variables in the models | Overlap vs. GER alone | Overlap vs. Dyspepsia alone | ||
|---|---|---|---|---|
| OR (95% CI)* | OR (95% CI)‡ | OR (95% CI)* | OR (95% CI‡ | |
| Age, per 10 years † | 0.9 (0.8, 1.1) | 1.0 (0.9,1.1) | 0.9 (0.8, 1.0) | 0.9 (0.8,1.1) |
| Female gender † | 1.5 (1.0, 2.4) | 1.7 (1.0,2.7) | 1.0 (0.6, 1.6) | 1.0 (0.6,1.6) |
| Mean SSC score † | 1.9 (1.4, 2.5) | 1.8 (1.3,2.5) | 1.6 (1.2, 2.1) | 1.6 (1.1,2.2) |
| Visiting a physician > 5 times † | 1.2 (0.7, 2.0) | 1.1 (0.6,1.9) | 1.0 (0.6, 1.8) | 1.1 (0.6,1.9) |
| Medication | ||||
| Proton pump inhibitors (PPIs) † | 1.5 (1.0, 2.4) | 1.7 (1.0,2.7) | 2.4 (1.5, 3.8) | 2.7 (1.6,4.4) |
| Ca++ blocker † | 1.6 (0.7, 3.9) | 1.8 (0.7,4.7) | 1.0 (0.4, 2.2) | 1.0 (0.4,2.6) |
| Antidepressants † | 1.1 (0.7, 1.8) | 1.2 (0.7,2.1) | 0.8 (0.5, 1.3) | 0.8 (0.5,1.4) |
| Antispasmodic † | 2.2 (0.8, 5.9) | 2.6 (0.9,7.5) | 1.6 (0.6, 4.0) | 1.7 (0.7,4.6) |
| Narcotic pain † | 1.1 (0.7, 1.9) | 1.2 (0.7,2.1) | 1.1 (0.6, 1.8) | 1.1 (0.6,2.0) |
| IBS § | 2.2 (1.3, 3.6) | 2.2 (1.3,3.7) | 1.0 (0.6, 1.7) | 1.1 (0.6,1.9) |
| SSC items# | ||||
| Headaches | 1.2 (1.0, 1.5) | 1.1 (0.9,1.4) | 1.2 (0.9, 1.5) | 1.2 (0.9,1.6) |
| Backaches | 1.2 (1.0, 1.5) | 1.2 (1.0,1.5) | 1.0 (0.8, 1.2) | 1.0 (0.8,1.3) |
| Insomnia | 1.4 (1.1, 1.7) | 1.3 (1.1,1.6) | 1.3 (1.1, 1.6) | 1.3 (1.0,1.6) |
| General stiffness | 0.8 (0.6, 1.0) | 0.8 (0.6,1.0) | 1.0 (0.8, 1.2) | 0.9 (0.7,1.2) |
| Dizziness | 1.2 (0.9, 1.6) | 1.2 (0.9,1.5) | 1.3 (1.0, 1.6) | 1.2 (0.9,1.5) |
| Weakness | 1.1 (0.9, 1.4) | 1.2 (0.9,1.5) | 0.9 (0.7, 1.2) | 1.0 (0.9,1.3) |
, Odds ratios for overlap (OR [95%CI]); SSC, somatic symptom checklist; IBS, irritable bowel syndrome
Weighted estimates to accommodate age and sex association with survey response.
Adjusted for age (per 10 years), gender, SSC, constipation, diarrhea and IBS
Adjusted for age (per 10 years), gender, total SSC score, constipation and diarrhea
Adjusted for age (per 10 years), gender, constipation, diarrhea and IBS
Discussion
This study has shown that the prevalence of dyspepsia-GER overlap was 4% in Olmsted County, Minnesota. This overlap of dyspepsia and GER was greater than expected by chance, suggesting the overlap group may represent a distinct syndrome.
