NOT ALL SYNCOPE IS VASOVAGAL SYNCOPE, EVEN WITH A NORMAL HEART
Cardiologists are trained to assess syncope patients for life-threatening causes. This usually involves a detailed evaluation to exclude valvular heart diseases, myocardial diseases, and cardiac arrhythmia. After these potentially lethal causes of syncope have been excluded, most cases are ascribed to vasovagal syncope (VVS). Cardiologists working in a syncope clinic or in a tilt table laboratory quickly realize that there are other causes for syncope and presyncope, including neurogenic orthostatic hypotension (nOH) and postural tachycardia syndrome. This article highlights some contrasting clinical characteristics between nOH and VVS (Table 1), and then reviews the clinical evaluation and management of nOH. The clinical characteristics of postural tachycardia syndrome are reviewed elsewhere in this issue.
Table 1.
Clinical comparison of vasovagal syncope and neurogenic orthostatic hypotension
|
Features |
Vasovagal Syncope |
Neurogenic Orthostatic Hypotension |
|---|---|---|
| Typical age | Any age; first episode usually in second or third decade |
>50 y |
| Gender (% female) | 60% | 40% |
| Symptoms with body position change |
After prolonged sitting or standing |
Immediately with sitting or standing |
| Syncope | +++ | ++ |
| Presyncope | + | +++ |
| Orthostatic Hypotension | +/− (usually only at time of faint) |
+++++ |
| Hemodynamic pattern with head-up tilt |
Sudden drop in BP and HR | Early and progressive decline in BP |
Abbreviations: BP, blood pressure; HR, heart rate.
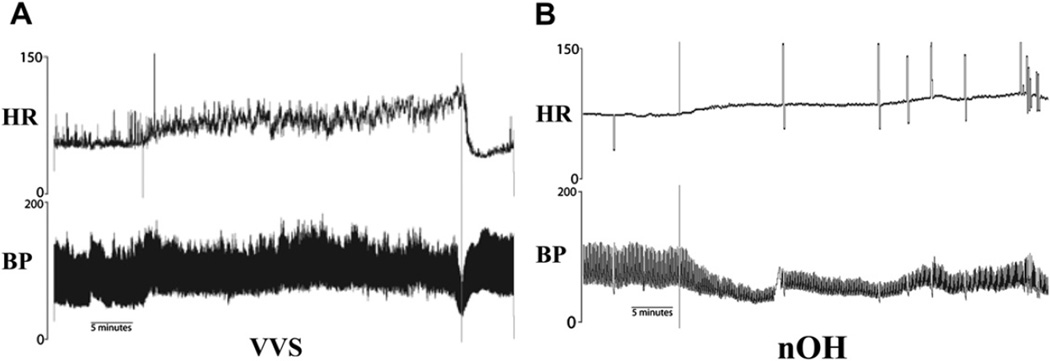
A striking difference between nOH and VVS is the different hemodynamic patterns during tilt table tests. These disorders each can have a distinct hemodynamic pattern during tilt table testing. During head-up tilt patients with VVS often hold a steady blood pressure (BP) for several minutes (often >10 minutes) after head-up tilt, before they develop symptoms and drop their BP rapidly (Fig. 1A). Patients with nOH are not able to maintain BP with head-up tilt. Their BP starts to fall immediately and orthostatic hypotension usually develops within 2 to 3 minutes of tilt (see Fig. 1B).
Fig. 1.
Head-up tilt test traces from a patient with VVS and nOH. (A) With VVS, the heart rate (HR) and BP increase a little bit at the onset of tilt, and they are maintained for more than 25 minutes before a sudden precipitous drop in BP before the table is returned to the supine position. (B) With nOH, the BP falls almost immediately when the table is tilted up with only minimal changes in HR. (From Raj SR, Sheldon RS. Head-up tilt-table test. In: Saksena S, Camm AJ, editors. Electrophysiological disorders of the heart. 2nd edition. New York: Saunders; 2012. p. 73–6–73–8; with permission.)
HEMODYNAMIC PHYSIOLOGY OF STANDING: HEALTHY AND ORTHOSTATIC HYPOTENSION
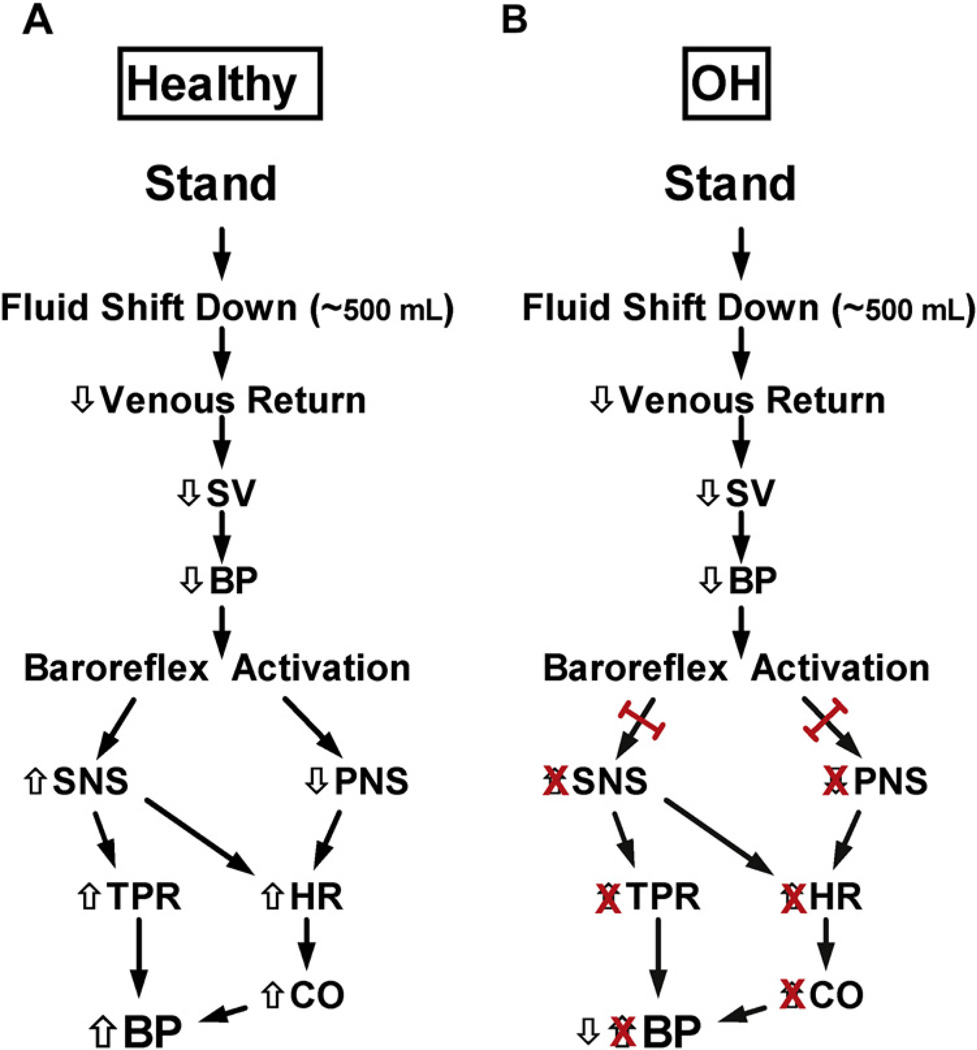
With the assumption of an upright posture, there is a downward shift of approximately 500 mL of blood to the dependent areas (mainly abdomen and legs). This gravitational shift in blood results in decreased venous return, decreased cardiac output, and eventually decreased BP (Fig. 2A).1 This “unloads” the baroreceptors, and triggers a reflex sympathetic activation with a resultant increase in heart rate (HR) and systemic vasoconstriction (countering the initial decline in BP). In a healthy individual, the net effect of assumption of upright posture is an increase in HR of 10 to 20 bpm, a minimal change in systolic BP, and an approximately 5 mm Hg increase in diastolic BP.
Fig. 2.
