Abstract
Purpose
To determine whether proton radiation affects coagulation.
Material and methods
Ferrets were exposed to solar particle event-like proton radiation at doses of 0, 25, 100, or 200 centigray (cGy), and dose rates of 50 cGy/minute (high dose rate or HDR) or 50 cGy/hour (low dose rate or LDR). Plasma was isolated from blood collected prior to radiation exposure and at 3–7 h post-radiation. Prothrombin time (PT) assays and activated partial thromboplastin time (aPTT) assays were performed as were mixing studies to determine the coagulation factors involved.
Results
HDR and LDR exposure led to statistically significant increases in PT values. It was determined that the HDR-induced increase in PT was due to Factor VII, while Factors II, V, and VII contributed to the LDR-induced increase in PT values. Only acute LDR exposure caused an increase in aPTT values, which remained elevated for 48 h post-irradiation (which was the latest time assayed in these studies). Mixing studies revealed that Factor IX contributed to the increased aPTT values. A majority of the animals exposed at the LDR had an International Normalized Ratio approaching or surpassing 2.0.
Conclusions
PT/aPTT assays resulted in increased clotting times due to different coagulation factors, indicating potential radiation-induced coagulopathy.
Keywords: Radiation, haematology – radiation, low dose rate
Introduction
Future plans of National Aeronautics Space Administration (NASA) include manned space flights occurring over extended periods of time (Lawler 2010). These ‘exploration class’ missions have augmented the risk of astronaut exposure to space radiation from a solar particle event (SPE) and highly energetic, charged (HZE) particles (Hellweg and Baumstark-Khan 2007). The possibility of increased exposure to SPE radiation, which can include HZE particles, has put an emphasis on the acute biologic effects of radiation exposure. There is a known risk of relatively high dose SPE radiation exposure to astronauts that may result in symptoms of the acute radiation syndrome, which could threaten mission completion. An SPE involves the release of particles (protons, electrons, heavy ions) with energies greater than 10 milli-electronvolt/nucleon (MeV/n) (Turner and Baker 1998). Protons are the major component of SPE radiation; therefore, ground-based SPE radiation research is focused on the biological consequences of proton radiation at the appropriate energies, doses, and dose-rates occurring during an SPE. Doses absorbed by tissues can vary for different SPE; appropriate model systems have been developed to calculate the radiation doses that could have been received by astronauts during previous SPE (Hu et al. 2009). For instance, it has been estimated that the August 1972 SPE could have delivered doses to the skin of 269 centiGray (cGy) in a spacecraft and 3215 cGy during extra-vehicular activity (EVA), as well as doses of approximately 46 and 138 cGy doses to blood forming organs (BFO) in a spacecraft and during EVA, respectively.
Although information relating to the biological effects of SPE-like radiation is limited, the effects resulting from exposure to other types of ionizing radiation have been extensively documented. Effects of SPE radiation on cardiovascular functions are of interest in space radiation research due to the fact that a number of charged particles from an SPE have the ability to penetrate an astronaut ’ s spacesuit and body. The estimated radiation doses could be deleterious to the heart and vasculature as they can approach or potentially exceed the NASA space radiation permissible exposure limits. Detrimental cardiovascular effects of radiation have been reported in the literature on the atomic bomb survivors (Shimizu et al. 1999, Preston et al. 2003), those exposed to fall-out from the Chernobyl accident (Ivanov 2006), radiological technicians (Muirhead et al. 2009), and cancer patients (Early Breast Cancer Trialists ’ Collaborative Group 2002, Clarke et al. 2005). There are also numerous cardiovascular clinical manifestations that have been associated with irradiation; some of which include coronary artery disease (Hancock et al. 1993, Reinders et al. 1999, Carr et al. 2005), pericarditis (Stewart and Fajardo 1984, Arsenian 1991), myocardial dysfunction (Glanzmann et al. 1998), valvular disease (Carlson et al. 1991, Hull et al. 2003), and vascular changes such as vessel rupture and arterial stenosis (Fajardo and Lee 1975, Lipshultz and Sallan 1993, Patel et al. 2005).
One of the understudied areas of radiation research involves the effects of radiation on blood coagulation. The coagulation pathway is comprised of interlocking components that generate a fibrin-rich blood clot, which halts bleeding. Primary hemostasis involves the activation of platelets at the site of injury by exposing collagen to blood, consequently allowing von Willenbrand factor (vWF) to bind to collagen and tether platelets to the vasculature wall. The coagulation pathway, or what is commonly known as secondary hemostasis, occurs simultaneously on the negatively charged surface of activated platelets to generate a fibrin-rich thrombus (Hoffman and Monroe 2001). The exposure of tissue factor (TF) at the site of vascular injury, and its subsequent binding with Factor VII (extrinsic pathway), initiates a thrombin (Factor II) burst that consequently leads to fibrin clot generation through the activation of a series of vitamin K-dependent serine proteases. However, it is the activation of coagulation factors, such as Factor V and VIII, by thrombin that becomes the driving force in developing a stable fibrin clot as part of the intrinsic pathway (Furie and Furie 1988, Davie et al. 1991).
