Abstract
Progress in developing treatments for diabetic neuropathy is slowed by our limited understanding of how disturbances in metabolic substrates- glucose and fatty acids- produce nerve injury. In this review, we present the current oxidative stress hypothesis and experimental data that support it. We identify weaknesses in our understanding of diabetes-disordered metabolism in the neurovascular unit; i.e. in critical cell types of the microvascular endothelium, peripheral sensory neurons, and supporting Schwann cells. Greater understanding of peripheral nervous system bioenergetics may provide insight into new drug therapies or improvements in dietary interventions in diabetes or even pre-diabetes.
Diabetic neuropathy (DN) is a frequent and severe complication of diabetes. It is more common and persistent in patients with type 2 than type 1 diabetes (Young, et al., 1993) and, over time, DN affects approximately 60% of all patients with the disease (Vincent and Feldman, 2004). DN is characterized by progressive, lengthdependent loss of peripheral nerve axons in a stocking and glove (distal to proximal) pattern (Said, 2007), resulting in pain, decreased sensation and eventually complete loss of sensation. In the United States, DN is the leading cause of diabetes-related hospital admissions and non-traumatic amputations (Boulton, et al., 2005; Edwards, et al., 2008; Feldman, 2008; Feldman, et al., 2003). DN predominantly occurs in a distal symmetrical pattern, with progressive skin denervation (reduced intraepidermal nerve fiber density) (Said, 2007; Sullivan, et al., 2007) over the duration of diabetes (Shun, et al., 2004), and a proximal-distal graded loss of myelinated fiber density observed in the peripheral nerves (Johnson, et al., 1986).
The condition of diabetes mellitus is characterized by hyperglycemia. For decades, the persistent or recurrent periods of hyperglycemia have been thought to produce the microvascular complications of diabetes including neuropathy, determined through epidemiological studies (Diabetes Control and Complications Trial Research Group, 1993 (Chisholm, 1993), Epidemiology of Diabetes Interventions and Complications, 1999 (1999)). More recent studies also suggest a link between dyslipidemia, a common risk factor for macro- and microvascular disease, and diabetic neuropathy (Vincent, et al., 2009). Hyperglycemia and dyslipidemia are predicted to produce gross aberrations in global energy balance and metabolism that could lead to cellular injury and neuropathy. Energy deficits are also predicted to underlie the pathophysiological pattern of distal to proximal injury through impaired ability to produce energy at the extremities of long axons far from the cell body. Despite the facts that energy metabolism is generally well understood and that there has been substantial research into bioenergetic failures that produce DN, there is little experimental data to support the hypothesis for the fundamental cellular mechanisms behind nerve injury. This lack of a clear mechanism or mechanisms has prevented the development of effective targeted treatments to prevent or reverse DN. In this paper, we consider the oxidative stress theory of diabetes complications and available bioenergetics data obtained in the peripheral nervous system (PNS) in order to identify areas that may be fruitful for developing greater understanding of DN.
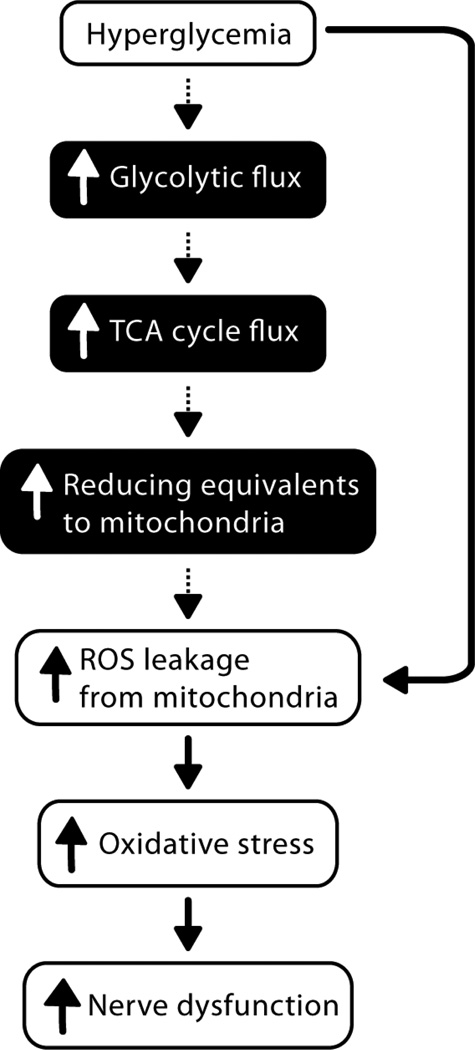
An association between hyperglycemia-derived production of reactive oxygen species (ROS) and the development of DN is well-accepted (Brownlee, 2001; Tomlinson and Gardiner, 2008), with a primary mechanism contributing to the onset and progression of DN suggested to be related to hyperglycemia-induced overproduction of superoxide (O2•−) by the mitochondrial electron transport chain (Brownlee, 2001; Nishikawa, et al., 2000) (Fig. 1). In systemic hyperglycemia, elevated glucose content in sciatic nerve (SCN) (Obrosova, et al., 2005) (Kishi, et al., 1999; Thurston, et al., 1995), and increased oxidative stress in plasma, SCN and dorsal root