Abstract
Cyclooxygenase-2 (COX-2) is a prostanoid-synthesizing enzyme that is critically implicated in a variety of pathophysiological processes. Using a COX-2-deficient mouse model, we present data that suggest that COX-2 has an active role in liver ischemia/reperfusion (I/R) injury. We demonstrate that COX-2-deficient mice had a significant reduction in liver damage after I/R insult. The inability of COX-2 −/− to elaborate COX-2 products favored a Th2-type response in these mice. COX-2−/− livers after I/R injury showed significantly decreased levels of IL-2, as well as IL-12, a cytokine known to have a central role in Th1 effector cell differentiation. Moreover, such livers expressed enhanced levels of the anti-inflammatory cytokine IL-10, shifting the balance in favor of a Th2 response in COX-2-deficient mice. The lack of COX-2 expression resulted in decreased levels of CXCL2, a neutrophil-activating chemokine, reduced infiltration of MMP-9-positive neutrophils, and impaired late macrophage activation in livers after I/R injury. Additionally, Bcl-2 and Bcl-xL were normally expressed in COX-2−/− livers after injury, whereas respective wild-type controls were almost depleted of these two inhibitors of cell death. In contrast, caspase-3 activation and TUNEL-positive cells were depressed in COX-2−/− livers. Therefore, our data support the concept that COX-2 is involved in the pathogenic events occurring in liver I/R injury. The data also suggest that potential valuable therapeutic approaches in liver I/R injury may result from further studies aimed at identifying specific COX-2-derived prostanoid pathways.
Liver transplantation has become one of the most effective therapeutic approaches against end-stage liver diseases. However, despite the improvements in surgical techniques, perioperative care, and immunosuppressive therapies, ischemia/reperfusion (I/R)4 injury remains a major problem in liver transplantation. I/R injury, an Ag-independent event, causes up to 10% of early transplant failures and can lead to a significantly higher incidence of acute and chronic rejections (1). Hepatic I/R insult is observed in many clinical situations other than transplantation, such as hepatectomy, shock, and cardiac arrest. Hepatocellular damage caused by I/R is the result of complex interactions between various inflammatory mediators (2, 3). A better understanding of the molecular pathophysiology of I/R injury may eventually lead to advanced therapeutic strategies that could improve the success rate of organ transplantation.
Cyclooxygenase (COX) catalyzes the conversion of arachidonic acid to PGH2 the common substrate for thromboxane A2 (TXA2), prostacyclin (PGI2), and PGE2 synthesis, which can be powerful proinflammatory factors (4). There are at least two cyclooxygenase isoenzymes, COX-1 and COX-2, that are encoded by genes (PGH synthase-1 and -2) located on different chromosomes (5). COX-1 is constitutively expressed in most cells and contributes to the synthesis of prostanoids involved in normal cellular functions, whereas COX-2 is undetectable in most tissues and its expression is up-regulated in pathological conditions, particularly in cells of the immune system (6–8). However, COX-2 inhibition has been shown to have a potent anti-inflammatory role (9), and there are paradoxical messages obtained in distinct experimental models (10). It has been reported that COX-2 inhibition reduced proteinuria and retarded the development of glomerulosclerosis in a model of diabetes and hypertension (11). COX-2-deficient mice have been useful to determine the function of COX-2 in variety of inflammatory responses. Whereas COX-2 null mice showed reduced susceptibility to ischemic brain injury (12) and to autoimmune arthritis (13), these mice developed lung fibrotic lesions in response to vanadium pentoxide with increased TNF-α expression (14).
In liver, COX-2 up-regulation has been linked to patients with chronic viral hepatitis B and C (15, 16), cirrhosis (17, 18), and hepatocellular carcinoma (17, 19). We have previously observed that COX-2 expression is up-regulated in damaged livers after I/R (20). Moreover, COX-2 inhibition has been shown to ameliorate liver I/R injury (21–23) and to reduced liver injury and hepatic microcirculatory dysfunction in response to LPS (24). In contrast, inhibition of COX-2 failed to attenuate hepatocellular injury in rats with endotoxemia (25), and COX-2-deficient mice showed more susceptibility to Con A-induced hepatitis (26). Furthermore, it has been reported that COX-2 inhibition blocked the effect of arachidonic acid, but not of ethanol, on the induction of collagen type I gene expression by stellate cells (27). Therefore, there is a growing body of evidence that the net effect of COX-2 inhibition depends on the underlying disease process and on the type of cells involved (28). In the present study, we used COX-2-deficient mice to gain further insight into the role of COX-2 in liver I/R injury. Our data provide evidence that COX-2 is an active player in liver I/R injury and that COX-2 deficiency favors a Th2-type immune response, disrupts neutrophil migration, impairs late macrophage activation, and, importantly, ameliorates liver injury after I/R.
Materials and Methods
Mice and model of hepatic I/R injury
Male COX-2−/− knockout (KO) mice (B6;129S7-Ptgs2tm1Jed/J), matched COX-2−/− wild-type (WT) littermates (B6129SF2/J), and male C57BL6 mice at 8–10 wk of age were obtained from Jackson Laboratory. Mice were housed in the UCLA animal facility under specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. A warm hepatic I/R model was performed as previously described (29). Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and injected with heparin (100 U/kg). Arterial and portal venous blood supplies were interrupted to the cephalad lobes of the liver for 90 min using an atraumatic clip. Mice were sacrificed at 6 and 24 h after reperfusion, and liver and blood samples were collected.
Celecoxib administration
Celecoxib (100 mg/kg; LKT Laboratories) was administered orally to C57BL6 mice 30 min before ischemia. The time of maximum plasma concentration (TMAX) and half-life (t1/2) for celecoxib are ~2 and 12 h, respectively (30). Control mice were treated with vehicle in a similar fashion to celecoxib administration. Celecoxib or vehicle administration had no effect in naive animals.
Assessment of liver damage
Serum glutamic-pyruvic transaminase (sGPT) and serum glutamic-oxaloacetic transaminase (sGOT) levels were measured with an autoanalyzer by Antech Diagnostics. Liver specimens were fixed with a 10% buffered formalin solution, embedded in paraffin, and processed for H&E staining.