Previous studies have established that radiation induces vWF secretion from endothelial cells (Sporn et al. 1984, Jahroudi et al. 1996). It has also been reported that TF is upregulated in response to radiation exposure, and it exerts a pro-coagulant effect up to 7 days post-radiation (Goldin-Lang et al. 2007). Radiation effects in an in vitro model system showed an increase in the activation of coagulation factors including Factors II, VII, VIII, IX, X, XI, XII, and XIII (Weisbach et al. 2007). In a proteomic study, results indicated overexpression of pro-coagulant proteins such as prothrombin-precursor, fibrinogen-alpha-chain, and high-molecular-weight kininogen (HMWK) two days after exposure to 300 cGy 137Cs γ-rays (Rithidech et al. 2009). At 7 days after exposure, however, there was decreased expression of the prothrombin-precursor, Factor XII, as well as HMWK overexpression. Weisbach et al. (2007) report a slight reduction in clotting time in irradiated, leukoreduced fresh frozen plasma (FFP), as observed in the study utilizing two different ex vivo assays to assess TF and the intrinsic pathway’s dependent fibrin generation; a prothombin time (PT) assay and an activated partial thromboplastin time (aPTT). Based on the limited studies that show increased activity in pro-coagulant proteins, as well as other pro-coagulant effects, such as decreased PT/aPTT in FFP, we hypothesized that ionizing radiation accelerates the time for clot formation in irradiated animals.
To determine whether SPE-like proton radiation could have effects on clotting time, experiments were performed in ferrets exposed to 110 MeV protons at doses and dose rates that mimic SPE-like proton radiation (50 cGy/hour [h] or 50 cGy/minute [min]). Ferrets were used as an outbred species, which are not expected to have a uniform response to radiation exposure; thus, it is hypothesized that the observed effects of SPE radiation in ferrets might closely resemble those that could occur in humans.
Materials and methods
Animals
Forty-five, 12–16 week old descented Fitch ferrets (0.3–0.8 kg; Marshall farms, North Rose, NY, USA) were housed at the Loma Linda University Medical Center (LLUMC) Animal Facility. Once acclimated, 3–5% isoflurane inhalation was used to anesthetize ferrets for jugular vein blood collection. The ferrets were randomly assigned to the various treatment groups in the studies. All animal procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of LLUMC and the University of Pennsylvania. All radiation/animal experiments were performed at LLUMC.
Radiation procedures
On the day of irradiation, the ferrets were placed in radiation chambers, approximately 16 × 24 × 9 cm. The radiation exposure was performed at the horizontal clinical beam-line at LLUMC using an incident proton beam of 155 MeV. A scattering system and modulator wheel were used to degrade the incident beam to 110 MeV. Two different dose rates were generated, a high dose rate (HDR) of 50 cGy/min and a low dose rate (LDR) of 50 cGy/h, with total body exposure of the following doses: 25, 100, and 200 cGy. Sham-irradiated ferrets were placed in the irradiation chambers with no radiation exposure for 7 h. Ferrets exposed at the LDR were irradiated for 30 min, 2 h or 4 h depending on dose, 25 cGy, 100 cGy or 200 cGy, respectively. The ferrets were removed from the chambers 3 h after the completion of radiation exposure, placed under anesthesia, and blood was drawn from both the irradiated and sham-irradiated control ferrets. To investigate changes in coagulation time induced by the 200 cGy dose of protons at different dose rates, one group of ferrets received a 200 cGy dose at the HDR (approximately a 4 min exposure) and were then restrained in the chamber for a total of 7 h, corresponding to the 4 h LDR exposure, which was followed by a 3 h restraint time in the chamber. Blood was collected in all HDR and LDR groups 3 h post-irradiation except for the single group of ferrets exposed to the HDR who were bled at 7 h post-irradiation.
Plasma preparation
Ferret blood was immediately placed in a collection tube containing a 3.8% sodium citrate solution with a ratio of 9 parts of blood to 1 part of anticoagulant. The blood was fractionated by centrifugation at 3,000 gravitational force at 4° C for 15 min to allow the isolation of platelet-poor plasma, which was then stored at −80°C until analyzed.
PT/aPTT assays
Plasma was thawed and diluted 1:5 with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution (Invitrogen, Carlsbad, CA, USA) containing 0.1% bovine serum albumin (HBS-B; Fisher Scientific, Hampton, NH, USA). Diluted plasma was added 1:1 to a cuvette containing HBS-B, and incubated for one minute. TriniCLOT PT excel (Trinity Biotech, Wicklow, Ireland) was then added to the cuvette at a ratio of 1:1 (volume/volume). Clotting times were measured using a STart® 4 semi-automated hemostasis analyzer (Diagnostica Stago, Parsippany, NJ, USA). For the aPTT analysis, a second aliquot of plasma was diluted 1:3 with HBS-B, and equal parts of TriniCLOT Automated APTT reagent, HBS-B, and diluted plasma were added to a cuvette and incubated for 3 min. 50 microliters (μl) of calcium chloride was added to initiate clotting, and clotting times were measured using the STart® 4 semi-automated hemostasis analyzer.