ganglia (DRG) (Vincent, et al., 2004b), as well as in vitro hyperglycemia-induced DRG neuron mitochondrial injury (Vincent, et al., 2005; Vincent, et al., 2004a) all support to the prevailing hypothesis that glycolytic flux is increased in the diabetic PNS (Brownlee, 2001; Tomlinson and Gardiner, 2008), contributing to mitochondrial ROS generation. Furthermore, enhanced fatty acid oxidation and lipotoxicity is implicated in the pathophysiology of type 2 diabetic cardiomyopathy (van de Weijer, et al., 2011) and nephropathy (Murea, et al., 2010). However, experimental evidence for increased metabolic flux through glycolysis or beta-oxidation in diabetic PNS is lacking. Brain glucose utilization is more widely studied. Data suggest that neurons continue to metabolize glucose during hyperglycemia via non-glycolytic mechanisms, and that there is a complex interplay between astroglia and neurons in order to utilize metabolic substrates that is not well understood (Izawa, et al., 2009). Further understanding of the utilization of energy substrates in specific nerve cell types is critical.
Figure 1.
Prevailing view that excessive glycolytic metabolism is responsible for the generation of harmful reactive oxygen species (ROS), mitochondrial injury, and diabetic neuropathy. White boxes, experimental evidence derived from neuronal tissue and cells; black boxes, associations based on hypotheses derived from multiple, non-neuronal tissue and cell types.
The uptake and utilization of glucose are not insulin dependent in peripheral neurons (Greene and Winegrad, 1979). Glucose transporters (GLUT) are required for facilitated glucose uptake, peripheral neurons are dependent upon GLUT3 (Simpson, et al., 2008), and Schwann cells utilize both GLUT3 and GLUT1 for facilitated glucose uptake (Magnani, et al., 1996). To our knowledge, the effects of diabetes on this GLUT1 and GLUT3- mediated glucose transport have not been investigated. Glucose accumulates in SCN in alloxan-induced diabetes in rats at 26 weeks (Thurston, et al., 1995), and in streptozotocin (STZ)-induced diabetic rats at 4 weeks (Kishi, et al., 1999) and 6 weeks (Obrosova, et al., 2005), suggesting no impairment of facilitated glucose transport into the nerve in experimental diabetes. However, Kishi et al (Kishi, et al., 1999) demonstrate that despite this elevated SCN glucose content in STZ-diabetic rats after one month of diabetes, both nerve and DRG glucose uptake are reduced compared with controls. Thus, changes in glucose transporter expression and activity warrant consideration and future investigation in the detailed evaluation of peripheral nerve bioenergetics in diabetes. In the face of poor glucose control, if bioenergetics proves to be a key to neuron injury, an ability to target specific glucose transporters may be one way to protect peripheral neurons against an energy substrate crisis.
In his unifying hypothesis, based primarily on research in endothelial cells, Brownlee (Brownlee, 2001) proposed that ROS inhibition of GAPDH activity (Du, et al., 2000) leads to accumulation of glyceraldehyde-3- phosphate and upstream glycolytic metabolites, which are then diverted into alternative pathways including the advanced glycation end products pathway, PKC pathway, hexosamine pathway, and polyol pathway (Brownlee, 2001). Specifically, hyperglycemia is associated with overproduction of tricarboxylic acid (TCA) cycle-derived electron donors, subsequent increased mitochondrial O2•− production (Du, et al., 2001) and O2•−-induced inhibition of GAPDH in cultured endothelial cells (Du, et al., 2000). Work by Thurston and colleagues partially supports this theory in peripheral nerves: glucose-6-phosphate and fructose-1,6-bisphosphate are elevated following 26 weeks of alloxan-induced diabetes (Thurston, et al., 1995). However, in the same study, elevated nerve lactate and ATP lead to the conclusion that there is increased glycolysis and decreased TCA cycle activity, although no TCA cycle intermediates were measured or quantified (Thurston, et al., 1995) to better support this conclusion.