Myeloperoxidase (MPO) assay
MPO activity was evaluated as previously described (31). Frozen tissue was homogenized in an iced solution of 0.5% hexadecyltrimethyl-ammonium (Sigma-Aldrich) and 50 mmol/L potassium phosphate buffer solution (Sigma-Aldrich) with pH adjusted to 5. Samples were centrifuged at 15,000 rpm for 15 min at 4°C. Supernatants (100 µl) were mixed in a solution of hydrogen peroxide-sodium acetate and tetramethylbenzidine (Sigma-Aldrich). The absorbance change at 655 nm in 1 min was measured with PowerWave XS spectrophotometer (BioTek Instruments). The quantity of enzyme degrading 1 µmol/L peroxide per minute at 25°C per gram of tissue was defined as 1 U of MPO activity.
Immunohistochemistry
Liver specimens embedded in Tissue Tec OCT compound (Miles) and snap-frozen in liquid nitrogen were used for immunostaining as previously described (32). Appropriate primary Abs against mouse CD3 (17A2; Bio-Legend), CD4 (L3T4; BD Biosciences), macrophage Ag-1 (Mac-1, M1/70; BD Biosciences), Ly-6G (1A8; BD Biosciences), and MMP-9 (AF909; R&D Systems) were used at optimal dilutions. Bound primary Ab was detected using biotinylated anti-rat or anti-goat IgG and then streptavidin peroxidase-conjugated complexes (Vector Laboratories). Negative controls included sections in which the primary Ab was replaced with dilution buffer. Control sections from inflammatory tissues known to be positive for each stain were included as positive controls. The peroxidase reaction was developed with a DAB substrate kit (Vector Laboratories). The sections were evaluated blindly by counting the labeled cells in triplicates within 40 high-power fields per section. Dual staining was detected by immunofluorescence with Alexa Fluor 488-green anti-rat IgG (H+L) and Alexa Fluor 594-red anti-goat IgG (H+L) Abs (Invitrogen), and slides were analyzed using a Leica confocal microscope (UCLA Brain Research Institute, Confocal Microscope Core Facility).
RT-PCR and quantitative PCR
Total RNA was extracted with Trizol (Life Technologies). First-strand synthesis and PCR were performed as previously described (33). Transcripts were amplified with Platinum TaqDNA polymerase SuperMix (Invitrogen) on Gene Amp PCR system (Applied Biosystems). COX-1 primers (forward, 5′-CTTTGCACAACACTTCACCCACC-3′; reverse, 5′-AGCAAC CCAAACACCTCCTGG-3′), size 284 bp; COX-2 primers (forward, 5′-CCAGATGCTATCTTTGGGGAGAC-3′; reverse, 5′-GCTTGCATTGAT GGTGGCTG-3′), size 249 bp (12). PCR products were separated by electrophoresis on 1% agarose gels and stained with ethidium bromide. Quantitative PCR was performed in triplicates using SYBR GreenER qPCR SuperMix Universal (Invitrogen) and a Chromo 4 detector (Bio-Rad). The expression level of the gene of interest was calculated and normalized to β-actin.
Prostanoid production
Concentrations of TXB2, 6-keto-PGF1α (the stable hydrolysis products of TXA2 and PGI2, respectively), and PGE2 in liver extracts were determined using commercial enzyme immunoassay kits (Cayman Chemical) according to the manufacturer’s instructions.
ELISA
Cytokine concentrations in liver extracts (40 µg of total protein) and in cell culture supernatants were measured by a sandwich ELISA (eBioscience) assay according to the manufacturer’s instructions (29). The conversion of tetramethylbenzidine by HRP was detected by measuring the absorbance at 450 nm using an ELISA plate reader (BioTek Instruments). Mouse rIL-2, rIL-10, and rTNF-α from their respective ELISA kits were used as standards. Final results were expressed as picograms of cytokine per milliliter of liver extracts or per milliliter of cell supernatants.
Western blot and zymography analyses
Snap-frozen liver tissue was immediately homogenized as previously described (31). Protein content was determined using a BCA protein assay kit (Pierce Chemical).
For Western blots, 40 µg of protein in SDS-loading buffer was electrophoresed through 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The gels were then stained with Coomassie blue to document equal protein loading. The membranes were blocked with 5% dry milk and 0.05% Tween 20 (USB) in TBS and incubated with specific primary Abs against Bax, Bcl-2 (Cell Signaling Technology), Bcl-2 (Abcam), and inducible NO synthase (iNOS) (Millipore). The filters were washed and then incubated with HRP-conjugated secondary Abs, followed by detection with SuperSignal West Pico chemiluminescent substrate (Pierce). After development, membranes were stripped and re-blotted with an Ab against β-actin (Abcam). Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D image analysis software).
Gelatinolytic activity was detected in liver extracts at a final protein content of 100 µg by 10% SDS-PAGE contained in 1 mg/ml gelatin (Invitrogen) under nonreducing conditions. After SDS-PAGE, the gels were soaked twice with Novex zymogram renaturing buffer (Invitrogen) for 30 min each time, rinsed in water, and incubated overnight at 37°C in Novex zymogram developing buffer (Invitrogen). The gels were then stained with Coomasie brilliant blue R-250 (Bio-Rad) and destained with methanol-acetic acid-water (20:10:70). A clear zone indicates the presence of enzymatic activity. Positive controls for MMP-9 (BIOMOL) and prestained m.w. markers (Kaleidoscope prestained standards; Bio-Rad) served as standards. Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D image analysis software).
Caspase-3 activity
Caspase-3 activity was determined in liver samples using the ApoAlert caspase-3 colorimetric assay kit (Clontech) according to the manufacturer’s instructions. OD measurements at 405 nm were performed using a microplate reader (BioTeK Instruments). Caspase activity was expressed in units with 1 U being the amount of enzyme activity liberating 1 pmol of p-nitroanilide/min.
TUNEL assay
The TUNEL assay was performed on 5-µm cryostat sections using the In Situ Cell Death detection kit (Roche) according to the manufacturer’s protocol. TUNEL-positive cells were detected under light microscopy. Terminal transferase was omitted as a negative control. Positive controls were generated by treatment with DNase 1 (30 U/ml in 40 mmol/L Tris-Cl (pH 7.6), 6 mmol/L MgCl2, and 2 mmol/L CaCl2 for 30 min). Additionally, CD45/TUNEL dual staining was detected by immunofluorescence using an anti-CD45 mAb (30–F11; BD Biosciences), and slides were analyzed using a Leica confocal microscope (UCLA Brain Research Institute, Confocal Microscope Core Facility).