Mixing studies
To determine deficiencies of particular coagulation factors, ferret plasma was prepared as reported in the PT/aPTT methods section and was mixed with Factor deficient plasma or pooled normal plasma (PNP; Valley Biomedical, Winchester, VA, USA). Factor deficient plasma for the following factors were used: Factor II, V, VII, VIII, IX, X, XI, or XII (Haematologic Technologies Inc, Essex Junction, VT, USA). The factor deficient plasma were independently mixed with plasma samples from ferrets taken pre- or post-irradiation exposure (1:1). The mixed samples were then assayed for PT and aPTT values (described above).
Results from these studies are reported as statistically significant when a factor deficient plasma sample mixed with a plasma sample from an irradiated ferret (with an originally statistically significant PT/aPTT value) still produced a statistically significant increase in clotting time, when compared to the appropriate control values. Thus, the mixing studies are confirming that the experimental plasma samples are not sufficient in a particular factor and continue to display a sustained increase in clotting time after the addition of a plasma sample having a deficiency in a particular factor. For example, if a sample from an irradiated ferret was deficient in Factor II, then introducing Factor II deficient plasma does not correct the significant increases in PT values observed.
Statistical analysis
The data were analyzed using PRISM 5.0 for Mac OS X software (Graphpad, La Jolla, CA, USA). The results are presented as the mean ± standard error of the mean (SEM). The International Normalized Ratios (INR) were calculated using the following equation:
The International Sensitivity Index (ISI) is unique to each reagent and lot number, and is calibrated to each individual instrument by Trinity Biotech. As emi-automated ISI mechanical value of 1.89 was used for these calculations and ferret pre-irradiation PT by experimental groupings were used to calculate the PT ratio (PTtest/PTnormal). The results were initially analyzed to confirm a normal distribution. Upon confirmation, data were analyzed to determine whether there were statistically significant differences between treatment groups using paired t-tests (InStat v. 3.1 software, Graphpad). The differences between treatment groups were considered statistically significant at p < 0.05.
Results
Effect of animal restraint on clotting times
PT and aPTT values were measured in ferrets exposed to irradiation and compared to the corresponding pre-irradiation PT and aPTT values. To determine whether the restraint of the animals (without radiation exposure) affects the blood clotting PT or aPTT values, sham-irradiated control ferrets were placed in chambers for up to a total of 7 h, to mimic the experimental design for irradiated ferrets (as described in Material and methods). The average PT time for ferrets prior to the 7-h incubation in the chamber was 19.5 ± 1.3 sec, compared to the post-sham-irradiation PT time of 21.7 ± 2.1 sec (data not shown). The pre-sham-irradiation aPTT average value was 29.5 ± 1.3 sec, while the average post-sham-irradiation value was 30.4 ± 1.9 sec (data not shown). Monitoring PT and aPTT values after a 7-h restraint, in the absence of radiation exposure, resulted in no statistically significant differences in clotting times, when comparing the pre-sham-irradiation and the post-sham-irradiation values.
HDR and LDR effects on the extrinsic pathway (PT)
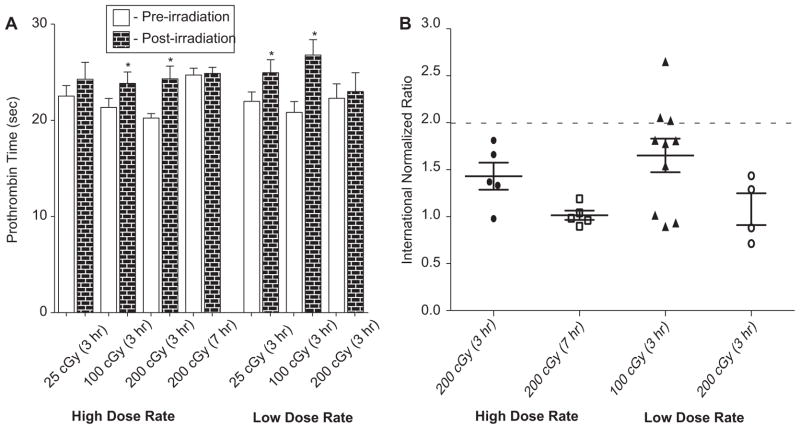
The next set of experiments was performed to determine the dose response relationship for 110 MeV protons administered at either the HDR or LDR. The average pre-irradiation PT value for all irradiated ferrets was 21.8 ± 0.4 sec (data not shown). At the 25 cGy dose (HDR), no statistically significant differences were observed when comparing the pre-irradiated value to the post-irradiated PT values (Figure 1A). 100 and 200 cGy exposures at the HDR, measured 3 h after radiation exposure, yielded a statistically significant increase in PT values when compared to their pre-irradiated value, with the 200 cGy dose resulting in a post-irradiation PT value of 24.3 ± 1.3 sec. Interestingly, the average PT value from the 200 cGy dose at the HDR, which was measured at 7 h post-irradiation, was not statistically significant from the pre-irradiated value (Figure 1A).
Figure 1.
