Abstract
Serotonin1A receptor (5-HT1AR) deficiency has been associated with anxiety and depression and mice with genetic receptor inactivation exhibit heightened anxiety. We have reported that 5-HT1AR is not only a genetic but also a maternal “environmental” factor in the development of anxiety in Swiss-Webster mice. Here we tested if the emergence of maternal genotype dependent adult anxiety is preceded by early behavioral abnormalities or if it is manifested following a normal emotional development. Pups born to null or heterozygote mothers had significantly reduced ultrasonic vocalization between postnatal day (P) 4 and 12 indicating an influence of the maternal genotype. The offspring’s own genotype had an effect limited to P4. Furthermore, we observed reduced weight gain in the null offspring of null but not heterozygote mothers indicating that a complete maternal receptor deficiency compromises offspring physical development. Except a short perinatal deficit during the dark period, heterozygote females displayed normal maternal behavior which, with the early appearance of ultrasonic vocalization deficit, suggests a role for 5-HT1AR during pre/perinatal development. Consistent with this notion, adult anxiety in the offspring is determined during the pre/perinatal period. In contrast to heterozygote females, null mothers exhibited impaired pup retrieval and nest building that may explain the reduced weight gain of their offspring. Taken together, our data indicate an important role for the maternal 5-HT1AR in regulating offspring emotional and physical development. Since reduced receptor binding has been reported in depression, including postpartum depression, reduced 5-HT1AR function in mothers may influence the emotional development of their offspring.
Keywords: ultrasonic vocalization, anxiety, emotionality, maternal effect, programming, prenatal
Introduction
The serotonin (5-HT) system has long been associated with neuropsychiatric disorders, such as anxiety and depression [Lucki, 1998; Murphy, 1990] with a prominent role for the 5-HT1AR in these conditions. 5-HT1AR deficit was found in panic and post-traumatic stress disorder patients and in depression, including postpartum depression [Lesch et al., 1992b; Lopez et al., 1998; Mann, 1999; Moses-Kolko et al., 2008]. Consistent with previous imaging studies [Arango et al., 2001; Drevets et al., 1999; Lopez et al., 1998], a recent report showed that mean 5-HT1AR binding potential is reduced by 26% in the mesiotemporal cortex and by 43% in the raphe in recurrent depression [Drevets et al., 2007].
In agreement with the association of the 5-HT1AR with anxiety/depression, mice lacking the receptor were found to display anxiety-like behavior such as increased fear and avoidance of open spaces, decreased exploration, and increased escape directed behavior in highly stressful situations [Groenink et al., 2003; Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998]. By using littermate and non-littermate breeding and postnatal/embryonic crossfostering, we have recently showed that increased anxiety in the receptor deficient Swiss-Webster (SW) mouse strain is transmitted from one generation to the next by a non-genetic mechanism [Gleason et al.]. Specifically, certain life-long anxiety behaviors and maladaptive stress responses in the 5-HT1AR deficient mouse line, previously thought to be due solely to offspring receptor deficiency, are in fact caused by a maternal 5-HT1AR deficiency.
Gestational stress and early-life adversity have long been proposed to increase the risk for psychopathology [Bale, 2005; Bogoch et al., 2007; Caspi and Moffitt, 2006; Champagne and Meaney, 2006; Hougaard et al., 2005; Nemeroff, 2004; Sandman et al., 1997; Sarkar et al., 2008; Weinstock, 2001]. An important feature of the SW 5-HT1AR deficient mouse model of anxiety in comparison with the above mentioned “gestational stress” and “postnatal maternal care” models is that the origin and molecular basis of the “maternal environmental” effect on offspring anxiety behavior is known and can be regulated by the partial or complete deletion of the 5-HT1AR in heterozygote (H) or null (KO) mothers, respectively [Gleason et al.]. These characteristics of the SW 5-HT1AR deficient mouse model may help the identification some of the mechanisms underlying maternal programming of offspring emotionality.
In our previous report we determined the behavioral consequences of the 5- HT1AR deficient maternal environment in adult mice, long after weaning [Gleason et al.]. Here we asked if behavioral consequences of the maternal receptor deficit can be detected during the period of mother-offspring interaction, between birth and weaning. In addition, we also studied maternal behavior in an attempt to associate it with specific offspring behaviors. A previous study with 5-HT1AR deficient mice on a mixed genetic background suggested an effect of the maternal genotype on early offspring behavior [Weller et al., 2003]. However, this study assessed the combined effect of the maternal and offspring genotypes, rather than the maternal genotype alone. The maternal genotype influenced the H and KO offspring behavior in opposite directions indicating a complex interaction between the maternal and offspring genotypes. To unequivocally show a maternal genotype effect, in the absence of the modifying effect of the offspring’s own genotype, here we studied wild type (WT) offspring raised in normal and receptor deficient maternal environments.