Isolation of splenocytes and in vitro COX-2 inhibition assay
Mice were sacrificed and spleens were removed aseptically. Spleens were carefully minced in ice-cold HBSS, and clumps of cells were dispersed by passage through a 22-gauge needle. Splenocytes were pelleted by centrifugation, and erythrocytes were hypotonically lysed. The spleen cells were then washed twice in RPMI and resuspended in RPMI 1640 containing 50 U/ml penicillin-streptomycin. The cells were counted, and viability was determined by using trypan blue exclusion dye. Splenocytes were activated in BD BioCoat T cell activation 96-well assay plates, anti-mouse CD3 (BD Biosciences) at a density of 2 × 105 cells/well. The final volume per well was 100 µl after the addition of the selective COX-2 inhibitor NS-398 (Calbiochem) or vehicle. Cell supernatants were collected after 3 h of incubation (37°C, 5% CO2), and ELISA detected IL-2 and IL-10 concentrations.
Data analysis
Data in the text and figures are expressed as means ± SEM. Two-group comparisons were analyzed by the two-tailed Student t test for independent samples. Probability values of <0.05 were considered statistically significant.
Results
COX-2 expression in liver I/R injury
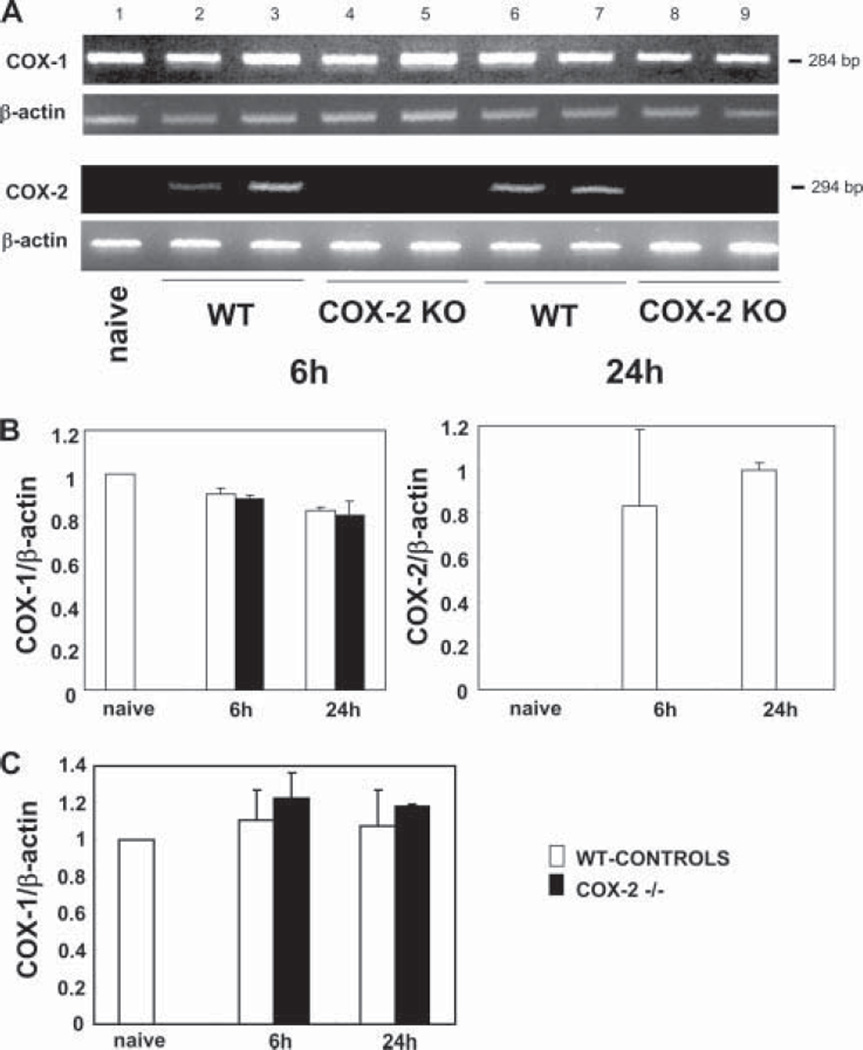
COX-2 mRNA expression was undetectable in naive COX-2 −/− livers as well as in naive WT livers. However, COX-2 mRNA was readily expressed in the WT control livers after 6 and 24 h of liver I/R injury, and it was indeed undetectable in the corresponding COX-2-deficient livers (n = 4–5/group (gr)) (Fig. 1). These results are consistent with our previous observations in a rat model of liver transplantation in which COX-2 was highly expressed after liver I/R injury (20). In contrast, COX-1 mRNA expression assessed by RT-PCR, and confirmed by real-time RT-PCR, was comparable in both COX-2-deficient and WT livers 6 and 24 h after I/R injury (n = 4/gr) (Fig. 1). Indeed, it has been previously indicated that in COX KO mice, expression of the intact COX isoform is not up-regulated to compensate for the lack of the knocked-out isoform, suggesting that each isoform has a distinct role (34).
FIGURE 1.
COX-1 and COX-2 mRNA expression in the liver of COX-2 null and WT mice after I/R injury. COX-2 mRNA expression (A) was readily detected in WT livers at 6 (lanes 2 and 3) and 24 h (lanes 6 and 7) of reperfusion following 90 min of warm ischemia. COX-2 mRNA expression was absent in COX-2−/− livers after 6 (lanes 4 and 5) and 24 h (lanes 8 and 9) of I/R injury and in naive WT livers (lane 1). In contrast, COX-1 mRNA expression was comparable in all studied groups. The densitometric ratios COX-1/β-actin mRNA and COX-2/β-actin mRNA are shown in B. Real-time PCR of COX-1 gene expression confirms that the mRNA levels of COX-1 were similar in both COX-2−/− and WT livers after reperfusion (C).