(A) Radiation exposure increases prothrombin time (PT) values in ferrets. Plasma was assayed for PT prior to radiation exposure (pre-irradiation) and 3 or 7 h post-radiation exposure. A statistically significant increase in PT value was observed for 25 and 100 cGy (LDR), as well as for the 100 and 200 cGy doses (HDR) at 3 h post-irradiation. For the 200 cGy dose (HDR) at 7 h post-irradiation, increased PT values were not observed. P-values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 5–12 animals from two independent experiments. (B) Radiation exposure results in a clinically significant INR in some of the irradiated ferrets. Three ferrets receiving 100 cGy at the LDR exposure had INR levels ≥ 2.0 while another three ferrets approached that level (INR of 1.75–2.0). While 200 cGy (HDR) led to statistically significant increases in PT values at 3 h post-irradiation, clinical significance (indicated by the INR value) of PT clotting was not evident. Error bars indicate the standard error of the mean (SEM) for n = 5–12 animals from two independent experiments.
At the LDR, both the 25 and 100 cGy doses resulted in a statistically significant increase in PT values. The 100 cGy dose (LDR) resulted in the largest increase in PT values observed among the doses and dose rates investigated. The average PT clotting time for 100 cGy (LDR) at pre-irradiation was 20.8 ± 1.1 which increased to 26.8 ± 1.6 sec (p < 0.05) post-irradiation exposure. No statistically significant differences were observed for animals exposed to 200 cGy (LDR).
Human PT values are commonly reported as an INR, which is defined as the patient’s ‘test’ PT value divided by the laboratory ‘normal ’ PT value, raised to the power of the ISI. A normal human INR is 1.0, and is of clinical concern at an INR ≥ 2.0 (Yuan et al. 2007, Ng 2009). An INR was calculated for animals exposed to the 200 cGy dose, at both dose rates, as well as the 100 cGy LDR dose, which resulted in the greatest response to SPE radiation in the studies reported here. Three out of 10 animals exposed to the 100 cGy dose at the LDR had an INR value of ≥ 2.0 (Figure 1B). The results for an additional 3/10 of the ferrets are of borderline clinical significance, with INR values approaching 2.0 (>1.75). Although the average PT value for animals exposed to 200 cGy at the HDR (when measured at 3 h post-irradiation) were statistically significant when compared to their pre-irradiated PT values, as shown in Figure 1A, the calculated INR for this dose group was not of clinical concern (INR < 2.0).
HDR and LDR effects on the intrinsic pathway (aPTT)
APTT values for irradiated ferrets are shown in Figures 2A and 2B. The average clotting time for all pre-irradiation samples was 31.9 ± 0.8 sec (data not shown). For the HDR exposure groups, there were no statistically significant differences observed when comparing the aPTT values from the pre-irradiation samples to the post-irradiation samples at 3–7 h (Figure 2A); however, there was a significant effect at 48 h post-irradiation. The LDR exposures, however, did result in statistically significant differences between the pre- and post-radiation samples for all doses evaluated: 25, 100, and 200 cGy. The aPTT values after radiation exposure were 39.2 ± 1.9 sec in the 25 cGy group, 36.6 ± 1.8 sec for the 100 cGy group, and 41.6 ± 5.5 sec for the 200 cGy group. APTT values remain increased for the 200 cGy dose group at 48 h post-irradiation, which was the last timepoint assayed. The data for 100 cGy LDR (48 h post-irradiation) were of borderline statistical significance (p = 0.08).
Figure 2.
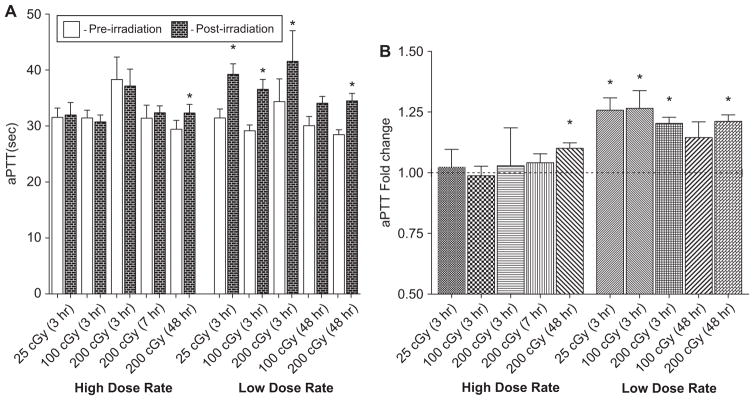
(A) LDR radiation leads to an increase in aPTT values in irradiated ferrets. Statistically significant effects were observed for radiation exposure only at the LDR exposures. P -values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 5–11 animals from two independent experiments. (B) LDR induces a measurable fold change in aPTT. Data were normalized by individual ferret pre-irradiation clotting times and reported in terms of fold change. The dotted line represents a standard control value of 1.0. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 5–11 animals from two independent experiments.
