Abstract
The retromer complex plays an important role in intracellular transport, is highly expressed in the hippocampus, and has been implicated in the trafficking of the amyloid precursor protein (APP). Nevertheless, the trafficking routes of the neuronal retromer and the role it plays in APP transport in neuronal processes remains unknown. Here we use hippocampal neuronal cultures to address these issues. Using fluorescence microscopy, we find that Vps35, the core element of the retromer complex, is in dendrites and axons, is enriched in endosomes and trans-Golgi network, and is found in APP-positive vesicles. Next, to identify the role the neuronal retromer plays in cargo transport, we infected hippocampal neurons with a lentivirus expressing shRNA to silence Vps35. By live fluorescence imaging, Vps35 deficiency was found to reduce the frequency, but not the kinetics, of long-range APP transport within neuronal processes. Supporting the interpretation that retromer promotes long-range transport, Vps35 deficiency led to increased APP in the early endosomes, in processes but not the soma. Finally, Vps35 deficiency was associated with increased levels of Aβ, a cleaved product of APP, increased co-localization of APP with its cleaving enzyme BACE1 in processes, and caused an enlargement of early endosomes. Taken together, our studies clarify the function of the neuronal retromer, and suggest specific mechanisms for how retromer dysfunction observed in Alzheimer’s disease affects APP transport and processing.
INTRODUCTION
The retromer is a coat-like complex of proteins comprised of a cargo recognition domain (Vps35/Vps26/Vps29), and a membrane association domain composed of a dimer of the sorting nexin proteins SNX1, 2, 5, or 6 (Bonifacino and Hurley, 2008). The retromer has been shown to act either in trafficking cargoes from the endosome back to the trans-Golgi network (TGN) (Seaman, 2005; Bonifacino and Hurley, 2008), or, in the case of polarized epithelial cells, in promoting long-range transport (Verges et al., 2004).
The retromer was first implicated in Alzheimer’s disease (AD) in a study that identified deficiencies in Vps35 and Vps26 in the hippocampal formation of patients with late-onset disease (Small et al., 2005). It was hypothesized that Vps10-containing transmembrane proteins might be part of the neuronal retromer pathway, mediating the sorting of the amyloid precursor protein (APP) away from the amyloidogenic endosomal system (Small et al., 2005). To date, three members of the family of Vps10-containing proteins have been shown to interact with the neuronal retromer-sortilin, SorL1 (also called SorLA or LR11) (Muhammad et al., 2008) and, most recently, SorCS1 (Lane et al., 2010). Several cell culture studies have shown that selective silencing of Vps35 (Small et al., 2005), Vps26 (Rogaeva et al., 2007), SorL1 (Rogaeva et al., 2007), or SorCS1 (Lane et al., 2010) causes elevations in Aβ, a cleaved product of APP associated with AD pathophysiology (Hardy and Selkoe, 2002), and similar observations have been made in animal models with genetically-induced deficiencies in Vps35 (Muhammad et al., 2008), Vps26 (Muhammad et al., 2008), SorL1 (Andersen et al., 2005; Dodson et al., 2006), or SorCS1 (Lane et al., 2010). Additionally, genetic variations in SorL1 (Rogaeva et al., 2007) or SorCS1 (Reitz et al., 2011) increase the risk of developing late-onset AD.
Besides implicating retromer dysfunction in AD pathogenesis, these studies suggest that APP is a cargo that is also trafficked by retromer. Indeed, this prediction was recently confirmed in non-neuronal cell lines, showing that the retromer complex is involved in endosome-to-TGN trafficking of APP (Vieira et al., 2010). Nevertheless, despite high expression levels in the brain (Haft et al., 2000), in particular the hippocampal formation (Muhammad et al., 2008), a detailed characterization of the neuronal retromer has not yet been completed. More importantly, as neurons are highly polarized, it is unknown whether the neuronal retromer is involved in long-range transport of cargo and if so how.
We used cultured hippocampal neurons to address these series of questions. After establishing in fixed neurons the distribution of Vps35, the core element of the retromer, we turned to live imaging to investigate the role the retromer plays in long-range transport. We focused on the distribution APP, to understand the general role the neuronal retromer plays in cargo transport, but also to clarify the link between retromer dysfunction and AD pathophysiology. Results of our studies suggest that, akin to polarized epithelial cells, the retromer can act to promote the long-range transport of cargo, and suggests that retromer dysfunction causes elevations in Aβ production by redistributing APP to endosomes in neuronal processes.