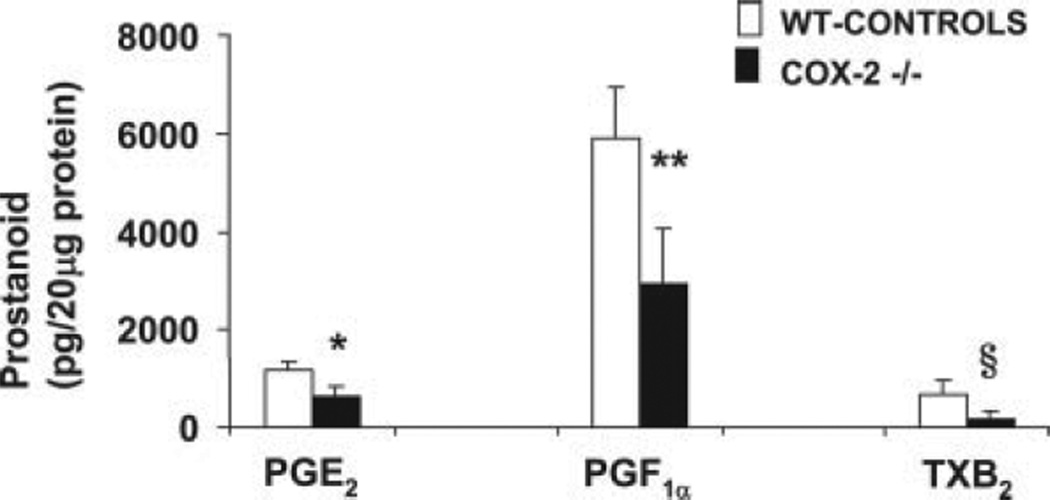
Decreased prostanoid synthesis in COX-2-deficient livers after I/R injury
To determine whether the lack of COX-2 expression is associated with a reduction in prostanoid synthesis in liver I/R injury, concentrations of the COX reaction products PGE2, PGF2α, and TXB2 were measured in livers 6 h after reperfusion. COX-2-deficient (2–, 3-, and 6-fold) and WT (4–, 6-, and 24-fold) livers showed increased levels of PGE2, PGF2α, and TXB2, respectively, as compared with naive livers. Although prostanoid synthesis in COX-2-deficient mice is likely due to the presence of COX-1, in WT mice prostanoid synthesis seems to reflect the presence of both COX isoforms. In this regard, the syntheses of PGE2 (pg/20 µg protein: 636 ± 192 vs 1167 ± 157; p < 0.008, n = 4/gr), PGF1α (pg/20 µg protein: 2944 ± 1134 vs 5923 ± 1023; p <0.004, n = 4/gr), and TXB2 (pg/20 µg protein: 169 ± 117 vs 652 ± 291; p < 0.04, n = 4/gr) were significantly reduced in COX-2-deficient livers at 6 h of I/R injury as compared with respective WT controls (Fig. 2). These data indicate that prostanoid synthesis is noticeably decreased in COX-2-deficient mice after liver I/R injury.
FIGURE 2.
Prostanoid synthesis in COX-2 and WT livers after I/R injury. The concentrations of COX reaction products PGE2, 6-keto-PGF2α, and TXB2 were significantly reduced in COX-2-deficient livers as compared with respective controls at 6 h after I/R injury (*, p < 0.008, **, p < 0.004, and §, p < 0.04).
Reduced I/R injury response in livers from COX-2-deficient mice
There were no apparent differences in transaminase levels and liver histology between naive COX-2−/− and naive WT mice. We then studied the liver injury produced by I/R in COX-2-deficient mice. Mice were sacrificed at 6 and 24 h after liver I/R injury. COX-2−/− mice showed significant less liver damage, as evidenced by the reduced transaminase levels (sGPT (U/L): 2639 ± 1930 vs 15,466 ± 7,552; p < 0.003; and sGOT (U/L): 1502 ± 1125 vs 15,355 ± 11,344; p < 0.03, n = 5– 6/gr) at 6 h after I/R injury (Fig. 3A). A sustained effect was observed in the COX-2−/− mice, with sGPT (U/L: 206 ± 45 vs 1244 ± 600; p < 0.01, n = 4 –5/gr) and sGOT (U/L: 349 ± 3 vs 974 ± 193; p < 0.01, n = 4 –5/gr) levels depressed at 24 h after I/R injury (Fig. 3A). Whereas control livers were characterized by elevated sinusoidal congestion at 6 h after reperfusion, COX-2 KO livers showed reduced sinusoidal congestion but some focal necrotic areas as well (Fig. 3B). However, at 24 h after I/R injury, the histological preservation was restored in the COX-2−/− livers, contrasting with extensive areas of necrosis (>60%) observed in the respective controls (Fig. 3B). These findings suggest that COX-2 is an active participant in the pathogenesis of I/R injury.
FIGURE 3.
Liver transaminases and histological preservation in COX-2−/− and WT mice. sGPT and sGOT levels (IU/L) (A) were measured in the blood samples taken at 6 and 24 h after I/R injury. sGPT and sGOT levels in the COX-2−/− mice were significantly lower than those in the respective WT control littermates at both 6 (*, p < 0.003, §, p < 0.03) and 24 h (**, p < 0.01). Representative H&E staining of livers at 6 and 24 h post-I/R injury (B). At 6 h, control WT livers were mostly characterized by elevated sinusoidal congestion (A), and COX-2−/− livers (B) showed reduced sinusoidal congestion and some focal necrotic areas. At 24 h, whereas WT livers (C) showed extensive signs of necrosis, COX-2−/− livers (D) had very good histological preservation (×100, H&E stain).
COX-2 inhibition with celecoxib was effective in protecting against liver I/R injury
We performed additional experiments in WT C57BL6 mice treated with celecoxib, a selective COX-2 inhibitor. Mice treated with celecoxib before I/R injury showed a marked decrease in liver damage at 6 h after I/R injury (sGPTs (U/L): 4944 ± 3177 vs 14,523 ± 700; p < 0.003; and sGOTs (IU/L): 2796 ± 1425 vs 13,700 ± 735; p < 0.002; n = 5/gr) as compared with respective vehicle-treated control mice. Therefore, these results support our observations in COX-2-deficient mice and are in agreement with previous studies in rats (22, 23) showing that COX-2 inhibition ameliorates liver I/R injury.