To indicate the amount of variation from individual pre-irradiated aPTT values as well as to compare the effects of the two dose rates, the data were normalized by individual pre-irradiation clotting times and reported in terms of fold change, with the pre-irradiated control = 1.0 (Figure 2B). 25, 100, and 200 cGy LDR exposure resulted in increased fold changes of 1.26 ± 0.05, 1.27 ± 0.07, and 1.20 ± 0.02, respectively. At 48 h post-irradiation (200 cGy), APTT values remain increased with a fold change of 1.21 ± 0.03.
Determination of individual factors involved in radiation-induced clotting times
To study particular coagulation factors involved in the increased clotting times, mixing studies were conducted. First, PNP was mixed with ferret plasma obtained either at the pre- or post-irradiation timepoints. PNP contains all the essential factors that contribute to the extrinsic, intrinsic, and common (where the two pathways converge) pathways of coagulation; therefore, if experimental plasma samples, taken from ferrets at a post-irradiation time point, are deficient in any factor, the mixing of PNP would supplement the deficient factor and correct for the observed abnormal radiation-induced clotting time. The results of these studies indicated that mixing plasma samples taken from ferrets at post-irradiation timepoint(s) with PNP samples led to PT values comparable to the pre-irradiation PT values (data not shown).
Next, factor deficient plasma samples were mixed with the plasma samples isolated from the irradiated ferrets and PT values were determined. Deficiencies were observed in Factors II, V, and VII for the 100 cGy dose group at the LDR (p < 0.05), as was evidenced by an increase in PT values (Figure 3A). Factor X was not deficient when the pre- and post-irradiation plasma PT values were compared. The 200 cGy HDR exposure group resulted in increased PT values when Factor VII deficient plasma samples were mixed with the irradiated ferret plasma samples (Figure 3B). All other factors evaluated were not significantly deficient and therefore did not result in a statistically significant increase in PT values in the 200 cGy HDR exposure group.
Figure 3.
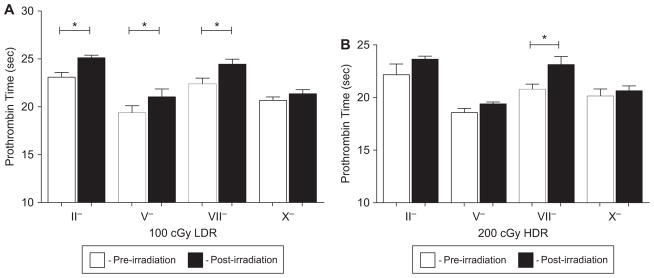
(A) LDR radiation induces an increase in PT due to Factors II, V, and VII deficiency. Plasma was isolated from ferrets exposed to 100 cGy (LDR) pre- and post-radiation (3 h) and was mixed with factor deficient plasma. Deficiencies in Factors II, V and VII were observed. P-values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 4–8 animals from two independent experiment. (B) Factor VII contributes to the HDR radiation-induced increases in PT values. Mixing studies determined that Factor VII deficient plasma results in a statistically significantly increase in PT values while the PT values after mixing with Factor II, V, and X deficient plasma returned the PT value in the irradiated animals to comparable pre-irradiation times. Pre- and post-irradiation (3 h) plasma were mixed with factor deficient plasma and assayed for PT. P-values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 3–4 animals from one independent experiment.
Lastly, the intrinsic pathway was evaluated using the aPTT assay to determine the integrity of the factors contributing to the observed radiation-induced increase in aPTT values. The 100 cGy dose group (LDR) resulted in radiation-induced factor deficiencies in Factor VIII, IX, X, XI, and XII (Figure 4A). Factor IX deficient plasma resulted in the largest increase in clotting time when the pre- and post-irradiation aPTT values were compared (39.4 ± 2.2 to 47.0 ± 1.4 sec, respectively). While exposure to 200 cGy at the LDR resulted in statistically significant aPTT values, the only factor deficiency observed was Factor IX, with a pre- and post-irradiation aPTT value of 37.9 ± 1.5 and 42.2 ± 1.6 sec (Figure 4B), respectively.
Figure 4.
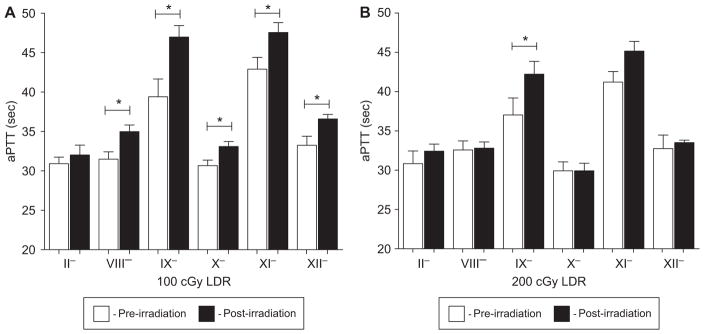
(A) Ferrets irradiated with 100 cGy (LDR) were observed with Factor VIII, IX, X, XI, and XII deficiency. Pre- and post-irradiation (3 h) plasma were mixed with factor deficient plasma and assayed for aPTT. Statistically significant deficiencies were observed with Factors VIII, IX, X, XI, and XII. APTT is reported in seconds. P-values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 4–8 animals from two independent experiment. (B) Factor IX deficiency resulted in increases in aPTT values observed in ferrets exposed to 200 cGy (LDR). Pre- and post-irradiation (3 h) plasma were mixed with factor deficient plasma and assayed for aPTT. Statistically significant deficiencies were observed solely with Factor IX. APTT is reported in seconds. P-values were determined using a paired t-test. *p < 0.05. Error bars indicate the standard error of the mean (SEM) for n = 4 animals from one independent experiment.