Physiology of standing: healthy and orthostatic hypotension. (A) With standing, there is a downward shift of approximately 500 mL of blood, which results in decreased venous return, decreased stroke volume (SV), and eventually decreased BP. This “unloads” the baroreceptors, and triggers reflex sympathetic nervous system (SNS) activation with an increase in HR and systemic vasoconstriction (countering the initial decline in BP). In a healthy individual, the net effect of assumption of upright posture is an increase in HR of 10 to 20 bpm, a minimal change in systolic BP, and an approximately 5 mm Hg increase in diastolic BP. (B) In patients with OH, the SNS and parasympathetic nervous system (PNS) cannot be adequately engaged, so the counterregulation is absent, and a drop in BP occurs. CO, cardiac output; TPR, total peripheral resistance.
In patients with nOH (see Fig. 2B), the efferent limb of the baroreflex cannot be adequately engaged. This can result in a lack of sympathetically mediated vasoconstriction (and increase in vascular resistance) and a fixed HR, instead of the expected increased HR. Together, this physiology results in a lack of counterregulation and a drop in BP. If the cerebral perfusion pressure falls below a critical threshold (as can happen with systemic hypotension), then adequate cerebral blood flow is not maintained (despite cerebral autoregulation) and syncope or presyncope result.
NEUROGENIC ORTHOSTATIC HYPOTENSION
nOH is defined as a sustained decrease in systolic BP greater than or equal to 20 mm Hg or diastolic BP greater than or equal to 10 mm Hg within 3 minutes of the assumption of an upright posture,2 although in almost all cases these diagnostic thresholds are met within 2 minutes.3,4 More recent guidelines for patients with severe supine hypertension raise the threshold to a reduction in systolic BP greater than or equal to 30 mm Hg.2 “Delayed orthostatic hypotension” has also been described with orthostatic symptoms occurring after 3 minutes, and a slow gradual drop in BP that crosses the threshold BP drop after 3 minutes of upright posture.2,5 Delayed nOH may represent a mild form of sympathetic nervous system failure.
The National Institutes of Health–funded longitudinal Framingham Heart study in the United States found that nOH accounted for 9.4% of all-cause syncope in their cohort.6 A European study reported that up to 24% of syncope-associated complaints were associated with nOH during emergency room visits.7
nOH is more prevalent with increasing age, with an estimated prevalence rate of 18% in patients greater than or equal to 65 years of age,8 and also represents a common cause of hospitalization among this age group.9 Recent studies have found that the presence of nOH is a strong predictor of future cardiovascular events.10
Clinical Features of nOH
Symptoms of nOH usually occur on assumption of upright posture (usually standing, occasionally even while seated) and most usually resolve (or improve greatly) very rapidly on resumption of a recumbent posture. Common upright symptoms include lightheadedness, blurred vision, weakness, fatigue, cognitive impairment, nausea, palpitations, tremulousness, headache, presyncope, and syncope.2 These symptoms are often incapacitating. In addition to the orthostatic symptoms, patients can often have other noncardiovascular autonomic symptoms, including gastroparesis, constipation, bladder dysfunction, and anhidrosis (although the loss of sweating can be patchy and may be reported as increased sweating over the face and chest). nOH and autonomic nervous system failure may also occur in movement disorders that present with extrapyramidal signs or cerebellar ataxia.