COX-2 deficiency impaired Bcl-2 and Bcl-xL down-regulation in liver I/R injury
Bcl-2 and Bcl-xL are considered to be important inhibitors of cell death (35). Bcl-2 and Bcl-xL were readily detected in naive livers of both COX-2 −/− and WT mice. However, whereas the COX-2 −/− livers expressed virtually normal levels of the so-called anti-apoptotic markers at 6 h of I/R injury, the respective control livers showed significantly reduced levels of both Bcl-2 (0.83 ± 0.14 vs 0.08 ± 0.09, p < 0.01; n = 4/gr) and Bcl-xL (1.38 ± 0.11 vs 0.58 ± 0.19, p < 0.01; n = 4/gr) (Fig. 4, A and B). Additionally, the enzymatic activity of caspase-3, a proapototic marker, was reduced at 6 h after reperfusion in the COX-2 −/− mice as compared with the respective WT controls (35.3 ± 3.2 vs 45.8 ± 8.4 U/g, p < 0.05; n = 4/gr) (Fig. 4C). The changes in the apoptotic networks were correlated with the numbers of TUNEL-positive cells detected in the livers. Indeed, COX-2 −/− mice showed significantly lower numbers of TUNEL-positive cells (16.2 ± 3.3 vs 73.6 ± 7.9, p < 0.01; n = 4/gr), with hepatocyte morphology, as compared with respective controls at 6 h of hepatic I/R injury (Fig. 5A). Indeed, most of the TUNEL-positive cells were negative for the pan-leukocyte marker CD45 (Fig. 5B). These results support the concept that COX-2 deficiency is associated with liver protection from I/R injury.
FIGURE 4.
Apoptotic markers in COX-2−/− and WT mice. A, Bcl-2 and Bcl-xL were readily expressed in naive livers (lane 1) and in COX-2−/− livers at 6 h post-I/R injury (lanes 5–7). In contrast, the levels of these inhibitors of cell death were markedly depressed in 6-h WT controls (lanes 2–4). B, Ratios of Bcl-2/β-actin and of Bcl-xL/β-actin in WT and COX-2-deficient livers after 6 h of I/R injury. C, Caspase-3 activity was significantly depressed in COX-2−/− livers at 6 h when compared with controls (*, p < 0.01, **, p < 0.05).
FIGURE 5.
TUNEL staining in COX-2−/− and WT mice. TUNEL-positive cells (A) were readily detected in WT livers and were significantly depressed in COX-2−/− livers at 6 h of hepatic I/R injury (*, p < 0.05). Dual staining with TUNEL reagents and Abs to CD45 (B) showed a virtual lack of TUNEL-positive cells that co-express CD45 in 6-h WT livers. Filled arrows denote TUNEL-positive cells (green), and open arrows indicate CD45+ cells (red).
COX-2 deficiency disrupted neutrophil but not mononuclear cell recruitment in liver I/R injury
We determined whether targeting COX-2 affected leukocyte infiltration in liver I/R injury. MPO activity (U/g), an index of neutrophil infiltration, was profoundly depressed in COX-2-deficient livers at 6 (2.0 ± 1.0 vs 13.7 ± 4.3, p < 0.01; n = 4–5/gr) and 24 h (1.2 ± 0.4 vs 3.4 ± 1.5, p < 0.01; n = 4–5/gr) of I/R injury as compared with respective controls (Fig. 6A). Moreover, the MPO activity results were correlated with the number of Ly-6G+ cells, a marker expressed primarily on granulocytes (36). COX-2 −/− livers showed significantly reduced numbers of Ly-6G neutrophils (6 h: 12.7 ± 0.7 vs 18.9 ± 1.2, p < 0.01; 24 h: 4.0 ± 1.0 vs 8.3 ± 1.2, p < 0.01; n = 4/gr) as compared with controls (Fig. 6B). However, the numbers of CD3 lymphocytes (6 h: 7.1 ± 0.5 vs 6.6 ± 0.8; 24 h: 1.6 ± 0.7 vs 1.7 ± 0.3; n = 4/gr) and Mac-1 cells (6 h: 22.3 ± 2.1 vs 20.7 ± 2.5; 24 h: 6.0 ± 1.0 vs 6.7 ± 0.6; n = 4/gr), a mouse macrophage Ag that is abundantly expressed on stimulated macrophages and, in lesser amounts, on granulocytes (37), were comparable in both COX-2 −/− and WT control livers after I/R injury (Fig. 7).
FIGURE 6.
Intrahepatic MPO enzyme activity and Ly-6G neutrophil infiltration in COX-2−/− and WT mice. MPO enzymatic activity (A), an index of neutrophil infiltration, was markedly reduced in the COX-2−/− mice at 6 and 24 h of reperfusion following 90 min of warm ischemia. Additionally, Ly-6G neutrophil infiltration (B) was lower in COX-2−/− livers as compared with controls at both 6 and 24 h post-I/R injury (*, p < 0.01). Arrows indicate Ly-6G cell labeling in liver specimens (immunostaining magnification ×200, 6 h).
FIGURE 7.
T and Mac-1 leukocyte infiltration in COX-2−/− and WT mice. Elevated infiltration of T (A) and Mac-1 (B) leukocytes in both COX-2−/− livers and respective controls at 6 h post-I/R injury is shown. In contrast, T and Mac-1 cell infiltration was minimal in both groups at 24 h after I/R injury. Arrows indicate leukocyte labeling in liver specimens (immunostaining magnification ×200, 6 h).
MMP-9 expression was reduced in COX-2-deficient livers after I/R injury
We have recently demonstrated that MMP-9 is expressed by leukocytes in damaged livers (33) and that MMP-9-specific inhibition impairs leukocyte migration in liver I/R injury (29). To evaluate whether COX-2 deficiency was linked to MMP-9 regulation in liver I/R injury, we performed zymography analyzes using SDS-PAGE/gelatin gels to access enzymatic activity in the liver specimens. As shown in Fig. 8, MMP-9 activity was almost undetectable in naive livers, and it was up-regulated in both COX-2-deficient livers and their respective controls, particularly at 6 h post-I/R injury; however, MMP-9 activity was reduced in COX-2 livers by ~1.5- to 2-fold when compared with controls. These results were correlated with the decreased number of MMP-9+ cells infiltrating the COX-2 −/− livers (25.0 ± 2.0 vs 33.3 ± 2.5, p < 0.01, n = 4/gr) at 6 h after I/R injury. Very few MMP-9+ cells were detected in livers at 24 h after I/R injury in both COX-2 −/− and control livers.
FIGURE 8.
Gelatin zymography and immunostaining for MMP-9 in COX-2−/− and WT livers. MMP-9 activity (A) was reduced in COX-2−/− livers both at 6 (lanes 4 and 5) and 24 h (lanes 8 and 9) as compared with respective controls at 6 (lanes 2 and 3) and 24 h (lanes 6 and 7) post-I/R injury. MMP-9 was virtually undetected in naive livers (lane 1). Additionally, MMP-9-positive leukocytes (B) were significantly depressed in COX-2−/− livers at 6 h after I/R injury (*, p < 0.01).