Discussion
The results of these studies indicate that radiation exposure has varying effects on both arms of coagulation, increasing the blood clotting times in irradiated ferrets. The time interval between 3–7 h post-radiation exposure appears to be a crucial period for observing an increase in PT values in ferrets, suggesting that radiation exposure may have a temporal effect on the extrinsic cascade. Unlike the extrinsic pathway, the intrinsic pathway (measured by aPTT values) is solely affected by LDR exposure at 3–7 h post-irradiation. These results indicate that there appears to be a time frame after radiation exposure in which the clotting effects involving the extrinsic pathway can be measured. Additionally, a dose rate effect is observed, depending on the pathway studied, which can result in coagulopathies in the irradiated animals.
The doses used in these experiments ranged from 25–200 cGy. These doses are within the expected range of previously estimated SPE radiation doses to BFO. Radiation effects on PT values have been reported for radiation doses of 300 up to 3000 cGy (Weisbach et al. 2007, Rithidech et al. 2009). There are no data published using low doses (< 100 cGy) for such studies. The estimated dose from the 1972 SPE to BFO inside a spacecraft is (approximately) 46 cGy, nearly twice the lowest dose used in these experiments; during an EVA, the dose to BFO is expected to be approximately 138 cGy (Hu et al. 2009).
The INR data presented here suggest that SPE radiation exposure could pose a serious risk to astronauts. Recent publications report bleeding risks associated with trauma victims with an INR > 1.5 or 1.8 (Thomas and DeLoughery 2004, Yuan et al. 2007). As the INR levels reported here resulting from ferret exposure to SPE-radiation exceed the current limits in place for trauma patients in the clinic, our results suggest that trauma or injuries could pose a threat to astronauts. Complications of coagulopathies include, but are not limited to, bleeding into joints, muscle, and other tissues. Limited medical care while on an EVA increases the risk of hypocoagulability and in the situation of massive trauma, potential exsanguination. Some 70% of ferrets exposed to a 100 cGy (LDR) dose had an INR that would be deemed a risk for trauma victims (>1.5), indicating an INR in the clinically significant range. Not all the ferrets exhibited coagulopathies, presumably because ferrets represent an outbred model system, and are thus not expected to have a uniform response.
The acute aPTT measurements in this study were solely affected by the LDR exposures (Figure 2B). Increased aPTT values were observed 48 h post-irradiation when compared to pre-irradiation controls for both HDR and LDR exposures, suggesting that there could be long-term radiation-induced changes in hemostasis. While HDR exposures resulted in a delayed aPTT response, their effects were minor (representing approximately a 10%-fold increase) when compared to the changes observed for LDR exposures. Limited sample size for this endpoint could have contributed to the borderline statistical significance at the 100 cGy (LDR) 48 h time-point. One out of the five ferrets analyzed at 48 h post-irradiation did not have increased aPTT values. It is noteworthy that this one ferret was not affected by LDR exposure at the 3 h time-point either; the inclusion of this animal resulted in a higher p-value (p = 0.08). This variability in the ferret response is not surprising due to the outbred nature of the ferrets.
Differences between the dose rates are evident from the clotting studies, and become even more noteworthy when the factor deficiencies were evaluated in the mixing studies. A summary of all the radiation affected factors, with corresponding pathways, are reported in Table I. When evaluating the integrity of the extrinsic pathway, the HDR exposure causes a Factor VII deficiency (Figure 3B), while LDR exposure affected Factors II, V, and VII (Figure 3A). It is difficult to separate which factor deficiency (II, V, or VII) specifically causes the observed clinically significant INR, as the combination of all three contribute to the hypocoagulable state. Factor VII deficiency may be critical to the temporal effect of radiation on PT values which was observed in the 200 cGy doses when plasma was isolated at 3 h or 7 h post-irradiation. Factor VII has a half-life of 3–6 h (Pehlivanov et al. 2004), which may contribute to the increase in PT values observed at 3 h post-irradiation, but not at 7 h post-irradiation.
Table I.