RESULTS
Localizing the retromer complex within neurons
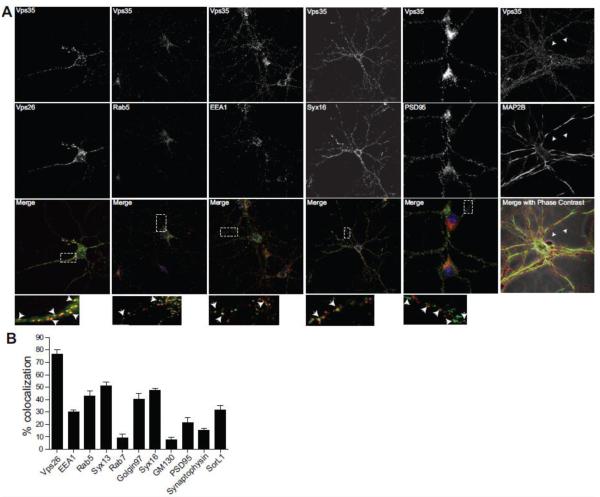
Confocal microscopy was used to identify the subcellular localization of the neuronal retromer complex and its co-localization with various intracellular markers. As in other cell types, Vps35 and Vps26, members of the retromer complex, were highly co-localized in puncta distributed throughout the neuron (Fig. 1A). Similar to non-neuronal retromer, neuronal retromer (as detected with an anti-Vps35 antibody) co-localized with markers of the early endosomes (EEA1, Rab5 and syntaxin13) and TGN (syntaxin16 and Golgin97), but to a lower extent with a marker of the late endosome/lysosome, Rab7, or the cis-Golgi (GM130; Fig. 1A-B). Importantly, proteins of the retromer complex were found, not only in the soma, but also throughout neuronal processes, including both dendrites and axons, as defined by presence and absence of MAP2 immunoreactivity in processes, respectively (Fig. 1A). Vps35 did not co-localize with markers of the presynaptic terminal (synaptophysin) and dendritic spines (PSD95; Fig. 1A-B), suggesting that the retromer complex is excluded from the synapse. On high magnification, however, the retromer complex was often found juxtaposed to PSD95 suggesting that a pool of the retromer complex may be found at the base of dendritic spines (Fig. 1A).
Figure 1. Vps35 localizes on endosomes and trans-Golgi Network (TGN) throughout the neuron.
A, 14 day old mouse primary hippocampal neurons from postnatal day 0 mice were fixed and stained for endogenous Vps35 (red) and the indicated organelle or synaptic markers (green) using antibodies, and imaged using confocal microscopy (Zeiss LSM 700). Arrows in the inset show regions of colocalization. B, Colocalization index for the indicated stainings. Values denote Means ± SEM (n ≥ 25 fields). Scale bars are 10 μm in all panels.
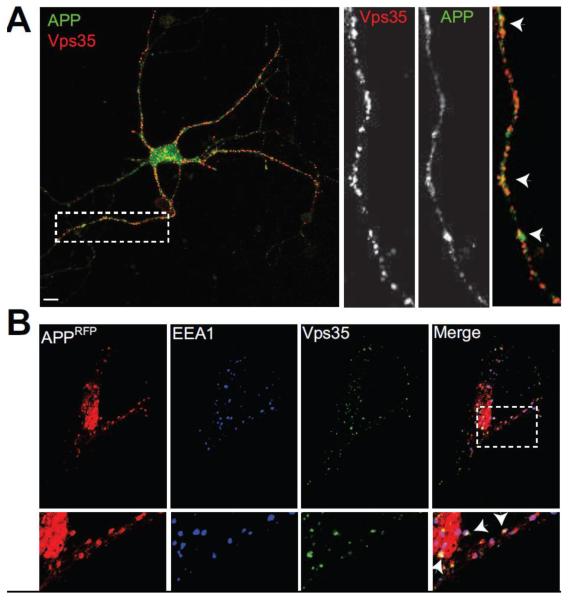
Finally, supporting studies that have implicated retromer in APP transport, we used confocal microscopy to show that Vps35 partially colocalizes with APP (Fig. 2) and SorL1 in neurons (Fig. 1B; ~35% colocalization for both). To provide additional evidence in support of the relationship between APP and retromer in a heterologous system, HeLa cells were transfected with APP-mRFP and stained with anti-Vps35 and anti-EEA1 antibodies. As in hippocampal neurons (Fig. 1), Vps35 colocalized with EEA1- and APP-positive early endosomes in HeLa cells (Fig. 2B; inset). In contrast, little colocalization was found between APP and TGN markers in this cell line (Supplemental Fig. 1).
Figure 2. Vps35 colocalizes with Amyloid Precursor Protein.
A, Hippocampal neurons (14 DIV) were fixed and stained for endogenous Vps35 (red) and the Amyloid Precursor Protein (APP) (green). Neurons were imaged as in Fig. 1. Inset shows colocalization between APP and Vps35 occurring also in neuronal processes. B, HeLa cells were transfected with APP-mRFP (red), and stained for endogenous Vps35 (green) and the early endosomal protein EEA1 (blue), 24 hours post transfection. Merge shows extensive colocalization between Vps35 and APP occurring on early endosomes. N=15 fields from at least three independent experiments. Scale bars are 10 μm.