Materials and Methods
Animals
5-HT1AR H mice were backcrossed more than 10 times to the SW genetic background as described previously [Parks et al., 1998]. Then, H mice were intercrossed to produce littermate 5-HT1AR KO and WT mice. Since our goal was to test offspring with different parental 5-HT1AR genotypes, WT mice were derived not only from H parents (as above) but also from WT parents. To achieve this, a separate WT line was established from WT mice born to H parents. Since maternal effects can be trans-generational that usually disappear after 3 generations [Whitelaw and Whitelaw, 2006], the 4th generation of WT mice was used. Similarly, KO animals were derived both from H and KO parents. To reduce the possibility of a genetic drift between the separate lines, these WT and KO mice were used to generate H parents that served as parents for littermate WT and KO mice (Fig. 1A). Previous experiments showed that this breeding strategy is suitable to separate offspring and maternal 5-HT1AR genotype effects [Gleason et al.].
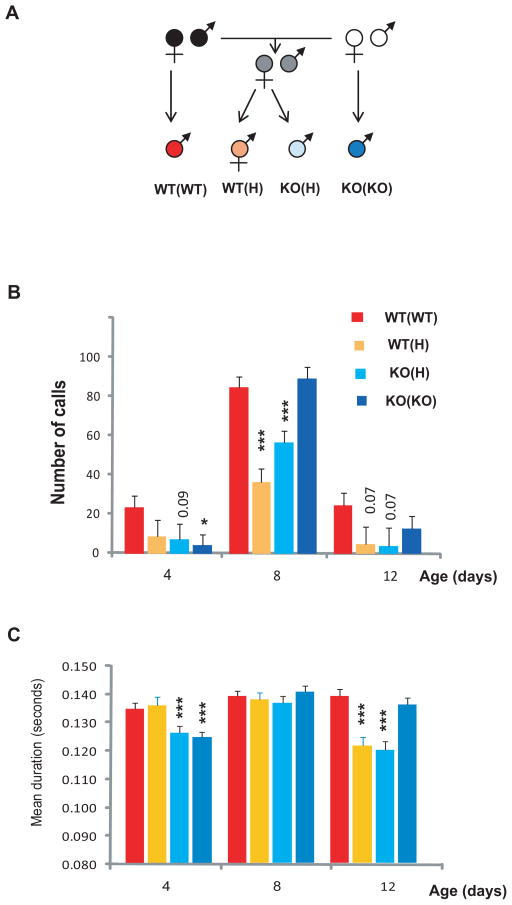
Figure 1.
Ultrasonic vocalization of WT(WT), WT(H), KO(H) and KO(KO) pups. A. Breeding strategy. B. Number of calls in a 5 minutes period analyzed by two way ANOVA followed by LSD post hoc test (statistically significant differences from the WT(WT) values within the same age groups are labeled as * p<0.05, *** p<0.001). Numbers indicate a trend. C. Mean duration of vocalization analyzed by two-way ANOVA and LSD posthoc test. Color coding of the columns is the same as in panel B.
Animals were maintained in standard cages (22 × 16 × 14 cm) under 12 hour light/dark cycle conditions (lights on from 06.00 to 18.00 hours) with standard rodent food pellets and water freely available. Each cage contained one male and one female of the same genotype and one litter. Offspring (1–20 days old) was used to study pup behavior and development. Experiments were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Genotyping
After the last test (postnatal day 20) genotype of offspring of 5-HT1AR H parents was determined, as previously described [Parks et al., 1998]. Briefly, DNA samples were obtained from a small section of tail (approximately 0.5 cm) by using DirectPCR Lysis Reagent (Viagen Biotech Inc., LA). PCR was performed in 20-ul reactions with 30 cycles of 94°C/15 sec., 60°/30 sec. and 72°C/30 sec. using WT forward (5′-AGT GCA GGC AGG CAT GGA TAT GTT-3′), WT reverse (5′-CCG ATG AGA TAG TTG GCA ACA TTC TGA-3′), KO forward (5′-CTT TAC GGT ATC GCC GCT CCC GAT TC-3′) and KO reverse (5′-TGC AGG ATG GAC GAA GTG GAA GTG CAG CAC A-3′) primers. Amplified bands were 238 and 400 bp long for the WT and null allele, respectively.
Behavioral tests
All testing took place during the first three postnatal weeks (until weaning) as outlined in supplementary Table 1. At the end of each test day pups were weighed and were numbered with a black marker. At P5-6 all pups were marked permanently by tattooing of their paws. All testing occurred within the testing room and temperature during experiments was held constant at 22 (±0.3) degrees Celsius. The investigator (AV) was blinded to the maternal and offspring genotype.
To include animals into the behavioral tests, the following criteria were applied. First, the litter size was set between 6 and 9 pups; bigger litter size was reduced by removing both males and females to maintain an approximately 50-50% gender ratio and litters with less then 6 pups were excluded (litter size was not statistically different between the different groups at birth; also, neonatal or early postnatal death was minimal and was not different between the groups). Second, pups from HxH crosses were included only if both WT and KO males were represented in the litter. Third, only litters of multipara mothers were included. Fourth, in each test, animals (in each group) were derived from at least 5 different mothers but overall 18 WT, 13 KO and 25 H parental litters were studied.