A summary of radiation-induced factor deficiencies. Factors are classified by assay (pathway) they are found in, and whether the pathway is PT (extrinsic) and/or aPTT (intrinsic).
| Factor | Factor Deficiency
|
||
|---|---|---|---|
| Pathway | Dose | Dose Rate | |
| Factor II | PT/aPTT | 100cGy | LDR |
| Factor V | PT | 100cGy | LDR |
| Factor VII | PT | 100cGy | LDR |
| 200cGy | HDR | ||
| Factor VIII | aPTT | 100cGy | LDR |
| Factor IX | aPTT | 100cGy | LDR |
| 200cGy | LDR | ||
| Factor X | PT/aPTT | 100cGy | LDR |
| Factor XI | aPTT | 100cGy | LDR |
| Factor XII | aPTT | 100cGy | LDR |
The mixing studies performed to evaluate the integrity of the intrinsic pathway suggested that there were coagulation factor deficiencies in Factor VIII, IX, X, XI, and XII, as shown by the increased aPTT values in plasma samples isolated from the 100 cGy dose LDR group (Figure 4A). Factor VIII, XI, and XII are all part of the intrinsic cascade. While Factor IX is referenced as part of the common pathway upon activation from the complex of TF and Factor VII, the predominant form of activation is through the intrinsic cascade. Therefore, in mixing studies, Factor IX signaling is associated with the aPTT cascade. Factor X is a member of the common pathway, where the intrinsic and extrinsic pathways converge. We report that Factor X deficiency is observed in plasma samples obtained from ferrets exposed to LDR radiation in which the PT values were increased, but Factor X is not observed to be deficient when irradiated ferret plasma samples were evaluated for alterations in PT values. This discrepancy may be attributed to mutations caused in Factor X that could exclusively impact aPTT and not PT, which has been reported previously (Denson 1969, Denson et al. 1970, Mannucci et al. 2004).
Plasma isolated from the animals exposed to the 200 cGy dose (LDR) were Factor IX deficient (Figure 4B). The half-life of Factor IX is 18–24 h (White et al. 1997, 1998); therefore, deficiencies in Factor IX could lead to an increase in aPTT values beyond 3 h after SPE radiation. This question will be assessed in future experiments.
In conclusion, SPE-like proton radiation causes a disruption in secondary hemostasis, as is evidenced by increases in PT and aPTT values measured post-irradiation and compared to pre-irradiation values. The intrinsic pathway was solely affected by LDR exposure at 3–7 h post-irradiation, as evidenced by the radiation-induced increase in aPTT values. Mixing studies were used to assess the integrity of the coagulation factors involved in each of the pathways. The LDR exposed ferrets resulted in plasma samples which were deficient in a number of factors affecting all pathways (Table I). The only observation from the plasma isolated from ferrets exposed to HDR radiation was Factor VII deficiency, affecting the extrinsic pathway. At the highest dose tested, 200 cGy at the LDR, only Factor IX deficiency was observed to cause an increase in aPTT values. Future studies will be focused on the determination of the concentration of various affected factors, long-term effects of radiation exposure on Factor IX deficiency, and estimating the risk of coagulopathy.
Acknowledgments
We would like to thank the investigators from LLUMC for helping us with these studies; in particular, we thank Drs Daila Gridley, Andrew Wroe and James Slater for allowing us to use the proton treatment and therapy center for our ferret proton exposures. We would also like to thank investigators from the Armed Forces Radiobiology Research Institute, including Drs Gregory King and Alexandra Miller, as well as Rafael Rives and Laura Mininson, and from the University of Pennsylvania, Dr Jolaine Wilson, for their assistance in sharing ferret tissue, and in the planning and performance of these studies. Lastly, we would like to thank Dr Rodney Camire and Alexandria Savage for their assistance with the PT/aPTT assays. Ferret blood samples were obtained through the NSBRI CARR grant ‘tissue sharing’ activities. The NSBRI is funded through NASA NCC 9-58. This work was also funded by NIH Training Grant 2T32CAO9677.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arsenian MA. Cardiovascular sequelae of therapeutic thoracic radiation. Progress in Cardiovascular Disease. 1991;33:299–311. doi: 10.1016/0033-0620(91)90022-e. [DOI] [PubMed] [Google Scholar]
- Carlson RG, Mayfield W, Normann S. Radiation-associated valvular disease. Chest. 1991;99:538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- Carr ZA, Land CE, Kleinerman RA. Coronary heart disease after radiotherapy for peptic ulcer disease. International Journal of Radiation Oncology, Biology, Physics. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. The Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry. 1991;30:10363. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Denson KW. Abnormal forms of factor X. The Lancet. 1969;2:1256. doi: 10.1016/s0140-6736(69)90791-0. [DOI] [PubMed] [Google Scholar]
- Denson KW, Lurie A, De Cataldo F, Mannucci PM. The factor-X defect: Recognition of abnormal forms of factor X. British Journal of Haematology. 1970;18:317–27. doi: 10.1111/j.1365-2141.1970.tb01446.x. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Radiotherapy for early breast cancer. Cochrane Database of Systematic Reviews. 2002:CD003647. doi: 10.1002/14651858.CD003647. [DOI] [PubMed] [Google Scholar]