Neuronal retromer promotes long-range transport of APP in processes
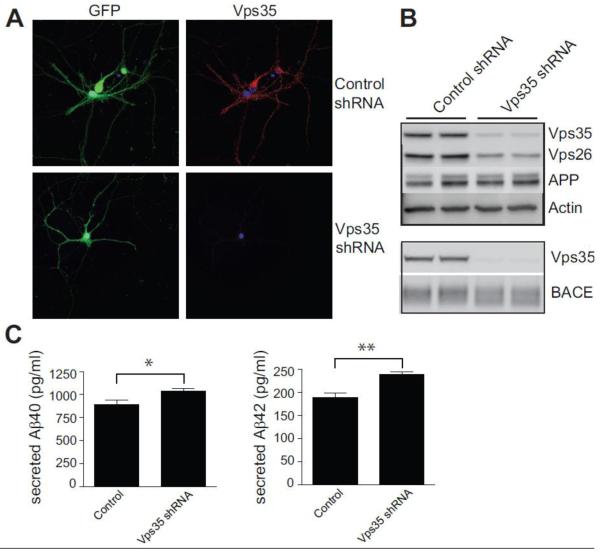
The observation that Vps35 is found in processes raised the possibility that the neuronal retromer might play a role in long-range cargo transport. To investigate this possibility we developed a shRNA lentivirus directed against Vps35. Infection of primary hippocampal neurons with this virus resulted in greater than 80% reduction in Vps35 relative to control virus (Fig. 3). In agreement with other studies (Verges et al., 2004; Muhammad et al., 2008), a downregulation of Vps35 caused a concomitant downregulation of a second retromer protein, Vps26 (Fig. 3), suggesting a general destabilization of the retromer complex.
Figure 3. Vps35 deficiency increases Aβ-40 and 42 accumulation.
A, Hippocampal neurons (7 DIV) were treated with either control lentivirus or Vps35 shRNA lentivirus, both of which express GFP. Neurons were stained for Vps35 (red) seven days post infection. B, Neurons were treated with lentivirus as in A, and homogenized to obtain a total cell lysate. Western blot analysis shows significant reduction in Vps35 and Vps26 with no significant change in total APP (upper panel) or BACE (lower panel) levels. C, Murine Aβ-40 and 42 levels were measured in the media of either control treated or Vps35 shRNA treated neurons by ELISA (Invitrogen). Values denote Means ± SEM (n=9 from 3 independent experiments). *, p < 0.05. **, p < 0.01 using the Student’s t-test.
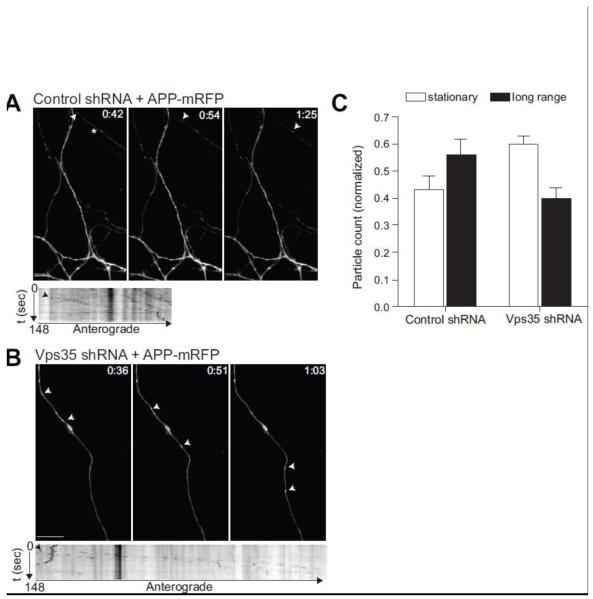
By transfecting Vps35-deficient neurons with APP-mRFP, we find that Vps35 deficiency causes a relative decrease in the number of long-range moving APP positive particles (Fig. 4A-C; p=0.014) but does not affect the kinetics of APP’s long-range trafficking. APP was transported through neuronal processes with a relative velocity of 1-2 μm/sec in both control and Vps35 knockdown conditions (Fig. 4A-B; Movie 1A-B and data not shown). As previously described in epithelial cells (Verges et al., 2004), retromer might promote the long-range transport of cargo, although not necessarily found in long-range vesicles themselves. To investigate this issue in neurons, we first transfected neurons with a construct expressing a GFP-tagged version of human Vps35 (GFP-Vps35; Fig. 5). Exogenously expressed Vps35 was found to be localized throughout the soma and processes, a pattern similar to that seen by staining for endogenous Vps35 (Movie 2; see also Fig. 1). By live imaging the motility of Vps35 was characterized as short-ranged or stationary (Fig. 5, left panel; Movie 2), suggesting that the neuronal retromer is not found in long-range moving vesicles.