Pup ultrasonic vocalization (USV) test
USV tests were performed at P4, 8 and 12 (supplementary Table 1), between 11.00 and 15.00 hours. Cages were transferred to the testing room, where the mice (pups together with their mothers) were habituated for at least 60 minutes before testing. Pups were tested in random order. Each pup was separated from its littermates and parents, one at a time, and gently placed in the test chamber. Habituation time in the test chamber was 30 sec. before recording started. After the test pups were placed into a separate cage with a heating pad (37 °C) covered by several layers of paper tissue until all pups of the same litter were tested, after which they were placed back into their home cage. The bottom of the test chamber was cleaned with 30% ethanol between measurements.
The test chamber was made of a transparent plastic container with a Plexiglas bottom (diameter 23.5 cm) and with a built in bat detector (distance from bottom; 15 cm). Pups were tested for a 5 minutes period in which the number and duration of USVs were recorded. Calls within the frequency range of 80±4 kHz and longer than 100 ms in length, with an inter call interval of at least 10 ms, were detected by a Mini-3 bat detector and analyzed by Ultravox 2.0 (Noldus Information Technology, Wageningen, Netherlands). Before and after every test series a zero measure was performed to exclude background noise. Room temperature was measured at the beginning and end of each test day.
Nest quality
A 5-point nest-rating scale [Deacon, 2006] (supplementary Table 2) was used to score quality of nest building on days P1-10, 12, 13, 16 and 20 (supplementary Table 1). Cages were changed once per week and provided with fresh nesting material, consisting of 6 Nestlets (5 cm pressed cotton squares). Percentages of shredded/torn Nestlets (supplementary Table 2) was estimated and reflected in a score between 0 and 5.
Pup retrieval test
Retrieval tests were performed at P2, 6 and 10 (supplementary Table 1) between 10.30 and 12.30 hours. All tests were videotaped for later analysis. The female and male were temporarily removed from their home cage and kept in clean holder cages with water and food. Pups were carefully removed from the nest and scattered around the opposite end of the cage, as far away from the nest as possible. After 60 sec. of isolation, the female was reintroduced into the home cage. The latency to the retrieval of the first pup (in sec.) was recorded. The mean duration of retrieval per pup for all pups was also calculated. If a female had not completed the first retrieval within 5 minutes the test was terminated, resulting in a maximum score of 300 seconds. This time limitation was chosen because pilot studies indicated that retrieval of nine pups by a WT female is usually completed in less than a minute. The male was placed back in the home cage following testing.
Maternal observation
The procedure for assessing specific maternal behavior was partly adapted from previous work [Champagne et al., 2003; Gammie et al., 2007; Lerch-Haner et al., 2008; Myers et al., 1989]. Maternal observation was performed at P1, 3, 7, 13 and 20 (supplementary Table 1) and focused on arched-back nursing (ABN) and licking and grooming the pups (LG). Behavior was scored during two 15 min. observation periods, in light between 10.00 and 12.00 hours and in the dark (performed under infrared light) between 18.00 and 19.00 hours. Within each observation period ABN and LG were scored every minute, leading to 30 observations per female per test day. Proportion of time engaged in ABN and LG behavior, respectively, was determined for each day and each phase (light or dark).
Data analysis
One-way and two-way ANOVA analyses were used in various experiments as described in the text. This analysis was followed by LSD post hoc test in cases of significance (p<0.05).
Results
WT pups of 5-HT1AR deficient females have impaired ultrasonic vocalization
We used littermate and non-littermate animals in parallel that allows the detection of maternal and offspring genotype effects. Specifically, we compared the behavior of wild- type (WT) pups born to WT mothers (WToffspring(WTmother)), that have neither maternal nor offspring genotype effects with WT pups born to H mothers (WT(H)), which have only maternal genotype effects and with KO pups born to H or KO mothers (KO(H) and KO(KO)), which have both maternal and offspring genotype effects (Fig. 1A). We have previously shown that WT(H) mice express WT receptor mRNA levels in the hippocampus and cortex and embryonic and postnatal cross-fostering experiments excluded the possibility that the maternal effect on adult anxiety-like behavior was the result of a genetic effect. By using littermate WT(H) and KO(H) and non-littermate WT(WT) and KO(KO) mice, we could determine if specific pup behaviors and development in general are altered by the offspring or the maternal genotype.