Classification of nOH
nOH can be classified into disorders with either primary or secondary causes of autonomic failure. Secondary causes include most diseases that cause a peripheral neuropathy that can affect the peripheral small fibers (including the autonomic nerves) (Box 1). Although this list is extensive, the most common cause is diabetes mellitus, although autonomic failure is uncommon with diabetes in the absence of a glove and stocking neuropathy. Another cause of a potentially reversible neuropathy is amyloid. Autonomic failure is also commonly associated with movement disorders, including Parkinson disease and dementia with Lewy bodies. A more recently described cause of autonomic failure is autoimmune autonomic ganglionopathy. In this disorder, an autoantibody targeting the autonomic ganglia causes loss of function and autonomic failure.11 In some cases, treatments targeted at reducing the antibody burden improve or resolve the nOH.12–14
Box 1 Causes of nOH.
Primary autonomic failure
Multiple system atrophy (Shy-Drager syndrome)
Pure autonomic failure (Bradbury-Eggleston syndrome)
Dementia with Lewy bodies
Secondary autonomic failure
Diabetes mellitus
Parkinson disease
Amyloid
Autoimmune autonomic ganglionopathy
Guillain-Barré syndrome
Familial dysautonomia (Riley-Day syndrome)
Hereditary sensory autonomic neuropathy
Vitamin deficiency (e.g. vitamin B12)
Toxic neuropathy
Drug-induced neuropathy
Infectious neuropathy
Multiple system atrophy (Shy-Drager syndrome)
Multiple system atrophy (MSA) is a progressive, neurodegenerative disease characterized by a combination of pyramidal, extrapyramidal, cerebellar, and autonomic failure. MSA is a sporadic disorder with a prevalence of 4 to 5 per 100,000.15 It presents at a mean age of 54 years, and affects men and women with a slightly higher prevalence among men.16 The cause of MSA is unknown. Most patients with MSA present with autonomic complaints (e.g., bladder dysfunction, erectile dysfunction, constipation, or nOH), but develop motor symptoms within 2 years.17 Most patients with MSA (approximately 80%) have Parkinson-like motor features, with a smaller number having predominantly cerebellar motor symptoms.18 Patients with MSA can often be differentiated from patients with Parkinson disease by their poor response to therapy with levodopa. MSA presents with a very poor prognosis with a median survival of only 7 to 9 years19 from the point of diagnosis, and a progressively debilitating course until death.20
MSA can be grouped into three major diagnostic groups21: (1) definite MSA, for patients with autopsy demonstration of typical histopathologic features; (2) probable MSA, for patients with multiple features of autonomic failure in addition to extrapyramidal or ataxic motor symptoms; and (3) possible MSA, for patients with less classical clinical findings. Many patients do not receive the correct diagnosis of MSA while alive because of the difficulty in differentiating MSA from other disorders (e.g., Parkinson disease, or other rare movement disorders). The defining neuropathologic findings of MSA consist of degeneration of striatonigral and olivopontocerebellar structures accompanied by profuse distinctive glial cytoplasmic inclusions formed by fibrillized α-synuclein proteins.21–23 Clinically, one can only make a diagnosis of possible MSA or probable MSA. Table 2 summarizes some clinical features of MSA (central autonomic failure).
Table 2.
Central versus peripheral autonomic failure
| Central Autonomic Failure | Peripheral Autonomic Failure | |
|---|---|---|
| Pathologic Lesions | ||
| Lesion | Preganglionic | Postganglionic |
| Clinical features | ||
| Orthostatic hypotension | +++++ | +++++ |
| Supine hypertension | ~50% | ~50% |
| Falls | +++ | ++ |
| Olfactory function | Normal | Decreased |
| Nocturia | +++ | +++ |
| Laboratory testing | ||
| Supine plasma NE | Normal | Low |
| Standing plasma NE | Low | Low |
| Orthostatic plasma NE | Minimal or no increase | Minimal or no increase |
| Cardiac SNS innervations (123I-MIBG scan) |
Normal | Decreased |
| Quantitative sudomotor axonal reflex testing |
Can be normal | Abnormal at multiple sites |
| Thermoregulatory sweat test | Abnormal | Abnormal |
Abbreviations: MIBG, m-iodobenzylguanidine; NE, norepinephrine; SNS, sympathetic nervous system.
Differentiating MSA from Parkinson disease
Most patients with MSA have parkinsonian features, which can clinically mimic Parkinson disease. These include a resting tremor (more often bilateral in MSA than Parkinson disease); bradykinesia; rigidity; and postural instability. Both groups of patients can develop autonomic failure and orthostatic hypotension, although this typically occurs earlier in the course of MSA and later in the course of Parkinson disease.
One important distinguishing clinical feature is that patients with Parkinson disease usually experience a dramatic reduction in muscle stiffness with l-dopa replacement therapy (e.g., Sinemet), whereas patients with MSA often have a poor clinical response. The diagnosis often becomes clearer over time, because patients with MSA experience a more rapid clinical deterioration than patients with Parkinson disease.
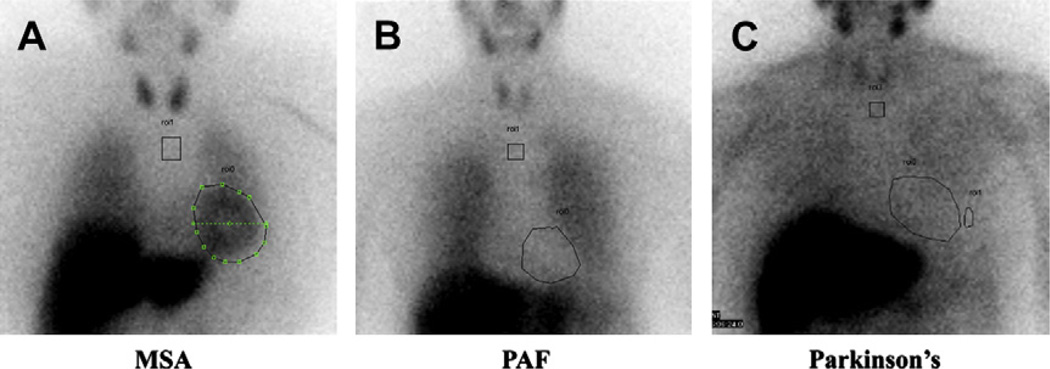
Although Parkinson disease and dementia with Lewy bodies have central nervous system features, the autonomic failure in Parkinson disease is caused by a peripheral autonomic neuropathy and behaves like pure autonomic failure (see Table 2).24 These disorders can manifest evidence of cardiac sympathetic nerve degeneration,25 similar to that shown in Fig. 3 using m-iodobenzylguanidine scans of the heart.