Figure 4. APP long-range movement in neuronal processes is affected by Vps35 deficiency.
Hippocampal neurons were treated with either control virus (A) or Vps35 shRNA lentivirus (B). Neurons were transfected with APP-mRFP 4 days post viral infection and imaged using an Olympus epi-fluorescence microscope 24-48 hours post transfection. Arrowheads indicate motile APP-mRFP containing organelles. Kymographs (lower panels) were generated using MetaMorph software. The star in A depicts the neuronal process for which the kymograph is shown below. Infected neurons were identified by GFP fluorescence (not shown in the figure). C, A repeated measures analysis of variance was performed including long-range versus stationary particles as the within-group factor and Vps35 kd versus control as the between-group factor. Results showed a significant interaction (F=7.1, p=0.014), such that Vps35 kd caused a relative reduction in long-range particles. N=4 independent experiments. Scale bars are 10 μm.
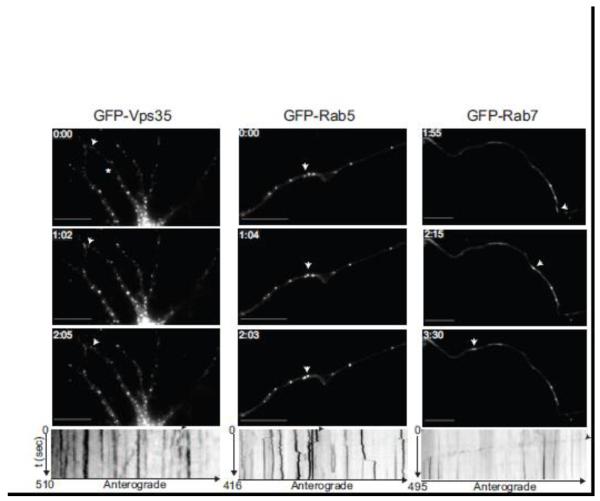
Figure 5. Vps35 exhibits short-range movement in hippocampal neurons.
Hippocampal neurons (9 DIV) were transfected with GFP-Vps35 (left panel), GFP-Rab5 (middle panel) or GFP-Rab7 (right panel), and imaged 24-48 hours post transfection. Time-lapse images were collected using a GFP filter every 5 seconds for the indicated times. Representative time-lapse images show Vps35 (left) positive puncta exhibiting a short-range jitter-like movement similar to Rab5 (middle), whereas Rab7 undergoes long-range movement in neuronal processes. White arrows in the images correspond to the black arrows in the kymographs below, highlighting either the local (for Vps35 and Rab5) or long-range movement (for Rab7). Kymographs were generated from the time-lapse movies using MetaMorph software. The star in Vps35 panel depicts the neuronal process for which the kymograph is shown below. N=3 for Rab5 and Rab7. N>3 for Vps35. Scale bars are 10 μm in all panels.
Previous studies have shown that in motor neurons Rab7, but not Rab5, is a marker of long-range transport vesicles (Deinhardt et al., 2006). Accordingly, we transfected neurons with GFP-Rab5 or GFP-Rab7. Rapid long-range movement of GFP-Rab7 (Fig. 5, right panel and Movie 4) was documented in the processes of hippocampal neurons, distinct from the movement described for Vps35. In contrast, the relatively stationary or short-range movement of GFP-Rab5 was similar to that observed for Vps35 (Fig. 5, left and middle panels; Movies 2 and 3).
Finally, we mapped the normal movement of APP by transfecting neurons with APP-mRFP. Interestingly, live imaging suggested that the movement of APP in neuronal processes is a composite of a Rab5-like stationary phase (likely reflecting the movement of early-endosomes) and a Rab7-like long-range phase (likely reflecting that of late endosomes) (Fig. 4A-B), although a significant pool of APP also traffics anterogradely with TGN-derived vesicles (Koo et al., 1990; Kaether et al., 2000), We then transfected with fluorescently tagged Vps35 and APP, and both proteins localized to axons and dendrites (Movie 5). While APP exhibited both long-range and short-range movements, in an anterograde as well as retrograde manner as previously described (Kaether et al., 2000), Vps35 undergoes mainly short-range movements. Similar results were obtained in HeLa cells transfected with APP-mRFP and GFP-Vps35 (data not shown).
Together, these results suggest that as in other polarized cells the neuronal retromer is absent from long-range moving vesicles, but nevertheless acts to promote long-range transport.