We measured ultrasonic vocalization (USV) in pups to determine if the emergence of adult anxiety is preceded by early behavioral abnormalities or if it is manifested following a normal emotional development. USV is a well established measure to assess pup-mother communication in rodents [Hahn and Lavooy, 2005]. When the behavior of the four littermate and non-littermate groups were analyzed by two way ANOVA, the number of calls in a 5 min. period showed a group effect (F3,441=9.56, p=0.000004), an age effect (F2,441=92.12, p=0.000001) as well as a group × age interaction (F6,441=3.48, p=0.0022, N=20–58 per group) (Fig. 1B). WT(H) pups exhibited significantly reduced number of ultrasonic calls at P8 as compared to WT(WT) pups. Since WT(H) pups are genetically wild-type but are exposed to the heterozygote maternal environment, their altered behavior is related to the H maternal genotype rather than the offspring’s own genotype. KO(H) pups, exposed to both maternal and offspring genotype effects also showed reduced number of calls at P8 (Fig. 1B). KO(KO) pups exhibited a USV deficit already at P4 but not later suggesting that the complete absence of the receptor in KO mothers as compared to the partial deficit in H mothers may modify the USV behavior by shortening its length and by shifting its onset to an earlier time point. No significant difference was found between WT(H) and KO(H) pups indicating that the offspring genotype has no major influence on the number of USV calls.
Mean duration of calls analyzed by two-way ANOVA also showed a group effect (F3,440=9.11, p=0.000001), an age effect (F2,440=18.99, p=0.000001) as well as a group × age interaction (F6,440=6.58, p=0.0022, N=21–57 per group) (Fig. 1C). Similar to the measure of the number of calls, both WT(H) and KO(H) but not KO(KO) pups had a deficit (i.e. shorter duration) in USV at P12 indicating a H maternal genotype effect (see similar patterns at P12 in Fig. 1C and at P8 and P12 in Fig. 1B). However, an offspring genotype effect was also present in this measure. Specifically, at P4, the two groups of KO pups (KO(H) and KO(KO)) had reduced USV duration as compared to the two groups of WT pups (WT(WT) and WT(H)). However, this offspring genotype dependent pattern disappeared by P8 and was replaced by a H maternal genotype dependent pattern at P12 as described above. Taken together, these data demonstrate that, depending on the measure and age of the pups, reduced ultrasonic vocalization is either H maternal or offspring genotype dependent.
Pups of KO females show reduced weight gain during postnatal development
Physical development of the offspring was determined by assessing pup weight during the first two postnatal weeks. We noticed that both male and female KO(KO) pups gained weight less rapidly than WT(WT) mice (Fig. 2). Weight gain showed a group effect (F3,1299=132.46, p=0.000001), an age effect (F9,1299=1277.65, p=0.000001) and a group × age interaction (F27,1299=6.38, p=0.000001, N=15–50 per group) by two-way ANOVA. Although KO(KO) pups were born with normal weight, their reduced weight was apparent from P3 (males) and P6 (females) (LSD post hoc analysis). The weight difference between KO(KO) and WT(WT) mice however disappeared by adulthood (not shown). KO offspring born to H females (KO(H)) as well as WT(H) offspring showed nearly normal weight gain, exhibiting only a slight decrease in weight gain at the end of the second postnatal week. The weight of KO(KO) offspring was also reduced from P4 and P6 in males and females, respectively when compared to WT(H) and KO(H) animals. Taken together, the reduced weight gain in KO(KO) pups as compared to WT(WT) but also to KO(H) mice indicate that this deficit is related to the KO maternal genotype.
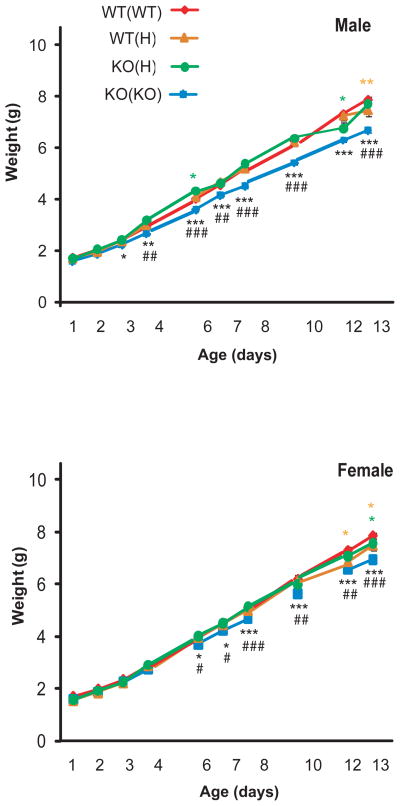
Figure 2.
Weight gain in KO(KO) is reduced between P3 and P13 in males (A) and between P6 and P13 in females (B) as compared to WT(WT) pups (black stars). The weight of WT(H) and KO(H) pups differ from that of WT(WT) animals at some postnatal days but not through the postnatal period (green and brown stars). Result of LSD post hoc analysis is indicated as: * p<0.05, ** p<0.01, *** p<0.001. Weight gain in the KO(KO) group is also reduced when compared to that of the KO(H) group (highlighted by #) indicating that only a complete receptor deficiency in the mother leads consistently to reduced weight.