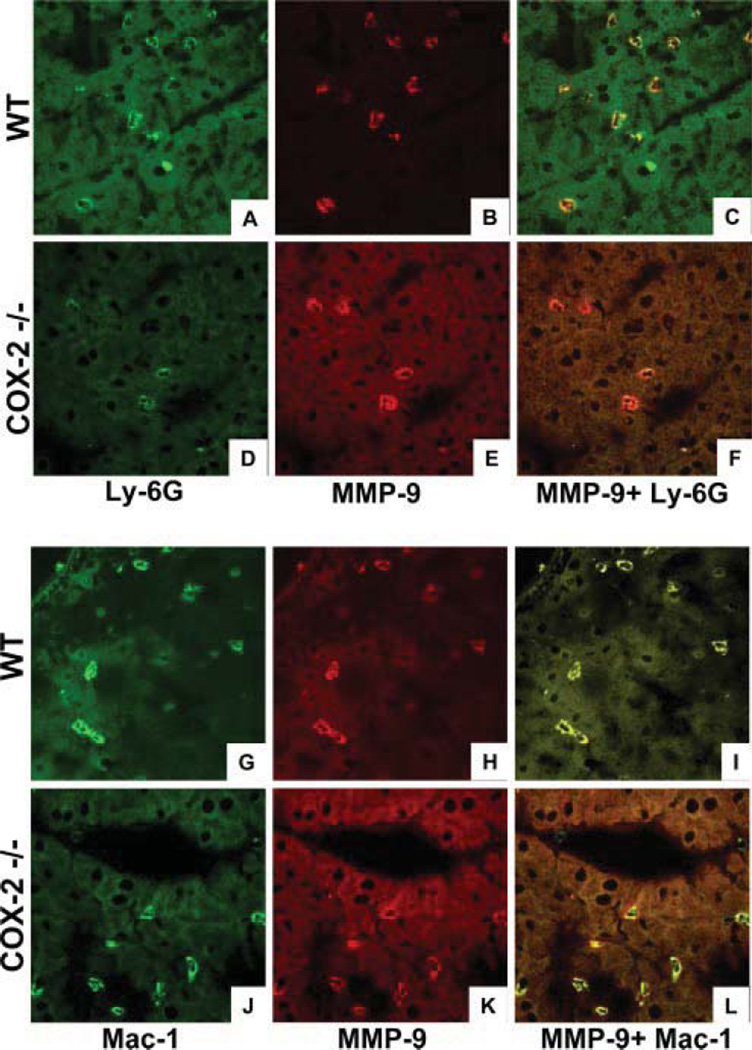
Cellular infiltration by double-positive MMP-9 Mac-1 leukocytes was comparable in both COX-2 −/− and control livers at 6 h after I/R injury (Fig. 9), suggesting that COX-2 deficiency virtually did not affect MMP-9 expression by macrophages in this liver model. In contrast, there was a noticeable reduction in MMP-9+ Ly-6G neutrophils infiltrating COX-2 −/− livers as compared with respective controls at 6 h after I/R injury (Fig. 9). Overall, these results suggest that COX-2 deficiency was associated with decreased levels of neutrophil-derived MMP-9.
FIGURE 9.
Colocalization of MMP-9 and leukocyte markers in livers after I/R injury. All panels represent confocal optical sections of immunostained COX-2−/− and WT livers at 6 h post-I/R injury. Ly-6G (A and D) and Mac-1 (G and J) cells were stained in green (Alexa Fluor 488), MMP-9 was labeled in red (Alexa Fluor 594) (B, E, H, and K), and cell colocalization of both Ly-6G/MMP-9 (C and F) and Mac-1/MMP-9 (I and L) markers are shown in a yellow-orange. Although MMP-9 + Ly-6G neutrophils were profoundly reduced in COX-2−/− livers, MMP-9 + Mac-1 macrophages were present in comparable numbers in both COX-2−/− and control livers after I/R injury.
COX-2 −/− deficiency down-regulated the expression of neutrophil-activating CXCL2 chemokine in liver I/R injury
Leukocyte transmigration across endothelial and extracellular matrix (ECM) barriers is dependent on adhesive and focal matrix degradation mechanisms, as well as on expression of cell-activating chemokines. MCP-1 is a member of the C-C chemokine family with chemotactic activity for monocytes (38). In our experimental settings, MCP-1 was predominantly up-regulated in both COX-2 −/− and WT control livers at 6 h after I/R injury (MCP-1/β-actin mRNA: 3.4 ± 0.4 vs 2.9 ± 0.1; n = 4/gr), a time point when Mac-1 leukocyte infiltration was elevated in both groups of mice (Fig. 8). CXC chemokines are considered to act predominantly on neutrophils (39). In mice, the two major CXC chemokines are cytokine-induced neutrophil chemoattractant (KC/CXCL1) and macrophage inflammatory protein-2 (MIP-2/CXCL2). Although no major variations were detected on CXCL1 expression (CXCL1/β-actin mRNA, 6 h: 2.5 ± 0.5 vs 3.0 ± 0.7; 24 h: 1.5 ± 1.0 vs 2.0 ± 0.2; n = 4/gr) between COX-2 −/− and WT mice, CXCL2 expression was significantly reduced in COX-2 −/− livers at both 6 (CXCL2/β-actin mRNA: 9.1 ± 0.7 vs 11.1 ± 0.7, p < 0.05; n = 4/gr) and 24 h (CXCL2/β-actin mRNA: 3.1 ± 3.0 vs 10.0 ± 3.5, p < 0.05; n = 4/gr) after I/R injury. These results were confirmed by real-time PCR, which showed that the expression of MCP-1 and CXCL1 was comparable in both COX-2-deficient and WT control livers, whereas the expression of CXCL2 was significantly decreased in COX-2 −/− livers at both 6 ( p < 0.03) and 24 h (p < 0.05) after I/R injury as compared with respective controls (Fig. 10).
FIGURE 10.