Neuronal retromer traffics APP away from EEA1- and BACE1-positive endosomes
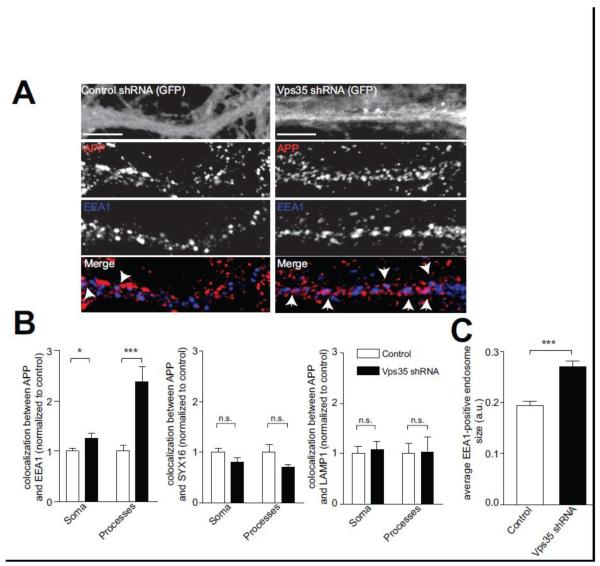
We reasoned that if the neuronal retromer acts to promote long-range transport in processes, Vps35 deficiency might cause an increase of APP in distal early endosomes. To test this, we examined the localization APP in Vps35 deficient neurons. Confirming the prediction, we find that APP is increased in EEA1-positive early endosomes in processes, and to a lesser extent, in the soma (Fig. 6A-B and Supplemental Fig. 2). In contrast, we do not find an increase of APP in late-endosome/lysosome, using the LAMP1 marker (Fig. 6B). To rule out any effect due to changes in protein levels, we assessed total protein using Western blotting. Vps35 deficiency produced no significant change in protein levels of endogenous APP, BACE (β-site APP-cleaving enzyme) or EEA1 (Fig. 3B and data not shown). Interestingly, in cultured hippocampal neurons, Vps35 deficiency resulted in a significant enlargement of early endosomes (as stained by EEA1), when compared to control conditions (Fig. 6C).
Figure 6. Vps35 deficiency shifts the localization of APP.
A, Hippocampal neurons were treated with control shRNA lentivirus (left panel) or Vps35 shRNA lentivirus (right panel). Neurons were fixed and stained for endogenous APP (red) and the early endosome marker EEA1 (blue). Diffuse GFP fluorescence marks the infected neuronal processes (top most panel) and arrows mark points of colocalization. B, Quantification of the colocalization between APP and EEA1 or APP and LAMP1 in control treated (white bars) or Vps35 shRNA treated (black bars) neurons was determined as done in Fig. 1. Values denote Means ± S.E.M (n>20 fields for Soma and 19 fields for Processes, from 3 independent experiments). Scale bars are 5 μm. C, Quantification of average EEA1 positive endosome size in control and Vps35 shRNA treated neurons was done using ImageJ. Values denote Means ± S.E.M (N=23 from 2 independent experiments). *, p<0.05. ***, p<0.0001 using the Student’s t-test.
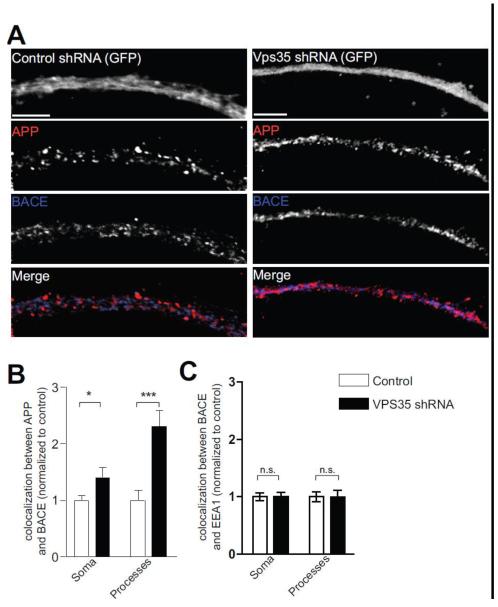
Next, we investigated the effect the redistribution of APP to early endosomes has on APP proteolytic cleavage. First, in agreement with previous studies (Muhammad et al., 2008; Sullivan et al., 2011), we find that Vps35 deficiency in cultured neurons causes a mild but significant increase in Aβ peptide, a cleaved product of APP (Fig. 3C). Second, guided by studies showing that retromer deficiency accelerates β-secretase cleavage of APP (Muhammad et al., 2008), we find that Vps35 deficiency increased the colocalization between APP and BACE1. Although observed throughout the neuron, the greatest increase in colocalization was observed in processes (p<0.001; Fig. 7A-B). A similar assessment carried out for BACE1 showed that the extent of BACE1 colocalization with EEA1 is not affected with retromer downregulation (Fig. 7C).
Figure 7. Vps35 deficiency increases the colocalization between APP and BACE1.