An overlap of dyspepsia and GER has been recognized but not well quantified, in contrast to a number of studies that have described the overlap of GER and IBS.24-26 Kennedy et al.26 showed that IBS, GER symptoms, and symptomatic bronchial hyper-responsiveness occur more frequently together than expected in the general population. In a systematic review of the overlap between dyspepsia and GERD, Nastaskin et al.25 showed a substantial overlap between IBS and GERD; after eliminating GERD in the community, the prevalence of IBS in non-GERD subjects was low (5.1%). The possible explanation of this close relationship of IBS and GERD is that these two disorders share a common etiology leading to a shared pathophysiology, such as visceral hyperalgesia.27 Similarly, dyspepsia has been reported to be a common gastrointestinal comorbid conditions in patients with IBS,28 and the significant overlap of these two conditions may be explained by a common pathophysiology, such as visceral hypersensitivity.29
The Rome II committee2 suggested the exclusion of predominant heartburn in patients with functional dyspepsia. They concluded, based primarily on expert opinion, that patients with typical heartburn as a dominant complaint almost invariably have gastroesophageal reflux disease and should be distinguished from patients with dyspepsia. The Rome III committee concurred with this recommendation.1 However, several studies showed that GER symptoms commonly co-exist in a considerable proportion of patients with dyspepsia9, 30 and vice versa.11, 12 The current study has demonstrated that the overlap of dyspepsia and GER is not explained by chance. Other data support this interpretation. A community study in Belgium undertook a survey of dyspepsia symptoms and overlapping GER symptoms in a random sample of 2025 subjects.30 They showed that reflux symptoms in subjects with dyspepsia overlapped in 34%, and they were more likely to have more severe and a higher frequency of dyspeptic symptoms compared to dyspeptic subjects without heartburn. Moreover, a three factor structure was identified by factor analysis, with one factor including reflux, belching, discomfort, and epigastric pain. These results suggest that a unique subset with dyspepsia and reflux may share a common pathophysiology, although whether this relates to visceral hypersensitivity or impaired gastric accommodation or another mechanism is unknown.31, 32 In 247 patients with dyspeptic symptoms, a negative upper gastrointestinal endoscopy, and without dominant symptoms of heartburn, Tack et al.9 also showed that 23% of these patients had abnormal pH monitoring (acid exposure >5% of time). Moreover, heartburn-negative dyspeptic patients with abnormal esophageal acid exposure did not differ from those with normal acid exposure, in terms of demographic characteristics or the prevalence of potential pathophysiological mechanisms, including Helicobacter pylori infection, gastric emptying, hypersensitivity to gastric distention, and gastric accommodation. However, they did observe that symptoms of epigastric pain were more prevalent in dyspeptic patients with pathological esophageal pH monitoring. Although this study population was from a major referral center, it suggests that about one-quarter of dyspeptic patients have GERD. Our study also showed a high prevalence of reflux symptoms (about 42%) among dyspeptic patients, but we also found that both EPS and PDS had more overlap with GER than expected by chance, a new observation. Other studies9, 33 have been based on selected patients rather than community samples and did not look at EPS and PDS separately.
Our study also found several putative risk factors for dyspepsia-GER overlap compared to GER alone or dyspepsia alone. Most notably, a high somatization score was independently associated with dyspepsia-GER overlap vs. GER alone or dyspepsia alone, which is consistent with other observations. Psychological stressors can probably promote both gastroesophageal reflux34 and dyspepsia.35 Among items in the SSC, we specifically found that self-reported insomnia was a risk factor for dyspepsia-GER overlap compared to both dyspepsia alone and GERS alone. In fact, many studies have reported a significant association between sleep disturbances and reflux disease as well as functional gastrointestinal disorders which might be bidirectional.36, 37 However, we failed to find an association of excess physician visits with dyspepsia-GER overlap although psychological factors might drive consultation behavior among subjects with GERD38 and dyspepsia.39 Interestingly, of the medications assessed, we found that only PPIs were associated with dyspepsia-GER overlap vs. both dyspepsia alone and GERS alone, suggesting that subjects who have more upper gastrointestinal symptoms are more likely to take PPIs. This observation is also consistent with a study reporting the overlap reflux-dyspeptic group had more frequent and more severe symptoms (dyspepsia and reflux).30
The presence of IBS was also associated with dyspepsia-GER overlap compared to GER alone. This is consistent with other studies, where the prevalence of IBS has ranged from 10 to 50% in GERD17, 24 and 20 to 50% in dyspepsia.33, 40 We speculate that a similar pathophysiological mechanism is responsible for all three conditions, but further studies are needed.