Chemokine gene expression in COX-2−/− and WT livers. Real-time PCR of chemokine gene expression shows that the mRNA levels of MCP-1, a monocyte chemoattractant, were up-regulated in both COX-2−/− and WT livers after reperfusion. However, whereas the expression of CXCL1, a neutrophil chemoattractant, had little variation between COX-2−/− and control livers, the expression of the neutrophil-activating CXCL2 chemokine was significantly reduced in COX-2−/− livers at 6 and 24 h post-I/R injury as compared with controls. Data were normalized to actin gene expression (*, p < 0.03, **, p < 0.05).
COX-2 deficiency induced Th1/Th2 cytokine imbalance in liver I/R injury
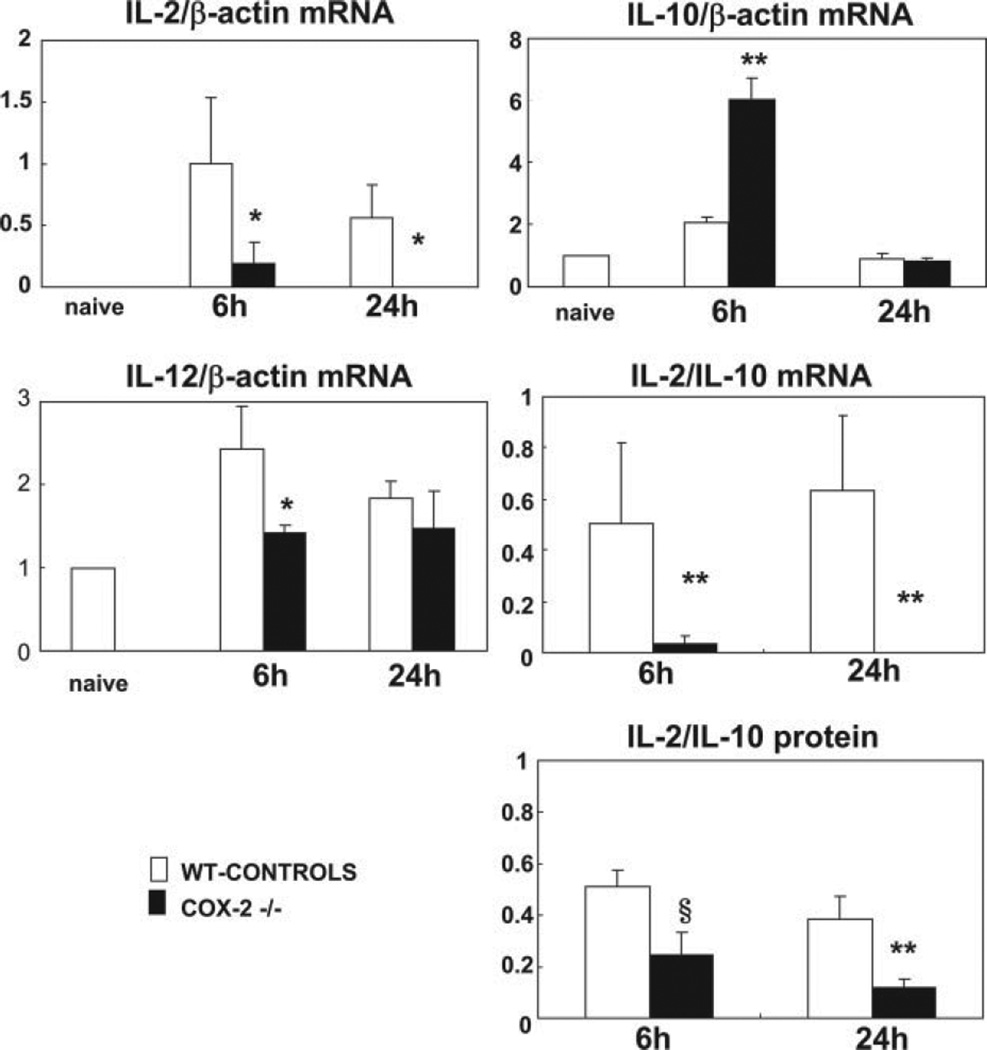
It has been shown that T lymphocytes mediate hepatic I/R injury responses (40). In COX-2 −/− mice, T cell infiltration was virtually unchanged after I/R injury. We next evaluated whether COX-2 deficiency affected cytokine profiles in liver I/R. Interestingly, IL-2 mRNA expression, a Th1-type cytokine, was profoundly depressed in COX-2 KO mice livers at 6 (IL-2/β-actin mRNA: 0.19 ± 0.16 vs 1.00 ± 0.53; p < 0.05; n = 4/gr) and 24 h (IL-2/β-actin mRNA: ND vs 0.56 ± 0.27; p < 0.05; n = 4/gr) after reperfusion as compared with respective controls (Fig. 11). IL-2 expression was also depressed at protein level in COX-2-deficient livers, particularly at 24 h post-I/R injury (IL-2 (pg/ml): 5.1 ± 0.9 vs 11.1 ± 1.2, p < 0.006; n = 4/gr). In contrast, IL-10 mRNA expression, a Th2-type cytokine that has been reported to be a global suppressor of immune responses as well as an immunoregulator of the Th cell responses (41), was significantly increased at mRNA (IL-10/β-actin: 6.04 ± 0.69 vs 2.06 ± 0.18, p < 0.01; n = 4/gr) and protein (IL-10 (pg/ml): 48.4 ± 13.1 vs 19.0 ± 3.4, p < 0.01; n = 4/gr) levels in COX-2 KO mice livers after 6 h of reperfusion (Fig. 11). Therefore, the ratios of IL-2:IL-10 mRNA and IL-2:IL-10 protein expression were significantly depressed severalfold in COX-2 −/− mice at both 6 and 24 h of liver I/R injury (Fig. 11). Moreover, the expression of IL-12 (IL-2/β-actin mRNA: 1.4 ± 0.09- vs 2.4 ± 0.5-fold, p < 0.05; n = 4/gr), which has a central role in promoting the differentiation of naive T cells into mature Th1 effector cells (42), was significantly depressed in COX-2 −/− deficient livers after 6 h of I/R injury (Fig. 11).
FIGURE 11.