A, Hippocampal neurons were treated with either control shRNA (left panel) or Vps35 shRNA (right panel) as in Fig. 3. Neurons were fixed and stained for endogenous APP (red) and BACE1 (blue). B-C, Colocalization between APP and BACE1 (B), or between BACE1 and EEA1 (C), in control treated (white bars) or Vps35 shRNA treated (black bars) neurons was determined as in Fig. 1C, Values denote Means ± S.E.M (n=17 fields for Soma and 19 fields for Processes, from 3 independent experiments). *, p<0.05. ***, p<0.001 using the Student’s t-test. Scale bars are 5 μm.
DISCUSSION
To begin understanding the role of the neuronal retromer, we first mapped the localization and colocalization of its core component, Vps35, with organelle markers within hippocampal neurons. Prior studies addressing mammalian retromer function in relation to APP trafficking and AD have used primarily non-neuronal cells (Vieira et al., 2010). Here we provide the first cell-based evidence for retromer function in a neuronal setting. The series of fluorescence microscopy studies suggest that, as in other cell types (Seaman, 2005; Bonifacino and Hurley, 2008), neuronal Vps35 is found primarily in endosomes and the TGN. Importantly, Vps35 was found in the three distinct compartments of neurons (cell body, axons and dendrites). This distribution, together with previous observations in epithelial cells, showing involvement of retromer in transcytosis of cargoes such as PIGR (Verges et al., 2004), raised the possibility that neuronal retromer might be involved in long-range transport. Indeed, previous studies have established that APP undergoes both anterograde and retrograde transport in processes (Koo et al., 1990), and that altering this transport affects amyloidogenic processing of APP (Brunholz et al., 2011). Additionally, previous studies have expressed exogenous PIGR in neurons, showing that PIGR undergoes long-range transport in neuronal processes (Ikonen et al., 1993).
Our findings suggest that retromer plays a role in long-range transport of APP. Specifically, we report that retromer deficiency caused a decrease in the frequency, but not the velocity, of APP particles found to undergo long-range transport in neuronal processes. Furthermore, retromer deficiency was found to increase APP in the early endosomes within processes, which is consistent with the notion that retromer deficiency reduced the sorting and delivery of APP into long-range moving particles. Importantly, studies have established that Aβ is actively produced by the cleavage of APP in early endosomes of distal neuronal processes (Kinoshita et al., 2003; Rajendran et al., 2006). This is in agreement with findings suggesting that early endosomes are major sites of APP processing (as reviewed in Small and Gandy 2006), in part due to the presence of a highly active pool of BACE1 in these organelles. Consistent with this interpretation, our results show that retromer deficiency increases the colocalization of APP with its cleaving enzyme BACE1. Thus, by trapping more APP in the early endosomes of dendrites or axons, retromer deficiency can lead to accelerated APP processing. This scenario is consistent with an increasing number of studies implicating retromer and its cargoes in late onset AD.
These cargoes include sortilin, SorL1 and SorCS1, among others, and have also been involved in the binding and transport of APP (Andersen et al., 2005). It is however unclear whether APP missorting in processes of retromer-deficient neurons is caused by that of SorL1 and related members. Our data suggest that SorL1 is also missorted upon Vps35 silencing, but rather than exhibiting a greater localization in early endosomes, it is enriched on the TGN compartment (Supplemental Fig. 3). One potential explanation is that the endosomal pool of SorL1 may be preferentially degraded in lysosomes of Vps35-deficiency neurons, consistent with previous work on the mannose-6-phosphate receptor (Arighi et al., 2004) as well as the mild reduction of SorL1 protein levels found in Vps35-deficient neurons (data not shown).
Interestingly, we find that retromer deficiency causes an enlargement of EEA1-positive early endosomes. Endosomal enlargement was first linked to AD in seminal studies that examined neurons from postmortem brains (Cataldo et al., 2001; Nixon, 2005). Recent studies have used stem cell biology to convert fibroblasts to neurons from AD patients, either by direct induction in fibroblasts harvested from patients with presenilin mutations (Qiang et al., 2011) or via induced pluripotency stem cells from patients with APP duplications and sporadic disease (Israel et al., 2012). Remarkably, enlargement in early endosomes was found to characterize the neurons from all three AD populations. Thus, endosomal enlargement is increasingly viewed as an intracellular histological phenotype characteristic of AD. By documenting endosomal enlargement, our current studies further links retromer deficiency to AD (Small, 2008), and suggests that defects in sorting proteins out of the early endosome (Small and Gandy, 2006) might be a potential mechanism for why endosomes become enlarged.
In summary, our studies clarify the general function of the retromer in neurons, and by focusing on APP our results clarify how retromer dysfunction observed in AD can lead to amyloid accumulation. Interestingly, recent studies have linked Vps35 mutations to Parkinson’s disease (Vilarino-Guell et al., 2011; Zimprich et al., 2011), although it remains unknown whether these mutations cause a gain or loss of retromer function and whether they might interfere with binding of cargo specifically associated with Parkinson’s disease. In any case, retromer is now implicated in two neurodegenerative diseases, suggesting that the brain is particularly vulnerable to alterations in retromer function. By clarifying the function of the neuronal retromer our studies aide in better understanding how defects in neuronal retromer contribute to disorders of the brain.