Fig. 3.
Cardiac sympathetic nerve integrity. Cardiac sympathetic nerve integrity can be assessed with m-iodobenzylguanidine (MIBG) scans with a chest view at 4 hours postinjection. MIBG is taken up by intact presynaptic norepinephrine transporters on postganglionic sympathetic neurons, and is not present in the setting of significant postganglionic sympathetic neuropathy. In MSA (A), the lesions are central and preganglionic. Because there is not postganglionic involvement, the heart takes up MIBG avidly. In contrast, patients with pure autonomic failure (PAF) (B) have a significant postganglionic sympathetic neuropathy, and the heart cannot be seen because it cannot take up MIBG. (C) Scan of a patient with Parkinson disease. Although the motor features of Parkinson disease are caused by central nervous system lesions, the autonomic failure is caused by a peripheral postganglionic sympathetic neuropathy, with poor cardiac uptake of MIBG.
Pure autonomic failure (idiopathic orthostatic hypotension, Bradbury-Eggleston syndrome)
Pure autonomic failure was first described by Bradbury and Eggleston in 1925.26 They described patients with orthostatic hypotension who had an unchanging HR, supine hypertension, reduced sweating, impotence, nocturia, constipation, and anemia. These patients had failure of the sympathetic and parasympathetic limbs of the autonomic nervous system, and “denervation hypersensitivity” in response to pharmacologic agonists. Compression garments helped these patients, presumably by preventing the pooling of blood in the lower body.
Pure autonomic failure is a disease process primarily involving the peripheral autonomic nerves. Autonomic neurons develop aggregates of a housekeeping protein called α-synuclein in the cytoplasm, known as Lewy bodies. These Lewy bodies damage the neurons. Lewy bodies are also seen in Parkinson disease and dementia with Lewy bodies. In contrast, patients with MSA have inclusions in glial cells and not neurons.
The clinical characteristics of pure autonomic failure (compared with MSA) are shown in Table 2. Patients with pure autonomic failure tend to develop their symptoms in their seventh decade or later, and have a fairly good prognosis with a slow progression. As a result of the peripheral autonomic nerve damage, patients with pure autonomic failure have a significantly lower supine level of plasma norepinephrine than do healthy age-matched control subjects.27
Evaluation of nOH
A good history and physical examination remains the single most valuable diagnostic resource. Non-neurologic causes of orthostatic hypotension, such as dehydration, acute blood loss, and deleterious medications, should be identified because these can be reversed. nOH can also be mimicked by disorders with decreased cardiac output, such as cardiomyopathy, constrictive pericarditis, and aortic stenosis.28 The critical element of making a diagnosis of nOH involves obtaining orthostatic vital signs (BP and HR) in response to upright posture for at least 3 minutes, and ideally for 5 minutes.3 A diagnosis of nOH cannot be made in the absence of orthostatic vital signs. Box 2 lists several orthostatic and nonorthostatic symptoms commonly seen in patients with nOH. Initial blood tests should commonly include a complete blood count (to exclude severe anemia); electrolytes; serum vitamin B12 levels; and serum and urine immunoelectrophoresis (to exclude monoclonal proteins that might cause amyloid neuropathy).28
Box 2 Common symptoms with orthostatic hypotension.
Orthostatic symptoms (worse with upright posture)
Lightheadedness
Presyncope and syncope
“Coat-hanger” neck and shoulder discomfort
Cognitive slowing when upright
Headache
Visual graying
Dyspnea
Angina
Tremulousness
Leg muscle weakness
Falls
General symptoms (in any posture)
Fatigue
Weakness
Nausea
Urinary frequency
Nocturia
Constipation (can be severe)
Loss of sweating (may be patchy)
Heat intolerance
Autonomic function testing in nOH
Formal autonomic function testing can be done in special centers and is integral to the diagnosis of autonomic failure. Testing assesses the parasympathetic nervous system and the sympathetic nervous system. Cardiovagal function can be assessed with a quantitative assessment of HR excursion with respiration (sinus arrhythmia), and the HR response to a Valsalva maneuver.29 The sympathetic cholinergic nervous system can be assessed with sweat tests (the quantitative sudomotor axonal reflex test or a thermoregulatory sweat test).30 Quantitative sudomotor axonal reflex test involves the iontophoresis of acetylcholine at different locations in the arm and leg to assess the ability of that region to sweat when provoked (intact reflex is present), whereas the thermoregulatory sweat test determines if and where a person sweats in response to environmental heating. The sympathetic noradrenergic nervous system is assessed with an orthostatic challenge (5–10 minute head-up tilt) and the continuous beat-to-beat BP response to a Valsalva maneuver, a cold pressor test (hand in ice water for 60 seconds), and an isometric hand grip test.28,29 Although beat-to-beat assessments of BP can be accomplished with an arterial line, it is more common to use a noninvasive continuous BP system in most autonomic laboratories.