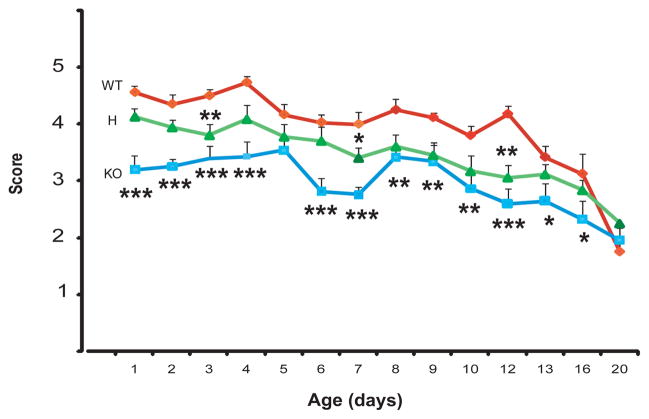
Nest quality is reduced in the KO but appears to be largely normal in the H female group
Certain well-coordinated maternal behaviors are involved in nurturing and rearing rodent pups, including nest building and pup retrieval [Gammie et al., 2007; Lerch-Haner et al., 2008]. Nest quality showed a genotype effect (F2,585=79.88, p=0.000001) and time effect (F13,585=24.61, p=0.000001, N=11–25 per group) by two-way ANOVA (Fig. 3). Nest quality of H females was slightly reduced as compared to WT mice (reduced in 3 time points out of the 14 measured; LSD post hoc analysis). However, nest quality of KO females was reduced throughout the entire postnatal period (Fig. 3) which may have contributed to the reduced weight gain of their KO(KO) pups (Fig. 2).
Figure 3.
Maternal nest building. Nest quality score ranged from 1 (low) to 5 (high) (see supplementary Table 2 for rating scale for nest quality) and analyzed by two way ANOVA. Differences from the WT values are labeled as * p<0.05, ** p<0.01, *** p<0.001 (LSD post hoc test).
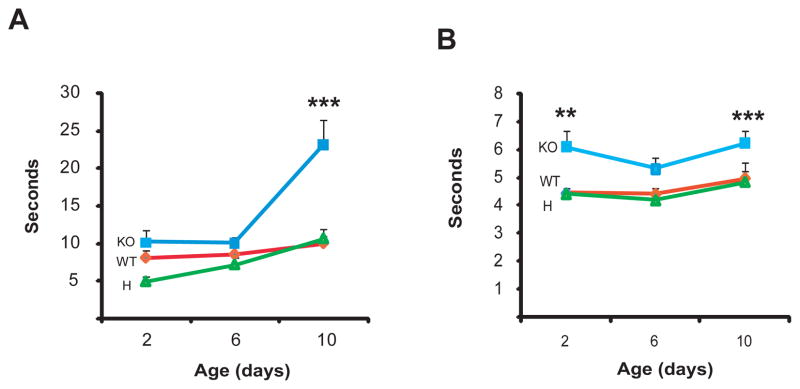
Pup retrieval behavior is altered in KO but not in H females
Pup retrieval is another measure of maternal behavior and two way ANOVA showed a genotype effect (F2,79=9.09, p=0.0003), a time effect (F2,79=9.16, p=0.0003) and a genotype × time interaction (F4,79=92.12, p=0.031, N=7–12 per group) in latency to retrieval (Fig. 4A). Mean duration of pup retrieval also showed a genotype effect (F2,78=16.41, p=0.000001, N=7–12 per group) by two-way ANOVA (Fig. 4B). Latency to retrieval and mean retrieval time of pups were not affected in H females while these measures were significantly increased in KO females. Therefore, retrieval, similar to nest building, is affected in the complete absence of the maternal receptor possibly contributing to the reduced weight gain of KO(KO) pups.
Figure 4.
Maternal performance in pup retrieval. A. Latency of retrieval (sec) analyzed by two way ANOVA. Differences from the WT values are labeled as ** p<0.01, *** p<0.001 (LSD post hoc test). B. Duration of retrieval. Significant differences are labeled as in “A”.