Materials and Methods
cDNA and antibodies
Human APP-mRFP and GFP-Vps35 (human) were generated by subcloning into pCDNA3.1 and pEGFP vectors respectively. GFP-Rab5 (human) was a kind gift from H. Stenmark. GFP-Rab7 (human) was provided by J. Gruenberg. Vps35-shRNA and corresponding control lentiviral constructs (System Biosciences) were kindly provided by Xinhua Lin and are described in (Belenkaya et al., 2008). Antibodies used: Vps35 (Abcam), Vps26 (Abcam and provided by Juan Bonifacino), APP (Abcam), EEA1 (Pierce), Rab5 (Synaptic systems), Syntaxin13 (Synaptic systems), Golgin97 (Molecular Probes), Syntaxin16 (Synaptic systems), GM130 (BD Biosciences), LAMP1 (Abcam), PSD95 (Synaptic systems), Synaptophysin (provided by Pietro De Camilli), MAP2B and SorL1 (BD Biosciences), and BACE1 (Millipore). All primary antibodies were used at a dilution of 1:200 from the specified vendors (unless otherwise stated). All secondary antibodies (AlexaFluor 488, AlexaFluor 555 and AlexaFluor 647) were obtained from Invitrogen, and used at 1:250-1:500.
Cell Culture
Hippocampi were dissected from postnatal day 0 wild type mice, trypsinized for 30 minutes at 37°C, then dissociated by passing through a Pasteur pipette and finally plated on poly-Ornithine coated 12 or 18 mm coverslips (imaging) or 6 well plates (ELISA) at a density of 40,000-50,000 cells/cm2. Media was changed 24 hours post plating to remove any cell debris. Neurons were cultured for 14 days (unless otherwise indicated) in Neurobasal-A media supplemented with B-27, Glutamax and u-FDU, in a 5% CO2 incubator. Vps35-shRNA lentivirus was generated by transfecting control or Vps35-shRNA vectors and pPACK-H1 packaging mix (System Biosciences) into HEK-293T cells using Lipofectamine 2000 as per manufacturer’s instructions. Media was collected 48-72 hours post transfection and spun in an ultra centrifuge at 22,000xg for 1.5 hours. Lentiviral pellet was re-suspended in Neurobasal-A media and used at 1000x for infection.
HeLa cells were grown at 37°C in a 5% CO2 incubator in MEM medium supplemented with Glutamine (Invitrogen), 10% fetal bovine serum (Invitrogen) and penicillin/streptomycin (Invitrogen). These cells were transiently transfected with the indicated cDNA(s) using the Fugene-6 reagent (Roche Diagnostics), using the manufacturer’s instructions, for 48h or 72h.
Confocal Microscopy
Mouse primary hippocampal neurons and HeLa cells cultured on coverslips were fixed in 4% PFA and permeabilized with 0.05-0.1% saponin in PBS/serum buffer. Images were captured with a Zeiss LSM 700 META confocal microscope equipped with a 63x Plan-Apochromat objective and HeNe1, HeNe2 and Argon lasers. Colocalization analysis was performed using the ImageJ software (NIH) and ZEN software (Zeiss).
Endosome size analysis was done using ImageJ. Briefly, the threshold for each image was adjusted to eliminate background and the ‘Analyze Particles’ function (in μm2) was used to calculate the size of all particles in the image. Average sizes of particles are reported.
Transfection and Live-cell Imaging
For APP-mRFP, GFP-Vps35, GFP-Rab5 and GFP-Rab7 imaging, mouse hippocampal neurons were transfected with 2 μg DNA per ml of media 9-10 days after plating, using Lipofectamine LTX (Invitrogen) as per manufacturer’s instructions. Transfection was carried out for 30 minutes at 37°C and transfection media was replaced with saved conditioned media. Neurons were imaged 24-48 hours post transfection. When indicated, Vps35 shRNA or control shRNA lentivirus was added 4 days prior to transfection for efficient knock-down. Neurons were imaged in 37°C imaging solution (119 mM NaCl, 3 mM KCl, 25 mM HEPES [pH. 7.2], 30 mM Glucose, 2 mM CaCl2, 2 mM MgCl2, 10 μM CNQX) using an Olympus IX81 inverted Epifluorescence microscope, with a 37°C heated stage and constant bubbling of 5% CO2 in a humidity controlling water bath. Images were captured at 3 second intervals for the individual constructs or 5 second intervals for GFP-Vps35 APP-mRFP double transfection. Images were captured using Slidebook software (Olympus). Kymographs were generated using MetaMorph software, briefly explained below. The purpose of Kymographs is to measure velocities of moving structures over time in 2D. Processes to be analyzed are selected using a segmented line tool. The length of the segment is represented as the x-axis on the kymograph, whereas the total time of the time-lapse movie is represented on the y-axis. The kymographs are generated in grayscale with background correction such that bright puncta are easily discernibly on the kymograph. A particle that moves along a track (neuronal process), can be seen as a slanting line on the kymograph. The greater the slope of the line the faster the velocity of the particle along the neuronal process. In each experiment there was a difference in the number of total particles. To normalize across all experiments, the number of stationary and long-range moving particles in each experiment was divided by the total number of particles in that experiment. This allowed for a statistical comparison to be completed between the groups.