Valsalva maneuver
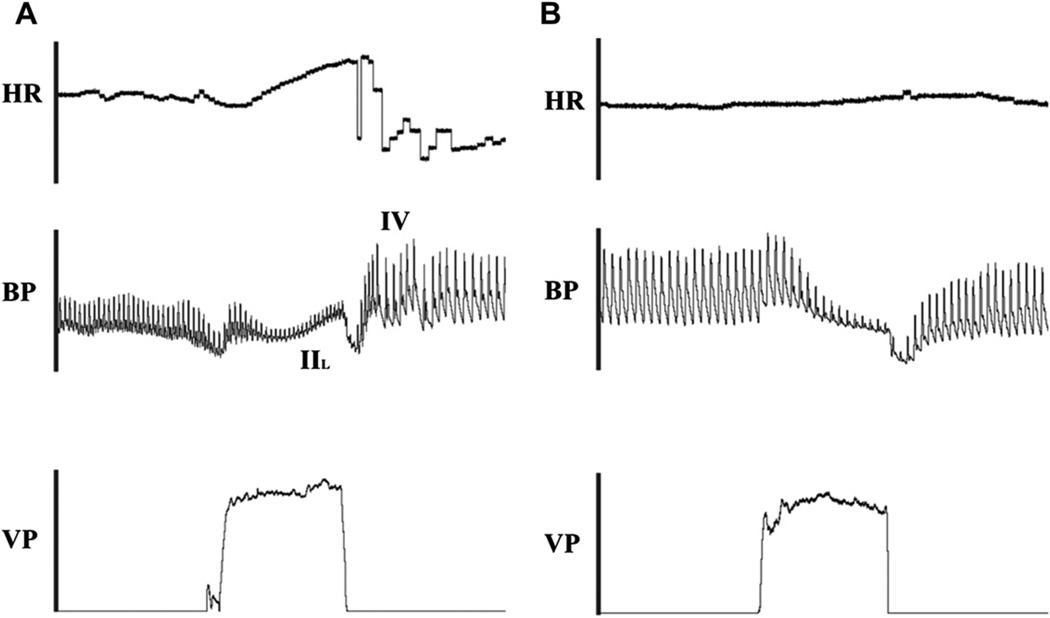
The Valsalva maneuver can be a very informative test in nOH. During continuous HR and BP monitoring, patients are asked to perform a forced expiratory effort at a standard expiratory pressure of 40 mm Hg for 15 seconds. The BP waveform consists of four well-defined phases. Phases I and II represent the strain phases, and phases III and IV are in recovery poststrain. Phases I and III deflections are caused by mechanical perturbations and represent changes in intrathoracic pressure at the beginning and the end of the Valsalva maneuver, whereas phases II and IV represent the clinically relevant stages.31 In healthy individuals, the BP and pulse pressure decrease initially during the strain phase of Valsalva because of diminished venous return (Fig. 4A). This hypotension is then sensed by intact baroreceptors, with resultant increased sympathetic tone. This leads to α-adrenergic receptor-mediated vasoconstriction and BP recovery in late phase II. With release of Valsalva, and normalization of venous return, there is an overshoot of BP in phase IV (combination of optimal venous return and vasoconstriction) before the BP returns to baseline.32
Fig. 4.
Valsalva maneuver. During the Valsalva maneuver, the patient is asked to blow and generate approximately 40 mm Hg of Valsalva pressure (VP) for 15 seconds while continuously monitoring HR and BP. In a healthy individual (A), the BP and pulse pressure initially decease because of the drop in venous return, but this hypotension triggers an increase in sympathoneurally mediated vasoconstriction. The BP starts to recover in late phase II (IIL). After release of VP (and with it increased venous return) the BP overshoots in phase IV (IV) before returning to baseline. A patient with nOH (B) might not be able to generate the appropriate sympathoneurally mediated vasoconstriction in response to the initial hypotension. Patients with nOH typically lack the late phase II BP recovery and the phase IV BP overshoot.
Patients with nOH are not able to generate an appropriate increase in sympathetically mediated vasoconstriction in response to the initial hypotension. They typically lack the late phase II BP recovery and the phase IV BP overshoot (see Fig. 4B). Rather, the BP slowly drifts back up to baseline after the Valsalva-induced hypotension.
Treatment of nOH
Treatment for nOH is largely empiric with only poor evidence from randomized, placebo-controlled drug trials.33 Patients who are asymptomatic may require no treatment, although they should be followed clinically for the development of symptoms. Symptomatic patients, however, can be treated with nonpharmacologic (Table 3) and pharmacologic (Table 4) measures.
Table 3.
Nonpharmacologic treatment of OH
| Intervention | Comments |
|---|---|
| Increase dietary salt intake | Recommend 10 g/day of sodium; goal is to promote fluid retention and blood volume expansion |
| Increase water and fluid intake | Target 2–3 L/day |
| Bolus water ingestion (osmopressor response) | Ingestion of 500 mL water within 2–3 min can acutely increase vascular resistance and BP in many patients; peak effect is between 20–40 min postingestion |
| Caffeinated beverages | |
| Physical maneuvers | |
| • Leg crossing • Squatting |
Increases standing time and may increase mean BP by 10–15 mm Hg May prevent loss of consciousness in emergencies |
| External pressure to lower body • Bandages firmly wrapped around the legs • Snugly fitted abdominal binders • Waist-high compression garments |
• Helps to decrease orthostatic venous pooling • Most venous pooling occurs in abdomen • Can be uncomfortable to wear (hot and itchy) and require help to put on in elderly patients • Start with 30–40 mm Hg pressure for compression garments • It can be difficult to adequately tighten abdominal binders |
Table 4.