Hexokinase directs glucose into the glycolytic pathway under normoglycemic conditions, however, there is a glucose concentration-dependent activation of aldose reductase in nerve (Greene and Lattimer, 1983; Greene, et al., 1987; Greene, et al., 1984). Thus, activation of the polyol pathway is a prominent metabolic feature of diabetic rat peripheral nerve (Obrosova, et al., 2005; Stevens, et al., 1994). Early in the course of diabetes, this polyol flux promotes NADPH depletion, decreased ATP production and neuron injury (Stevens, et al., 1994). Additionally, aldose reductase is localized to Schwann cells in the peripheral nerve (Kern and Engerman, 1982), and polyol pathway hyperactivity is associated with diminished energy flux in diabetic nerve (Greene and Lattimer, 1984; Greene and Lattimer, 1986). Hexokinase saturation and maximal glycolytic flux is one of the proposed mechanisms underlying the accumulation of nerve glucose and its direction into the polyol pathway in diabetes (Tomlinson and Gardiner, 2008); however, there appear to be no data in the literature on the degree of hexokinase activity in diabetic nerve. Gardiner and colleagues (Gardiner, et al., 2007) observed complex effects of STZ-induced diabetes on hexokinase I expression in rat DRG and suggested that metabolic flux through the glycolytic pathway is reduced in diabetes. Studies on excised rat retinas (Ola, et al., 2006) concluded that glucose metabolism downstream of hexokinase is not elevated by hyperglycemia or diabetes, but that intermediates of alternative glucose metabolism, such as those of the polyol pathway, are increased. In clinical trials, inhibitors of aldose reductase have generally failed to produce the desired results in decreasing the progression of neuropathy, although study data continue to suggest that improvements in the inhibitors and trial design may ultimately produce a therapeutic benefit (Tsai and Burnakis, 1993).
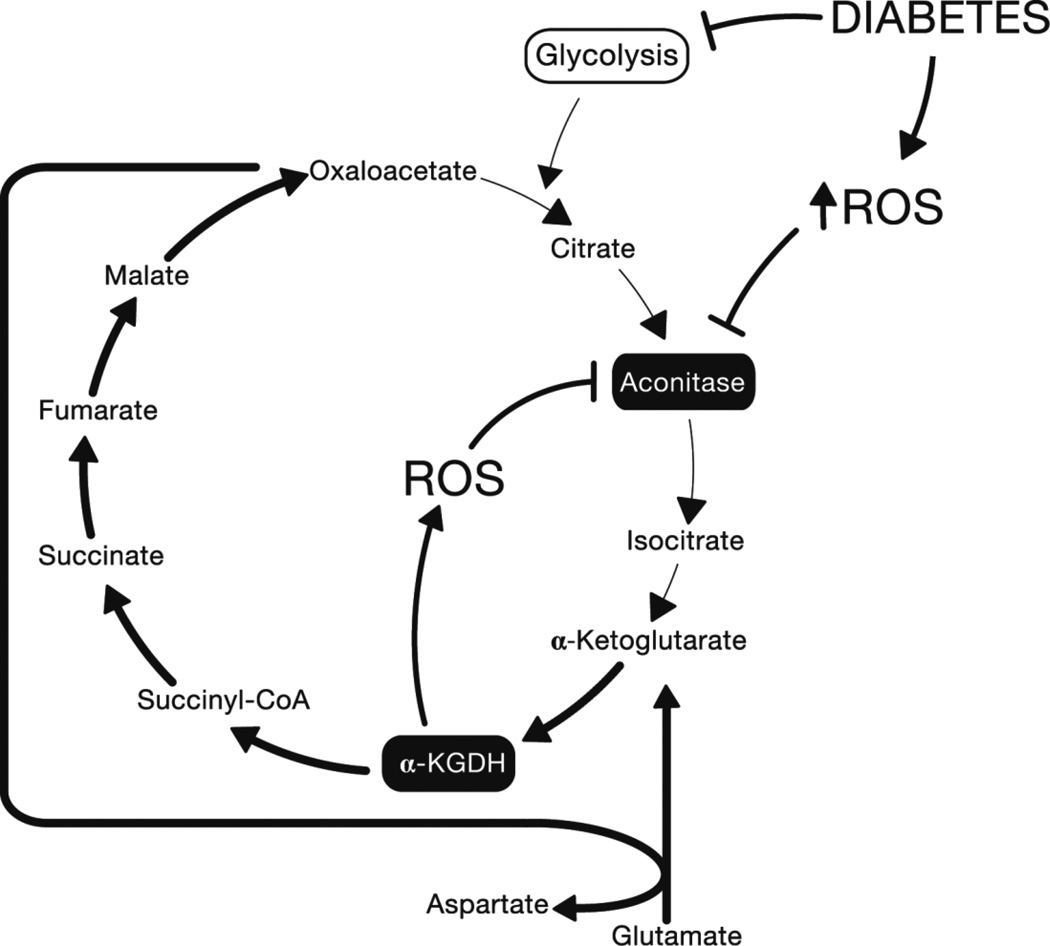
Work by Tretter and colleagues involving the relationship between key enzymes of the TCA cycle and oxidative stress suggests that in the absence of metabolite flux from glycolysis to the TCA cycle, ROS and subsequent further ROS generation by the TCA cycle itself (Tretter and Adam-Vizi, 2005) may contribute to mitochondrial dysfunction. Aconitase, catalyzing the citrate to isocitrate reaction in the TCA cycle, is inhibited by ROS, including O2•− (Gardner and Fridovich, 1992; Gardner, et al., 1995) and H2O2 (Tretter and Adam-Vizi, 2005). When aconitase is fully inhibited by H2O2 in nerve terminals, α-ketoglutarate dehydrogenase (α-KGDH; catalyzing the α-KG to succinyl-CoA reaction) remains functional and a segment of the TCA cycle (α-KG to