Aβ ELISA
Murine Aβ 1-40, 1-42 ELISA was performed as per manufacturer’s instruction (Invitrogen). Briefly, conditioned media from two-week old primary hippocampal cultures was collected and AEBSF (0.2 mg/ml) was added to prevent Aβ degradation, and kept on ice till the start of the experiment. Media volume was adjusted to account for variability due to evaporation (2 ml final volume per well of a six well plate). Media was spun at 1000xg at 4°C for 5 minutes to pellet any cell debris. ELISAs were carried out in duplicates, and the averaged values are reported in pg/ml.
Colocalization quantification
Percent colocalization was quantified using ImageJ software (N.I.H.). Briefly, the red and green channels were split and the threshold for each channel was adjusted to omit the background. The overlapping pixels were determined using the Image Calculator function (pixels overlapping in red AND green). The percent colocalization was calculated using the following equation: %colocalization=100×(overlapping pixels/total Vps35 positive pixels).
Supplementary Material
THE MAIN HIGHLIGHTS OF OUR STUDY ARE DETAILED BELOW.
-
-
RETROMER IS FOUND IN NEURONAL PROCESSES, PARTICULARLY IN DENDRITES
-
-
RETROMER PLAYS A ROLE IN LONG-RANGE TRAFFICKING OF AMYLOID PRECURSOR PROTEIN (APP)
-
-
RETROMER DEFICIENCY AFFECTS THE TRAFFICKING OF APP, LEADING TO Aβ ACCUMULATION
ACKNOWLEDGEMENTS
Funding for this project comes from The Alzheimer’s Association, NIH grants AG025161 (to S.A.S) and NS056049 (to G.D.P), and an anonymous grant. We thank Drs. Alim Muhammad, Samuel Frere and Zofia Lasiecka, for their intellectual input on preparing this manuscript. We also thank Dr. Xinhua Li for providing the Vps35 shRNA constructs, and Dr. Bonifacino for Vps26 antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunholz S, Sisodia S, Lorenzo A, Deyts C, Kins S, Morfini G. Axonal transport of APP and the spatial regulation of APP cleavage and function in neuronal cells. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo A, Rebeck GW, Ghetri B, Hulette C, Lippa C, Van Broeckhoven C, van Duijn C, Cras P, Bogdanovic N, Bird T, Peterhoff C, Nixon R. Endocytic disturbances distinguish among subtypes of Alzheimer’s disease and related disorders. Ann Neurol. 2001;50:661–665. [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Dodson SE, Gearing M, Lippa CF, Montine TJ, Levey AI, Lah JJ. LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:866–872. doi: 10.1097/01.jnen.0000228205.19915.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol Biol Cell. 2000;11:4105–4116. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Parton RG, Hunziker W, Simons K, Dotti CG. Transcytosis of the polymeric immunoglobulin receptor in cultured hippocampal neurons. Curr Biol. 1993;3:635–644. doi: 10.1016/0960-9822(93)90061-r. [DOI] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012 doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 2003;116:3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RF, Raines SM, Steele JW, Ehrlich ME, Lah JA, Small SA, Tanzi RE, Attie AD, Gandy S. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: evidence for involvement of SorL1 and the retromer complex. J Neurosci. 2010;30:13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tokuhiro S, Clark LN, Conrad C, Vonsattel JP, Hazrati LN, Palotas A, Lantigua R, Medrano M, I ZJ-V, Vardarajan B, Simkin I, Haines JL, Pericak-Vance MA, Farrer LA, Lee JH, Rogaeva E, George-Hyslop PS, Mayeux R. SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer’s disease risk. Ann Neurol. 2011;69:47–64. doi: 10.1002/ana.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Small SA. Retromer sorting: a pathogenic pathway in late-onset Alzheimer disease. Arch Neurol. 2008;65:323–328. doi: 10.1001/archneurol.2007.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel JP, Kim TW. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol. 2005;58:909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, Wells JM, Morin PJ. Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis. 2011;43:338–345. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges M, Luton F, Gruber C, Tiemann F, Reinders LG, Huang L, Burlingame AL, Haft CR, Mostov KE. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2004;6:763–769. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, Small SA, Gandy S, da Cruz ESEF, da Cruz ESOA. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.