Pharmacologic treatment for OH
| Drug | Dose | Mechanism of Action | Adverse Effect | Comments |
|---|---|---|---|---|
| Midodrine | 2.5–10 mg PO q4h × 3/d; can be taken on PRN basis |
α1-Adrenergic receptor agonist with vasoconstriction |
Supine hypertension, piloerection, urinary retention, scalp itch/ irritation |
FDA- approved agent for treatment of nOH Midodrine should not be taken within 4 h of lying down |
| Fludrocortisone | 0.05–0.2 mg PO daily | Fluorinated corticosteroid with affinity for the mineralocorticoid receptors; leads to increased renal Na+ retention, K+ excretion, and increased extracellular fluid retention |
Supine hypertension, headache, hypokalemia, cardiac edema |
Must follow serum potassium with chronic use, and may require potassium replacement Can worsen supine hypertension |
| Yohimbine | 5.4–10.8 mg PO TID | α2-Adrenergic receptor antagonist, with increased sympathoneural tone |
Irritability, hypertension, anxiety, scalp itch |
Although FDA approved, it is no longer manufactured Available through compounding pharmacies |
| Pyridostigmine | 30–60 mg PO TID | Acetylcholinesterase inhibitor that prolongs acetylcholine effect in autonomic ganglia to increase sympathetic tone |
Excessive salivation, increased peristalsis, nausea and vomiting |
Maximal effect seen when upright vs supine Can also help with chronic constipation |
| Caffeine/ergotamine combination |
100 mg/1 mg PO 1 tablet BID |
Antagonizes vasodilatory effects of endogenous adenosine |
Supine hypertension | Cannot use adenosine for SVT termination |
| Octreotide | 12.5–50 µg SQ BID | Somatostatin analogue that induces splanchnic vasoconstriction |
Severe seated hypertension | Peptide that requires refrigeration and multiple daily injections (like insulin) |
| Erythropoietin | 25–50 units/kg SQ 3/wk | Stimulates red blood cell production |
Hypertension, polycythemia | Can worsen supine hypertension Iron supplements required Must follow hematocrit carefully |
Abbreviations: BID, twice per day; FDA, US Food and Drug Administration; nOH, neurogenic orthostatic hypotension; PO, per os; PRN, as needed; SQ, subcutaneous administration; SVT, supraventricular tachycardia; TID, three times per day.
Nonpharmacologic Therapy for nOH
Medication withdrawal
In many cases, nOH can be made worse (or converted from asymptomatic to symptomatic) by prescribed medications (see Table 3). The BP of patients with nOH is very preload-dependent, so patients with nOH are exquisitely sensitive to diuretics and venodilators (e.g., nitrates). These medications should be withdrawn whenever possible. Direct vasodilators, including α-adrenergic antagonists (e.g., tamsulosin), are commonly used to treat benign prostatic hypertrophy. These agents worsen orthostatic hypotension, or even induce syncope in some individuals. Patients are routinely advised to hydrate aggressively. Because of the aforementioned preload dependence in patients with OH, these patients do not tolerate even mild volume depletion. We advise patients to drink water 2 to 3 L/day.
Postural maneuvers in nOH
The following postural maneuvers are useful in nOH:
Elevate head of bed: Renal perfusion pressure is often highest at night because of the higher supine BPs that these patients experience compared with seated or standing BPs during the day. This can lead to more nocturnal urine production and subsequently a nocturnal reduction in blood volume, resulting in worsened orthostatic hypotension on awaking in the morning. This problem can be blunted by elevating the head of the bed (effectively tilting the patient) at night, similar to the strategy used in patients with gastroesophageal reflux disease.
Physical counterpressure maneuvers: Patients should be encouraged to use physical countermeasures, such as standing up slowly and contracting the calf muscles while standing to encourage venous return from the legs. These easy changes can be effective maneuvers when orthostatic hypotension is not overly severe.
Waist-high compression stockings: In response to rising to upright posture, there is a significant downward shift of fluid from the thorax. Waist-high (panty hose style) compression garments are rated at different degrees of pressure. It is recommended to start with 30 to 40mmHg of compression to augment venous return.34 This can be too tight for some older patients to don these garments without assistance.
Abdominal binders: Most of the pooled blood with standing resides in the abdomen rather than the legs.35 Studies have shown a benefit to compression of the abdomen only with an abdominal binder.36 However, it can be difficult to tighten the abdominal binder enough to generate a significant amount of abdominal pressure.
The osmopressor response and nOH
In 1999, Jordan and colleagues37,38 first reported that rapid ingestion of approximately 500 mL of water by patients with nOH resulted in a subacute increase in systolic BP (>40 mm Hg on average). This pressor response peaks between 20 and 40 minutes postingestion. The pressor response relates to the oral ingestion, and not primarily blood volume expansion, because when 500 mL of D5W was given intravenously in the same patients, the BP increase was blunted.38 The pressor response was only half as strong when patients with nOH drank salt water compared with plain water, suggesting that the water’s hypoosmolality may be critical to this response.39 The BP increase is mediated by the sympathetic nervous system.38 The authors advise their patients to drink 500 mL of water rapidly (over 2 minutes) in the morning about 10 minutes before getting out of bed.
Pharmacologic Treatment of nOH
The pharmacologic therapy of nOH involves one of two main strategies: to expand the blood volume and to use short-acting pressor agents (see Table 4). The former raises the BP during day and night, whereas the latter raises the BP for a few hours during the period when the patient is upright (and needing BP support).
Volume expansion
Two methods of volume expansion are fludrocortisones and recombinant erythropoietin (EPO). Fludrocortisone promotes increased renal sodium reabsorption and ideally increases intravascular volume. Because fludrocortisone increases daytime and nighttime BP, it can exacerbate supine hypertension in susceptible individuals. Patients with severe autonomic failure may also have anemia. EPO could help with blood volume repletion and correction of symptomatic anemia. Biaggioni and colleagues40 showed that EPO use in patients with nOH improved standing BP and symptoms, but also noted that use was limited by exaggerated supine hypertension in some patients.