- Fajardo LF, Lee A. Rupture of major vessels after radiation. Cancer. 1975;36:904–913. doi: 10.1002/1097-0142(197509)36:3<904::aid-cncr2820360311>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Furie B, Furie B. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Glanzmann C, Kaufmann P, Jenni R. Caridac risk after mediastinal irradiation for Hodgkin’s disease. Radiotherapy Oncology. 1998;46:51–62. doi: 10.1016/s0167-8140(97)00125-4. [DOI] [PubMed] [Google Scholar]
- Goldin-Lang P, Niebergal F, Antoniak S, Szotowski B, Rosenthal P, Pels K, Schultheiss H-P, Rauch U. Ionizing radiation induces upregulation of cellular procoagulability and tissue factor expression in human peripheral blood mononuclear cells. Thrombosis Research. 2007;120:857–864. doi: 10.1016/j.thromres.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. Journal of Clinical Oncology. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: The astronauts’ risk from space radiation. Naturwissenschaften. 2007:94. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- Hoffman M, Monroe DM., III A cell-based model of hemostasis. Journal of Thrombosis and Haemostasis. 2001;85:958–965. [PubMed] [Google Scholar]
- Hu S, Kim M-HY, McClellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Physics. 2009;96:465–476. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- Hull MC, Morris CG, Pepine CJ. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. Journal of the American Medical Association. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- Ivanov V. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Physics. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- Jahroudi N, Ardekani AM, Greenberger JS. Ionizing Irradaition Increases Transcription of the von Willebrand Factor Gene in Endothelial Cells. Blood. 1996;88:3801–3814. [PubMed] [Google Scholar]
- Lawler A. Obama backs new launcher And bigger NASA budget. Science. 2010:327. doi: 10.1126/science.327.5961.18. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Sallan SE. Cardiovascular abnormalities in long-term survivors of childhood malignancy. Journal of Clinical Oncology. 1993;11:1199–1203. doi: 10.1200/JCO.1993.11.7.1199. [DOI] [PubMed] [Google Scholar]
- Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood. 2004;104:1243–1252. doi: 10.1182/blood-2004-02-0595. [DOI] [PubMed] [Google Scholar]
- Muirhead C, O’Hagan J, Haylock R. Mortality and cancer incidence following occupational radiation exposure: Third analysis of the National Registry for Radiation Workers. British Journal of Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng V. Liver disease, coagulation testing, and hemostasis. Clin Lab Med. 2009;29:265–282. doi: 10.1016/j.cll.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Patel DA, Kochanski J, Suen AW. Clinical manifestations of non-coronary atherosclerotic vascular disease after moderate dose irradiation. Cancer. 2005;106:718–725. doi: 10.1002/cncr.21636. [DOI] [PubMed] [Google Scholar]
- Pehlivanov B, Milchev N, Kroumov G. Factor VII deficiency and its treatment in delivery with recombinant factor VII. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2004;116:237–238. doi: 10.1016/j.ejogrb.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality. Radiation Research. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Reinders JG, Heijmen BJ, Olofsen-van Acht MJ. Ischemic heart disease after mantlefield irradiation for Hodgkin’s disease in long-term follow up. Radiotherapy Oncology. 1999;51:35–42. doi: 10.1016/s0167-8140(99)00026-2. [DOI] [PubMed] [Google Scholar]
- Rithidech KN, Honikel L, Rieger R, Xie W, Fischer T, Simon S. Protein-expression profiles in mouse blood-plasma following acute whole-body exposure to 137Cs γ-rays. International Journal of Radiation Biology. 2009;85:432–447. doi: 10.1080/09553000902820390. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Pierce DA, Preston DL. Studies of the mortality of atomic bomb survivors. Report 12, Part II. Noncancer mortality: 1950–1990. Radiation Research. 1999;152:374–389. [PubMed] [Google Scholar]
- Sporn IA, Rubin P, Marder VJ. Irradiation induces release of von Willebrand protein from endothelial cells in culture. Blood. 1984;64:567. [PubMed] [Google Scholar]
- Stewart H, Fajardo LF. Radiation-induced heart disease. Progress in Cardiovascular Disease. 1984;27:173–194. doi: 10.1016/0033-0620(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Thomas G, DeLoughery M. Coagulation defects in trauma patients: Etiology, recognition, and therapy. Critical Care Clinics. 2004;20:13–24. doi: 10.1016/s0749-0704(03)00089-7. [DOI] [PubMed] [Google Scholar]
- Turner RE, Baker JC. Solar particle events and the International Space Station. Acta Astronautica. 1998;42:107–114. doi: 10.1016/s0094-5765(98)00110-6. [DOI] [PubMed] [Google Scholar]
- Weisbach V, Strobel J, Hahn B. Effect of gamma irradiation with 30 Gy on the coagulation system in leukoreduced fresh-frozen plasma. Transfusion. 2007;47:1658–1665. doi: 10.1111/j.1537-2995.2007.01338.x. [DOI] [PubMed] [Google Scholar]
- White G, Beebe A, Nielsen B. Recombinant factor IX. Thrombosis and Haemostasis. 1997;78:261–265. [PubMed] [Google Scholar]
- White G, Shapiro A, Ragni M, Garzone P, Goodfellow J, Tubridy K, Courter S. Clinical evaluation of recombinant factor IX. Seminars in Hematology. 1998;35:33–38. [PubMed] [Google Scholar]
- Yuan S, Ferrell C, Chandler W. Comparing the prothrombin time INR versus the APTT to evaluate the coagulopathy of acute trauma. Thrombosis Research. 2007;120:29–37. doi: 10.1016/j.thromres.2006.07.002. [DOI] [PubMed] [Google Scholar]