Maternal behavior of 5-HT1AR deficient females is not impaired except a short perinatal deficit during the dark period
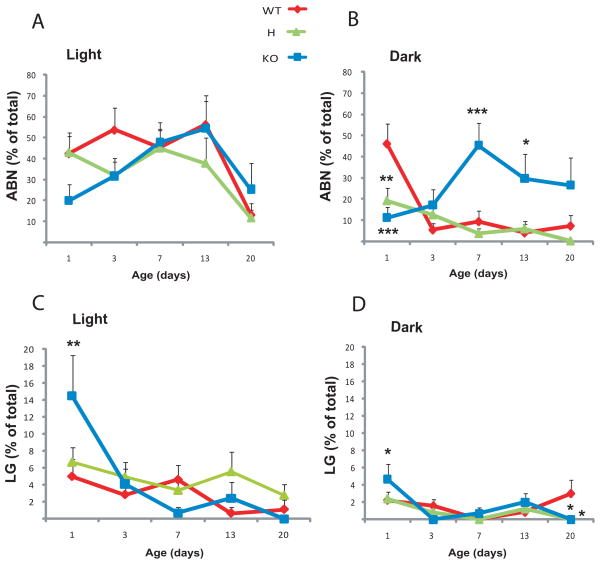
Arched-back nursing (ABN) and licking and grooming (LG) are well characterized maternal behaviors [Gammie et al., 2007; Lerch-Haner et al., 2008]. Extensive research implicated that maternal ABN and LG behaviors during the first postnatal week are critical for the appropriate neural development of the offspring [Bredy et al., 2003; Champagne et al., 2003; Francis et al., 1999; Liu et al., 1997] and reductions in maternal ABN and LG are related to increased adult anxiety-like behaviors in the offspring [Anisman et al., 1998; Calatayud et al., 2004; Caldji et al., 2000; Carola et al., 2006; Carola et al., 2008]. To test whether variations in ABN and LG contribute to the observed maternal genotype effects in the offspring of 5-HT1AR deficient mothers, ABN and LG were scored every minute during two 15 minutes observation periods, in the morning (light period) and evening (dark period). During the light period, WT females showed a fairly constant ABN behavior over time except a reduction toward P20 indicating that nursing of WT mothers was high initially but declined by the third week of postnatal life (Fig. 5A). A similar pattern of ABN was shown by the other groups indicating no genotype effect. In the dark, WT females initially (P1) had high ABN that was comparable to their behavior in light (Fig. 5B). However, ABN behavior decreased dramatically by P3 indicating that mothers during their active period spend relatively little time nursing their pups. Two way ANOVA of ABN in dark showed a genotype effect (F2,162=9.16, p=0.0002), time effect (F4,162=2.59, p=0.039) and genotype × time interaction (F8,162=4.74, p=0.00003, N=8–18 per group). Both H and KO females exhibited reduced ABN behavior at P1 that remained low throughout the postnatal period. However, because the sharp reduction of WT ABN behavior from P3, the low ABN of mutant mothers was no longer different from the control. Taken together, ABN behavior of H and KO mothers was deficient only perinatally and only during the dark period. Interestingly, KO females displayed significantly higher levels of ABN at P7 and P13. Also, KO females represented the only group that showed a difference, an increase, in LG behavior at P1 both in light and dark (Light: genotype × time interaction F8,184=2.14, p=0.03, N=9–19 per group; Dark: F8,159=1.76, p=0.087, N=8–17; Fig. 5C–D).
Figure 5.
Maternal ABN (A and B) and LG behavior (C and D) during the light (A and C) and dark periods (B and D). Analysis by two way ANOVA with posthoc LSD test. Differences from the WT values are labeled as * p<0.05, ** p<0.01, *** p<0.001.
Discussion
We have recently demonstrated that maternal 5-HT1AR deficiency results in increased adult anxiety and maladaptive stress response in outbred SW mice. As compared to inbred mice, outbred mice with genetic variability may better reproduce the complex interaction between genetic and environmental factors found in human populations vulnerable to anxiety disorders. Indeed, it has been reported that outbred strains tend to respond to adverse or stimulating environments while inbred lines, in particular the C57BL6 (B6), are resilient to these effects [Anisman et al., 1998; Millstein and Holmes, 2007]. Here we extended our previous study and demonstrate that the consequences of the matenal effect is already present during early postnatal development. We found two abnormalities in the offspring of 5-HT1AR deficient mothers: a deficit in USV behavior in the offspring of both H and KO mothers and delayed weight gain in the offspring of KO mothers. Although we do not know if the USV deficit in infant mice precedes the later developing anxiety-like behavior or if the adult behavioral deficit develops independently, our data show that a wide variety of behavioral abnormalities can be transmitted non-genetically from 5-HT1AR deficient mothers to genetically normal offspring.
The USV deficit, similarly to the adult anxiety-like behavior, may be linked to maternal 5-HT1AR deficit during the pre/perinatal period
Adult manifestations of the maternal genotype effect (anxiety and maldadaptive stress responses) were related to the pre/perinatal period [Gleason et al.]. The current study shows that the H and KO maternal genotype effects are apparent at P8 and as early as P4, respectively. This early onset suggests that the reduced USV behavior may also be related to the maternal receptor deficit during pre/perinatal development. Consistent with this notion, ABN and LG behavior of H mothers were normal through the postnatal period except a P1 ABN deficit during the dark period that, if significant enough to have an influence, also represents a perinatal effect. Nevertheless, we cannot exclude the possibility that the USV deficit is determined during the postnatal period and that it is an adaptation to impaired maternal communication, at least partly. Data indicate that the number of calls could reflect maternal responsiveness/unresponsiveness to react to pups’ needs [D’Amato et al., 2005]. A study conducted on genetically deaf mice demonstrated that cross-fostering of normal hearing pups to deaf dams induced a decrease in frequency of calling emitted by these pups [D’Amato and Populin, 1987]. Alternatively, the maternal milk may contain substance(s) such as cytokines that are under 5-HT1AR regulation and which have an effect on pup behavior. Maternal milk is rich in not only nutrients but also in various biologically active substances. As the production of gastric acid and pancreatic proteases in neonates is delayed [Blais et al., 2006; Koldovsky, 1985], milk-derived bioactive factors survive and retain biologic activity as they pass through the gastrointestinal tract. These factors are further protected from digestion by the presence of anti-proteases such as α1-antichymotrypsin and α1–antitrypsin in milk [Lindberg et al., 1982].