Short-acting pressor agents
Short-acting pressor agents include the following:
Midodrine: This is the only medication approved by the US Food and Drug Administration for the treatment of nOH. The metabolite is a vasoconstrictor and a venoconstrictor by α1-adrenoreceptor agonism. A pill starts working within 20 to 30 minutes and the effect lasts for about 4 hours. The patient should be instructed not to lie down within 4 hours of a dose. Midodrine should be started at 2.5 mg orally every 4 hours × 3/day, but can safely be increased to 10 mg/dose.
Yohimbine: This is an α2-adrenergic receptor antagonist. α2-Adrenergic receptors work to decrease sympathetic nervous outflow. By blocking the “brake” on sympathetic outflow, yohimbine facilitates increased sympathetic nervous system activity and unrestrained norepinephrine release from sympathetic neurons, inducing a pressor response to midodrine in patients with OH.41 Yohimbine (5.4–10.8 mg orally three times a day) is no longer manufactured, but is still available through compounding pharmacies.
Pyridostigmine: This is a peripheral acetylcholinesterase inhibitor that acts to increase acetylcholine concentrations in the autonomic ganglia. Pyridostigmine (60 mg orally three times a day) modestly reduces the fall in standing BP, but does not increase supine BP.42,43 Pyridostigmine also increases bowel motility in patients with autonomic failure who are often chronically constipated.
Norepinephrine reuptake transporter inhibitors: These can increase BP by increasing synaptic norepinephrine in sympathetic neurons. Atomoxetine can significantly increase BP in patients with MSA.27
Cafergot (caffeine, 100 mg, and ergotamine, 1 mg): This causes vasoconstriction through a nonsympathomimetic mechanism.
Octreotide (12.5–50 µg subcutaneously twice a day): This is an injectable peptide. It likely works through splanchnic vasoconstriction with resultant blood shunting into the central circulation.
Management of Supine Hypertension with nOH
Supine hypertension can often coexist with nOH, and is seen in approximately 60% of patients with nOH assessed at the Vanderbilt Autonomic Dysfunction Center.24 In patients with essential hypertension, sustained elevated BP is well-recognized to increase cardiovascular morbidity and mortality, but the consequences of chronic supine hypertension are not well documented,24 with the exception of accelerated renal dysfunction.44 In addition to long-term cardiovascular damage, patients with nOH with supine hypertension may have inappropriate nocturnal pressure natriuresis that can result in exaggerated volume depletion by early morning, and an exaggeration of morning symptoms.45 Treatment options for supine hypertension include sleeping with the head of the bed elevated 6 to 9 inches; a sweet desert at bedtime; nitroglycerine patch, 0.1 mg/h on at bedtime (off 10 minutes before getting up, can be titrated up as needed); and hydralazine, 25 to 50 mg orally at bedtime.
SUMMARY
Orthostatic hypotension is an important cause of syncope, especially in the older patients. Although there are multiple causes, the treatments are focused on improving symptoms. The mainstays of therapy are careful attention to hydration; bolus ingestion of water (osmopressor response); and the judicious use of short-acting pressor agents for the orthostatic hypotension. A significant proportion of patients can also have supine hypertension, which may necessitate the use of short-acting pressor agents overnight.
KEY POINTS.
Most patients who present with syncope have vasovagal (reflex) syncope, but neurogenic orthostatic hypotension can also cause syncope, especially in older patients.
Patients with neurogenic orthostatic hypotension have a fall in blood pressure greater than or equal to 20/10 mm Hg within 3 minutes of assumption of an upright posture.
Neurogenic orthostatic hypotension can often be differentiated from vasovagal syncope by its differing hemodynamic patterns during tilt table test and differing clinical characteristics.
Treatment of orthostatic hypotension focuses on improving symptoms through careful attention to hydration; bolus ingestion of water (osmopressor response); and the judicious use of short-acting pressor agents.
A significant proportion of patients with orthostatic hypotension can also have supine hypertension, which may necessitate the use of short-acting pressor agents overnight.
ACKNOWLEDGMENTS
The authors thank Dr David Robertson for his leadership in the field of autonomic disorders, and his training of numerous investigators in the field of autonomic physiology. They also thank Dr Emily M. Garland for her thoughtful review of this manuscript.
Research Funding: Supported in part by NIH grants R01 HL102387, P01 HL56693, and UL1 TR000445 (Clinical and Translational Science Award).