oxaloacetate) is maintained by glutamate, which is converted to α-KG via transamination (see (Tretter and Adam-Vizi, 2005) for comprehensive explanation). ROS-mediated inhibition of aconitase may cause this truncated TCA cycle to come into effect in diabetic tissues (Fig. 2). This truncated segment of the TCA cycle has been suggested to function in the absence of glucose (Erecinska, et al., 1996; Yudkoff, et al., 1994), and, it could be inferred, when glucose is metabolized by non-glycolytic pathways. Additionally, α-KGDH is itself a source of ROS production, the level of which increases when α-KG is utilized as a fuel source over glucose in isolated brain synaptosomes (Tretter and Adam-Vizi, 2004). Thus, in a state of oxidative stress and/or decreased glycolysis, aconitase is completely inhibited, and α-KGDH remains sufficiently active to deliver electron donors to the electron transport chain, but further contributes to the production of ROS (Fig. 2). We have demonstrated loss of aconitase activity in response to hyperglycemia in DRG neurons in vitro (Vincent, et al., 2005) but the consequences in regards to ongoing energy metabolism in these cells remain to be determined.
Figure 2.
Proposed relationship between diabetes, ROS and the TCA cycle.
In a state of oxidative stress and/or decreased glycolysis, aconitase is inhibited. α-KGDH remains sufficiently active to permit truncated TCA cycling and delivery of electron donors to the electron transport chain, but further contributes to the production of ROS. α-KGDH, alpha-ketoglutarate dehydrogenase; ROS, reactive oxygen species.
Thus, the prevailing theory that excessive glycolytic metabolism is responsible for the generation of harmful reactive oxygen species (ROS), mitochondrial injury, and DN (Brownlee, 2001; Tomlinson and Gardiner, 2008) is based on studies that are disparate, incomplete, and performed across multiple tissue and cell types. In addition, to our knowledge, there are no studies that address the interplay between glucose, fatty acids and peripheral nerve energy metabolism in diabetes, nor the relationships between microvascular, Schwann cell, and neuronal metabolism. With the development of quantitative mass spectroscopy techniques, further metabolite studies demand attention. An understanding of diabetic metabolic abnormalities distinct to the PNS may be crucial to developing effective treatments for DN-related peripheral nerve disease. These data, then, can more fully support the multiple mechanisms that are proposed to be involved in cumulative neuron and nerve injury in diabetes that include oxidative stress, loss of neurotrophic support, insulin resistance, myelin dysfunction, and inflammation (Vincent, et al., 2011).