Contrary to our initial expectation, the maternal 5-HT1AR deficit caused a reduction rather than an increase in USV calls. Isolation-induced distress calls have been widely accepted as an indication of anxiety and emotionality [Brunelli, 2005; Dichter et al., 1996; Winslow et al., 2000]. This notion is mainly based on pharmacological studies showing that anxiolytic drugs, such as opioids, benzodiazepines, 5-HT1AR agonists and serotonin reuptake inhibitors, reduce the number and duration of USV calls, whereas anxiogenic drugs, for example 5-HT1AR antagonists, produce increases in USV rates [Hard and Engel, 1988; Hodgson et al., 2008; Olivier et al., 1998; Winslow and Insel, 1990a, b, 1991]. However, as mentioned above, USV calls have also been considered as a communicative behavior between mother and offspring. Infant mice emit a wide variety of USV calls and some of them are specifically associated with mother-offspring dyadic interactions [Hahn and Lavooy, 2005]. In naturalistic environments rodent pup vocalization is interpreted as a means of communication between mother and her offspring since its first description [Zippelius and Schleidt, 1956]. A recent study found reduced USV in mice deficient for the potential autism susceptibility gene Foxp2 [Shu et al., 2005]. In humans, impaired communication of children with their mothers is the first sign of autism, a developmental disorder with both non-genetic and genetic underpinnings. Based on these data and because the genetic abnormality is in the mothers, we favor the hypothesis that the reduced USV behavior of WT(H) and KO(H) pups is secondary to the mother’s deficit and simply reflects an adaptation to a less responsive mother.
Importantly, a partial maternal 5-HT1AR deficit (in H mothers) was sufficient to elicit both the reduced USV and increased anxiety behavior in the offspring. In fact, the complete absence of the receptor in the mother does not exaggerate but only modifies the USV deficit by shortening its length and shifting its onset to an earlier time point. One possible explanation is that KO mothers have increased ABN later during the postnatal period that could normalize the USV deficit by P12.
Reduced weight gain in KO pups of KO mothers correlate with impaired maternal nest building and pup retrieval
Measuring weight gain in the pups revealed an additional maternal genotype effect. In contrast to the USV deficit however, reduced weight gain was associated with the complete lack of the receptor in the mother. By analyzing the maternal behavior of both H and KO females, we concluded that abnormal behaviors specific for KO mothers include impaired nest building and longer latencies to pup retrieval. These KO specific maternal behaviors may explain the reduced weight gain of their KO pups. KO mothers also had increased LG at P1 in both light and dark and increased ABN in dark at P7 and P13. Since these behaviors have traditionally been associated with maternal care, they are difficult to correlate with the reduced weight gain of the KO(KO) pups. Finally, KO pups of H mothers had a largely normal weight gain during postnatal life; thus, the offspring’s own receptor deficiency does not seem to play a role in the slower weight gain of KO(KO) pups.
A possible explanation for the lower weight of KO(KO) pups could be reduced nutrition intake. However, the presence of a milk band in KO(KO) pups between P1 and P5 indicated that the females were lactating and pups were fed. Another possibility is that the incomplete nest of KO mothers provided less insulation from the outside and required increased energy expenditure to maintain body temperature leading to a reduced weight.
Conclusion
Our data indicate an important role for maternal 5-HT1A receptor in regulating offspring emotional and physical development in SW mice. Since reduced receptor binding has been reported in anxiety [Lesch et al., 1992a; Lesch et al., 1992b] and depression [Drevets et al., 1999; Koran et al., 1996; Meltzer et al., 2004; Sargent et al., 2000] including postpartum depression [Moses-Kolko et al., 2008], reduced 5-HT1A receptor function in mothers may influence the emotional development of their offspring.
Supplementary Material
Acknowledgments
This work was supported by NIH 5R01MH058669.