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Gehrking JA, Hines SM, Benrud-Larson LM, et al. What is the minimum duration of head-up tilt necessary to detect orthostatic hypotension? Clin Auton Res. 2005;15:71–75. doi: 10.1007/s10286-005-0246-y. [DOI] [PubMed] [Google Scholar]
- 4.Raj SR. What is the optimal orthostatic stress to diagnose orthostatic hypotension? Clin Auton Res. 2005;15:67–68. doi: 10.1007/s10286-005-0265-8. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67:28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. [DOI] [PubMed] [Google Scholar]
- 6.Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 7.Sarasin FP, Louis-Simonet M, Carballo D, et al. Prevalence of orthostatic hypotension among patients presenting with syncope in the ED. Am J Emerg Med. 2002;20:497–501. doi: 10.1053/ajem.2002.34964. [DOI] [PubMed] [Google Scholar]
- 8.Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 9.Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120:975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Fagard RH, De CP. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension. 2010;56:56–61. doi: 10.1161/HYPERTENSIONAHA.110.151654. [DOI] [PubMed] [Google Scholar]
- 11.Vernino S, Low PA, Fealey RD, et al. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 12.Hollenbeck R, Black BK, Peltier AC, et al. Long-term treatment with rituximab of autoimmune autonomic ganglionopathy in a patient with lymphoma. Arch Neurol. 2011;68:372–375. doi: 10.1001/archneurol.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder C, Vernino S, Birkenfeld AL, et al. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–1590. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons CH, Vernino SA, Freeman R. Combined immunomodulatory therapy in autoimmune autonomic ganglionopathy. Arch Neurol. 2008;65:213–217. doi: 10.1001/archneurol.2007.60. [DOI] [PubMed] [Google Scholar]
- 15.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 16.Vanacore N, Bonifati V, Fabbrini G, et al. Epidemiology of multiple system atrophy. ESGAP Consortium. European Study Group on Atypical Parkinsonisms. Neurol Sci. 2001;22:97–99. doi: 10.1007/s100720170064. [DOI] [PubMed] [Google Scholar]
- 17.Parikh SM, Diedrich A, Biaggioni I, et al. The nature of the autonomic dysfunction in multiple system atrophy. J Neurol Sci. 2002;200:1–10. doi: 10.1016/s0022-510x(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 18.Wenning GK, Geser F, Stampfer-Kountchev M, et al. Multiple system atrophy: an update. Mov Disord. 2003;18(Suppl 6):S34–S42. doi: 10.1002/mds.10561. [DOI] [PubMed] [Google Scholar]
- 19.Bower JH, Maraganore DM, McDonnell SK, et al. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–1288. doi: 10.1212/wnl.49.5.1284. [DOI] [PubMed] [Google Scholar]
- 20.Schrag A, Wenning GK, Quinn N, et al. Survival in multiple system atrophy. Mov Disord. 2008;23:294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 21.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa T, Healy DG, Abou-Sleiman PM, et al. The alpha-synuclein gene in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2006;77:464–467. doi: 10.1136/jnnp.2005.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 24.Shibao C, Gamboa A, Diedrich A, et al. Management of hypertension in the setting of autonomic failure: a pathophysiological approach. Hypertension. 2005;45:469–476. doi: 10.1161/01.HYP.0000158835.94916.0c. [DOI] [PubMed] [Google Scholar]
- 25.Tipre DN, Goldstein DS. Cardiac and extracardiac sympathetic denervation in Parkinson’s disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med. 2005;46:1775–1781. [PubMed] [Google Scholar]
- 26.Bradbury S, Eggleston C. Postural hypotension with syncope: a report of three cases. Am Heart J. 1925;1:75–86. [Google Scholar]
- 27.Shibao C, Raj SR, Gamboa A, et al. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. doi: 10.1161/HYPERTENSIONAHA.107.089961. [DOI] [PubMed] [Google Scholar]
- 28.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615–624. doi: 10.1056/NEJMcp074189. [DOI] [PubMed] [Google Scholar]
- 29.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the autonomic nervous system. Luxembourg: Harwood Academic Publishers GmbH; 1995. pp. 25–59. [Google Scholar]
- 30.Low VA, Sandroni P, Fealey RD, et al. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- 31.Raj SR, Robertson D, Biaggioni I, et al. Abnormal valsalva maneuver is not always a sign of congestive heart failure. Am J Med. 2007;120:e15–e16. doi: 10.1016/j.amjmed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Sandroni P. Clinical evaluation of autonomic disorders. In: Robertson D, Biaggioni I, Burnstock G, et al., editors. Primer on the autonomic nervous system. 3rd edition. San Diego: Academic press; 2012. pp. 377–382. [Google Scholar]
- 33.Logan IC, Witham MD. Efficacy of treatments for orthostatic hypotension: a systematic review. Age Ageing. 2012;41:587–594. doi: 10.1093/ageing/afs061. [DOI] [PubMed] [Google Scholar]
- 34.Podoleanu C, Maggi R, Brignole M, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol. 2006;48:1425–1432. doi: 10.1016/j.jacc.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res. 2004;14:146–147. doi: 10.1007/s10286-004-0188-9. [DOI] [PubMed] [Google Scholar]
- 36.Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res. 2004;14:167–175. doi: 10.1007/s10286-004-0187-x. [DOI] [PubMed] [Google Scholar]
- 37.Jordan J, Shannon JR, Grogan E, et al. A potent pressor response elicited by drinking water. Lancet. 1999;353:723. doi: 10.1016/S0140-6736(99)99015-3. [DOI] [PubMed] [Google Scholar]
- 38.Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation. 2000;101:504–509. doi: 10.1161/01.cir.101.5.504. [DOI] [PubMed] [Google Scholar]
- 39.Raj SR, Biaggioni I, Black BK, et al. Sodium paradoxically reduces the gastropressor response in patients with orthostatic hypotension. Hypertension. 2006;48:329–334. doi: 10.1161/01.HYP.0000229906.27330.4f. [DOI] [PubMed] [Google Scholar]
- 40.Biaggioni I, Robertson D, Krantz S, et al. The anemia of primary autonomic failure and its reversal with recombinant erythropoietin. Ann Intern Med. 1994;121:181–186. doi: 10.7326/0003-4819-121-3-199408010-00004. [DOI] [PubMed] [Google Scholar]
- 41.Jordan J, Shannon JR, Biaggioni I, et al. Contrasting actions of pressor agents in severe autonomic failure. Am J Med. 1998;105:116–124. doi: 10.1016/s0002-9343(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 42.Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–518. doi: 10.1001/archneur.63.4.noc50340. [DOI] [PubMed] [Google Scholar]
- 43.Shibao C, Okamoto LE, Gamboa A, et al. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. doi: 10.1161/HYPERTENSIONAHA.110.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garland EM, Gamboa A, Okamoto L, et al. Renal impairment of pure autonomic failure. Hypertension. 2009;54:1057–1061. doi: 10.1161/HYPERTENSIONAHA.109.136853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanker MH, Bernsen RM, Ruud Bosch JL, et al. Normal values and determinants of circadian urine production in older men: a population based study. J Urol. 2002;168:1453–1457. doi: 10.1016/S0022-5347(05)64472-2. [DOI] [PubMed] [Google Scholar]