The strengths of the current study include the investigation of a random community sample. We obtained a representative sample of 3517 study participants. The subjects were not necessarily seeking health care for their gastrointestinal complaints, which should have minimized selection bias, and this sample provided an excellent opportunity to study the real relationship between dyspepsia and GER. However, our data may not be generalized to the whole U.S. population because the racial composition of this community is predominantly white. The prevalence of GER and dyspepsia may vary by ethnic group, but at a minimum our data are probably generalizable to the U.S. white population.
In conclusion, dyspepsia and GER overlap is common in the population and does not occur by chance. High somatization, especially insomnia and PPI use. independently predict dyspepsia-GER overlap vs. both dyspepsia alone and GER alone. Our study suggests that the overlap group may represent a distinct syndrome that might need alternate management strategies.
Acknowledgments
The authors wish to thank Lori R. Anderson for her assistance in the preparation of the manuscript.
Grant Support: This study made possible by the Rochester Epidemiology Project (Grant R01- AG034676 from the National Institute on Aging).
Footnotes
Disclosures: Dr. Talley and Mayo Clinic have licensed the Talley Bowel Disease Questionnaire.
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Stanghellini V, Heading RC, et al. Functional gastroduodenal disorders. Gut. 1999;45(2):II37–42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hungin AP, Hill C, Raghunath A. Systematic review: frequency and reasons for consultation for gastro-oesophageal reflux disease and dyspepsia. Aliment Pharmacol Ther. 2009;30:331–42. doi: 10.1111/j.1365-2036.2009.04047.x. [DOI] [PubMed] [Google Scholar]
- 4.Locke GR, 3rd, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Locke GR, 3rd, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–9. doi: 10.1136/gut.2005.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronkainen J, Aro P, Storskrubb T, et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population--the Kalixanda study. Aliment Pharmacol Ther. 2006;23:1725–33. doi: 10.1111/j.1365-2036.2006.02952.x. [DOI] [PubMed] [Google Scholar]
- 7.Enck P, Dubois D, Marquis P. Quality of life in patients with upper gastrointestinal symptoms: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST) Scand J Gastroenterol Suppl. 1999;231:48–54. doi: 10.1080/003655299750025264. [DOI] [PubMed] [Google Scholar]
- 8.Sarnelli G, De Giorgi F, Efficie E, et al. Correlation between oesophageal acid exposure and dyspeptic symptoms in patients with nonerosive reflux disease. Eur J Gastroenterol Hepatol. 2008;20:264–8. doi: 10.1097/MEG.0b013e3282f340b2. [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Caenepeel P, Arts J, et al. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut. 2005;54:1370–6. doi: 10.1136/gut.2004.053355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talley NJ, Meineche-Schmidt V, Pare P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies) Aliment Pharmacol Ther. 1998;12:1055–65. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Guillemot F, Ducrotte P, Bueno L. Prevalence of functional gastrointestinal disorders in a population of subjects consulting for gastroesophageal reflux disease in general practice. Gastroenterol Clin Biol. 2005;29:243–6. doi: 10.1016/s0399-8320(05)80756-0. [DOI] [PubMed] [Google Scholar]
- 12.Neumann H, Monkemuller K, Kandulski A, et al. Dyspepsia and IBS symptoms in patients with NERD, ERD and Barrett's esophagus. Dig Dis. 2008;26:243–7. doi: 10.1159/000121354. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Zinsmeister AR, Schleck CD, et al. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 16.Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Phillips SF, Melton J, 3rd, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 18.Rey E, Locke GR, 3rd, Jung HK, et al. Measurement of abdominal symptoms by validated questionnaire: a 3-month recall timeframe as recommended by Rome III is not superior to a 1-year recall timeframe. Aliment Pharmacol Ther. 2010;31:1237–47. doi: 10.1111/j.1365-2036.2010.04288.x. [DOI] [PubMed] [Google Scholar]