References
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Bale TL. Is mom too sensitive? Impact of maternal stress during gestation. Front Neuroendocrinol. 2005;26:41–49. doi: 10.1016/j.yfrne.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Blais DR, Harrold J, Altosaar I. Killing the messenger in the nick of time: persistence of breast milk sCD14 in the neonatal gastrointestinal tract. Pediatr Res. 2006;59:371–376. doi: 10.1203/01.pdr.0000199907.61549.94. [DOI] [PubMed] [Google Scholar]
- Bogoch Y, Biala YN, Linial M, Weinstock M. Anxiety induced by prenatal stress is associated with suppression of hippocampal genes involved in synaptic function. J Neurochem. 2007;101:1018–1030. doi: 10.1111/j.1471-4159.2006.04402.x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- Calatayud F, Coubard S, Belzung C. Emotional reactivity in mice may not be inherited but influenced by parents. Physiol Behav. 2004;80:465–474. doi: 10.1016/j.physbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Gross C. Identifying interactions between genes and early environment in the mouse. Genes Brain Behav. 2006;5:189–199. doi: 10.1111/j.1601-183X.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C. Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol Psychiatry. 2008;63:840–846. doi: 10.1016/j.biopsych.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Populin R. Mother-offspring interaction and pup development in genetically deaf mice. Behav Genet. 1987;17:465–475. doi: 10.1007/BF01073113. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Brunelli SA, Hofer MA. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol Behav. 1996;60:299–304. doi: 10.1016/0031-9384(95)02222-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Bethea ED, Stevenson SA. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007;8:17. doi: 10.1186/1471-2202-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason G, Liu B, Bruening S, Zupan B, Auerbach A, Mark W, Oh JE, Gal-Toth J, Lee F, Toth M. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc Natl Acad Sci U S A. 107:7592–7597. doi: 10.1073/pnas.0914805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason G, Liu B, Bruening S, Zupan B, Auerbach A, Mark W, Oh JE, Gal-Toth J, Lee F, Toth M. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0914805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Lavooy MJ. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35:31–52. doi: 10.1007/s10519-004-0854-7. [DOI] [PubMed] [Google Scholar]
- Hard E, Engel J. Effects of 8-OH-DPAT on ultrasonic vocalization and audiogenic immobility reaction in pre-weanling rats. Neuropharmacology. 1988;27:981–986. doi: 10.1016/0028-3908(88)90056-1. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson RA, Guthrie DH, Varty GB. Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol Biochem Behav. 2008;88:341–348. doi: 10.1016/j.pbb.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Andersen MB, Kjaer SL, Hansen AM, Werge T, Lund SP. Prenatal stress may increase vulnerability to life events: comparison with the effects of prenatal dexamethasone. Brain Res Dev Brain Res. 2005;159:55–63. doi: 10.1016/j.devbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Koldovsky O. Digestion and absorption of carbohydrates, protein, and fat in infant and children. In: Walker WAaW, JB, editors. Nutrition in Pediatrics. Basic Science and Clinical Applications. Boston: Little, Brown and Co; 1985. pp. 253–277. [Google Scholar]
- Koran LM, Cain JW, Dominguez RA, Rush AJ, Thiemann S. Are fluoxetine plasma levels related to outcome in obsessive-compulsive disorder? Am J Psychiatry. 1996;153:1450–1454. doi: 10.1176/ajp.153.11.1450. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008 doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Aulakh CS, Murphy DL. 5-HT1A receptor sensitivity in depression and anxiety disorders: molecular mechanisms of neuroadaption. Clin Neuropharmacol. 1992a;15(Suppl 1 Pt A):208A–209A. doi: 10.1097/00002826-199201001-00109. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wiesmann M, Hoh A, Muller T, Disselkamp-Tietze J, Osterheider M, Schulte HM. 5-HT1A receptor-effector system responsivity in panic disorder. Psychopharmacology (Berl) 1992b;106:111–117. doi: 10.1007/BF02253597. [DOI] [PubMed] [Google Scholar]
- Lindberg T, Ohlsson K, Westrom B. Protease inhibitors and their relation to protease activity in human milk. Pediatr Res. 1982;16:479–483. doi: 10.1203/00006450-198206000-00016. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril. 2008;89:685–692. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL. Neuropsychiatric disorders and the multiple human brain serotonin receptor subtypes and subsystems. Neuropsychopharmacology. 1990;3:457–471. [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004;38(Suppl 1):14–20. [PubMed] [Google Scholar]
- Olivier B, Molewijk HE, van der Heyden JA, van Oorschot R, Ronken E, Mos J, Miczek KA. Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci Biobehav Rev. 1998;23:215–227. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. J Neuroendocrinol. 2008;20:489–496. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Weller A, Leguisamo AC, Towns L, Ramboz S, Bagiella E, Hofer M, Hen R, Brunner D. Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev Psychobiol. 2003;42:194–205. doi: 10.1002/dev.10079. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. How lifetimes shape epigenotype within and across generations. Hum Mol Genet 15 Spec No. 2006;2:R131–137. doi: 10.1093/hmg/ddl200. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic and catecholaminergic reuptake inhibitors have opposite effects on the ultrasonic isolation calls of rat pups. Neuropsychopharmacology. 1990a;3:51–59. [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1990b;254:212–220. [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of the rat pup ultrasonic isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacology (Berl) 1991;105:513–520. doi: 10.1007/BF02244372. [DOI] [PubMed] [Google Scholar]
- Zippelius HM, Schleidt WM. Ultraschall-laute bei jungen mausen (Ultrasonic vocalization in infant mice) Naturwissenschaften. 1956;43:502–503. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.