References
- Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Chisholm DJ. The Diabetes Control and Complications Trial (DCCT). A milestone in diabetes management. Medical Journal of Australia. 1993;159:721–723. [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest JID - 7802877. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemiainduced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Deas J, Silver IA. Limitation of glycolysis by hexokinase in rat brain synaptosomes during intense ion pumping. Brain Res. 1996;726:153–159. doi: 10.1016/0006-8993(96)00324-1. [DOI] [PubMed] [Google Scholar]
- Feldman EL. Diabetic neuropathy. Curr Drug Targets. 2008;9:1–2. doi: 10.2174/138945008783431709. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Russell JW, Greene DA. Somatosensory Neuropathy. In: Porte D Jr, Sherwin RS, Baron A, editors. Ellenberg and Rifkin's Diabetes Mellitus. McGraw Hill: 2003. [Google Scholar]
- Gardiner NJ, Wang Z, Luke C, Gott A, Price SA, Fernyhough P. Expression of hexokinase isoforms in the dorsal root ganglion of the adult rat and effect of experimental diabetes. Brain Res. 2007;1175:143–154. doi: 10.1016/j.brainres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- Greene DA, Lattimer SA. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983;72:1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DA, Lattimer SA. Impaired energy utilization and Na-K-ATPase in diabetic peripheral nerve. Am J Physiol. 1984;246:E311–E318. doi: 10.1152/ajpendo.1984.246.4.E311. [DOI] [PubMed] [Google Scholar]
- Greene DA, Lattimer SA. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986;35:242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316:599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Greene DA, Winegrad AI. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes. 1979;28:878–887. doi: 10.2337/diab.28.10.878. [DOI] [PubMed] [Google Scholar]
- Greene DA, Yagihashi S, Lattimer SA, Sima AA. Nerve Na+-K+-ATPase, conduction, and myo-inositol in the insulin-deficient BB rat. Am J Physiol. 1984;247:E534–E539. doi: 10.1152/ajpendo.1984.247.4.E534. [DOI] [PubMed] [Google Scholar]
- Izawa Y, Takahashi S, Suzuki N. Pioglitazone enhances pyruvate and lactate oxidation in cultured neurons but not in cultured astroglia. Brain Res. 2009;1305:64–73. doi: 10.1016/j.brainres.2009.09.098. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Annals of Neurology. 1986;19:450–457. doi: 10.1002/ana.410190505. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Immunohistochemical distribution of aldose reductase. Histochem J. 1982;14:507–515. doi: 10.1007/BF01011860. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Schmelzer JD, Yao JK, Zollman PJ, Nickander KK, Tritschler HJ, Low PA. Alpha-lipoic acid: effect on glucose uptake, sorbitol pathway, and energy metabolism in experimental diabetic neuropathy. Diabetes. 1999;48:2045–2051. doi: 10.2337/diabetes.48.10.2045. [DOI] [PubMed] [Google Scholar]
- Magnani P, Cherian PV, Gould GW, Greene DA, Sima AAF, Brosius FC. Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism: Clinical and Experimental. 1996;45:1466–1473. doi: 10.1016/s0026-0495(96)90174-2. [DOI] [PubMed] [Google Scholar]
- 25.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5:2373–2379. doi: 10.2215/CJN.08160910. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;54:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF. Analysis of glucose metabolism in diabetic rat retinas. Am J Physiol Endocrinol Metab. 2006;290:E1057–E1067. doi: 10.1152/ajpendo.00323.2005. [DOI] [PubMed] [Google Scholar]
- Said G. Diabetic neuropathy--a review. Nat Clin Pract Neurol. 2007;3:331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MJ, Dananberg J, Feldman EL, Lattimer SA, Kamijo M, Thomas TP, Shindo H, Sima AAF, Greene DA. The linked roles of nitric oxide, aldose reductase and (Na + ,K + )-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. Journal of Clinical Investigation. 1994;94:853–859. doi: 10.1172/JCI117406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston JH, McDougal DB, Jr, Hauhart RE, Schulz DW. Effects of acute, subacute, and chronic diabetes on carbohydrate and energy metabolism in rat sciatic nerve. Relation to mechanisms of peripheral neuropathy. Diabetes. 1995;44:190–195. doi: 10.2337/diab.44.2.190. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alphaketoglutarate dehydrogenase. J Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360:2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SC, Burnakis TG. Aldose reductase inhibitors: an update. Ann Pharmacother. 1993;27:751–754. doi: 10.1177/106002809302700616. [DOI] [PubMed] [Google Scholar]
- van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011 doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011 doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL. New insights into the mechanisms of diabetic neuropathy. Rev Endocr Metab Disord. 2004;5:227–236. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Olzmann JA, Brownlee M, Sivitz WI, Russell JW. Uncoupling proteins prevent glucoseinduced neuronal oxidative stress and programmed cell death. Diabetes. 2004a;53:726–734. doi: 10.2337/diabetes.53.3.726. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine Reviews. 2004b;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–154. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]