- 19.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 20.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–47. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 21.Attanasio V, Andrasik F, Blanchard EB, et al. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7:247–57. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 22.Choung RS, Locke GR, 3rd, Zinsmeister AR, et al. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009;104:1772–9. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 24.Jung HK, Halder S, McNally M, et al. Overlap of gastro-oesophageal reflux disease and irritable bowel syndrome: prevalence and risk factors in the general population. Aliment Pharmacol Ther. 2007;26:453–61. doi: 10.1111/j.1365-2036.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 25.Nastaskin I, Mehdikhani E, Conklin J, et al. Studying the overlap between IBS and GERD: a systematic review of the literature. Dig Dis Sci. 2006;51:2113–20. doi: 10.1007/s10620-006-9306-y. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy TM, Jones RH, Hungin AP, et al. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43:770–4. doi: 10.1136/gut.43.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasiorowska A, Poh CH, Fass R. Gastroesophageal reflux disease (GERD) and irritable bowel syndrome (IBS)--is it one disease or an overlap of two disorders? Dig Dis Sci. 2009;54:1829–34. doi: 10.1007/s10620-008-0594-2. [DOI] [PubMed] [Google Scholar]
- 28.Talley NJ, Dennis EH, Schettler-Duncan VA, et al. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–9. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 29.Hillila MT, Siivola MT, Farkkila MA. Comorbidity and use of health-care services among irritable bowel syndrome sufferers. Scand J Gastroenterol. 2007;42:799–806. doi: 10.1080/00365520601113927. [DOI] [PubMed] [Google Scholar]
- 30.Piessevaux H, De Winter B, Louis E, et al. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol Motil. 2009;21:378–88. doi: 10.1111/j.1365-2982.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 31.Tack J. Is there a unifying role for visceral hypersensitivity and irritable bowel syndrome in non-erosive reflux disease? Digestion. 2008;78(1):42–5. doi: 10.1159/000151254. [DOI] [PubMed] [Google Scholar]
- 32.Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57:674–83. doi: 10.1136/gut.2007.127886. [DOI] [PubMed] [Google Scholar]
- 33.Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 25:1151–6. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- 34.Baker LH, Lieberman D, Oehlke M. Psychological distress in patients with gastroesophageal reflux disease. Am J Gastroenterol. 1995;90:1797–803. [PubMed] [Google Scholar]
- 35.Choung RS, Locke GR, Schleck CD, et al. Do distinct dyspepsia subgroups exist in the community? A population-based study. Am J Gastroenterol. 2007;102:1983–9. doi: 10.1111/j.1572-0241.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerson LB, Fass R. A systematic review of the definitions, prevalence, and response to treatment of nocturnal gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:372–8. doi: 10.1016/j.cgh.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Schey R, Dickman R, Parthasarathy S, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology. 2007;133:1787–95. doi: 10.1053/j.gastro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Johnston BT, Gunning J, Lewis SA. Health care seeking by heartburn sufferers is associated with psychosocial factors. Am J Gastroenterol. 1996;91:2500–4. [PubMed] [Google Scholar]
- 39.Koloski NA, Talley NJ, Huskic SS, et al. Predictors of conventional and alternative health care seeking for irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2003;17:841–51. doi: 10.1046/j.1365-2036.2003.01498.x. [DOI] [PubMed] [Google Scholar]
- 40.Locke GR, 3rd, Zinsmeister AR, Fett SL, et al. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]