Cytokine gene expression in COX-2−/− and WT livers. The expression of IL-2 mRNA, a Th1-type cytokine, was profoundly depressed in COX-2−/− livers as compared with controls at both 6 and 24 h post-I/R injury. In contrast, the anti-inflammatory IL-10 mRNA, a Th2-type cytokine, was significantly up-regulated in COX-2−/− livers at 6 h after liver I/R injury. Moreover, IL-12 mRNA, a cytokine required for the development of adaptive Th1 responses, was also significantly depressed in the COX-2−/− at 6 h post-I/R injury. Thus, COX-2 deficiency favored a Th2-type immune response in liver I/R injury, as indicated by the markedly decreased IL-2/IL-10 mRNA and protein ratios in the COX-2 null livers at 6 and 24 h post-I/R injury (*, p < 0.05, **, p < 0.01, and §, p < 0.001).
To further support our previous in vivo observations, we performed cell culture experiments to assess whether COX-2 inhibition can modulate cytokine expression in vitro. IL-2 (pg/ml) (ND vs 136 ± 7.5; n = 4/gr) and IL-10 (pg/ml) (420 ± 3.15 vs 498 ± 37.8, p < 0.03; n = 4/gr) expressions were up-regulated in anti-CD3-activated murine splenocytes (Fig. 12). However, the addition of NS-398, a selective COX-2 inhibitor (43), to the anti-CD3-activated splenocytes significantly depressed the expression of IL-2 (pg/ml) (10 µM: 108 ± 1.8 vs 136 ± 7.5, p < 0.01; 100 µM: 94.3 ± 4.5 vs 136 ± 7.5, p < 0.002; n = 4/gr) and increased the expression of IL-10 (pg/ml) (10 µM: 598 ± 46.4 vs 498 ± 37.8, p < 0.02; 100 µM: 766 ± 122 vs 498 ± 37.8, p < 0.03; n = 4/gr) by these cells (Fig. 12). Moreover, COX-2 inhibition with meloxicam also favored a shift toward a Th2 response in anti-CD3-activated murine splenocytes (n = 4/gr; data not shown). All together, these results suggest that COX-2 inhibition favors a Th2-dominant immune response in liver I/R injury.
FIGURE 12.
Cytokine expression in murine splenocytes. IL-2 and IL-10 expression were up-regulated in anti-CD3-activated splenocytes. However, the addition of NS-398, a COX-2-selective inhibitor, to the anti-CD3-activated splenocytes, significantly depressed the expression of IL-2 and increased the levels of IL-10 by these cells (*, p < 0.01, **, p < 0.002, ***, p < 0.02, and §, p < 0.03).
COX-2 deficiency impairs late macrophage activation
The expressions of iNOS and COX-2 are considered to be linked and to mediate two of the most prominent molecular mechanisms in inflammatory processes (44). Activated macrophages release high levels of NO as a result of iNOS up-regulation (45). We next evaluated whether COX-2 deficiency affected iNOS expression in liver I/R injury. As Fig. 13 shows, COX-2 −/− and WT livers were characterized by high iNOS expression at both mRNA and protein levels at 6 h after I/R injury. These results may explain, to a certain extent, the necrotic signs found in both COX-2 −/− and WT livers at 6 h. However, 24 h COX-2 −/− well-preserved livers showed significantly reduced levels of iNOS expression, whereas WT controls still had a sustained expression of this inducible enzyme at both mRNA (iNOS/β-actin: 0.07 ± 0.03 vs 0.52 ± 0.4, p < 0.01; n = 4/gr) and protein (iNOS/β-actin: 0.08 ± 0.01 vs 0.28 ± 0.1; p < 0.01; n = 4/gr) levels (Fig. 13). These results were correlated with TNF-α expression, a proinflammatory cytokine associated to I/R injury (46), that is mainly released by activated macrophages (47) and regulated by iNOS-derived NO (48). Indeed, TNF-α expression at both mRNA (TNF-αβ-actin mRNA: 1.1 ± 0.4 vs 2.0 ± 0.6, p < 0.05; n = 4/gr) and protein (pg/ml) (0.2 ± 0.5 vs 5.4 ± 2.8, p < 0.04; n = 4/gr) levels showed a significant decrease in 24-h COX-2−/− livers. These results suggest that macrophage activation was impaired in COX-2-deficient mice at 24 h after liver I/R injury.
FIGURE 13.
iNOS expression in COX-2−/− and WT livers. iNOS expression at mRNA (A) and protein (C) levels. iNOS is virtually absent in naive livers (lane 1), and it is similarly up-regulated in WT (lanes 2 and 3) and COX-2−/− (lanes 4 and 5) livers after 6 h of I/R injury. However, COX-2−/− livers express almost negligible levels of iNOS at 24 h of I/R injury (lanes 8 and 9), whereas respective WT livers (lanes 6 and 7) still express elevated levels of iNOS. B and D show the ratios of iNOS/β-actin mRNA and protein, respectively (*, p < 0.01).
Discussion
We used COX-2-deficient mice to investigate the role of COX-2 in the mechanisms of liver I/R injury. Our data demonstrate that COX-2−/− mice, compared with their WT counterparts, were significantly less susceptible to liver I/R reperfusion injury. COX-2−/− mice showed reduced sGPT and sGOT levels after I/R injury, which indicate that liver damage was reduced in these mice as compared with WT controls. Inflammatory processes are mediated by multiple molecular mechanisms, and COX-2 is a major inflammatory mediator (44). Our observation that the lack of COX-2 confers a protective role in liver I/R injury is supported by our own celecoxib studies, in which selective COX-2 inhibition ameliorated mouse liver I/R injury. This observation is also supported by other publications, in which COX-2 inhibition was beneficial in rat liver I/R injury (22, 23).
Bcl-2 and Bcl-xL play an important role in inhibition of apoptotic cell death and are essential for maintenance of major organ systems (49). Bcl-2 and Bcl-xL were readily detected in naive livers. However, whereas the expression of Bcl-2 and Bcl-xL in COX-2-deficient livers was not significantly different from that found in naive mice, it was profoundly reduced in WT mice. Thus, COX-2 expression may interfere with the maintenance of Bcl-2 and Bcl-xL patterns of expression in WT livers, perhaps making these livers more susceptible to apoptosis. Bcl-2 controls cytoplasmic events in part by blocking the activation of membrane-associated procaspases (50). Indeed, in our settings, caspase-3 activation was significantly reduced in COX-2−/− livers as compared with control littermates after I/R injury, and it was accompanied by a reduced number of TUNEL-positive cells observed in the COX-2-deficient livers. In this regard, it has been shown that PGE2 and PGF2α augment caspase-3 activation in ischemic brain (51) and increase the Bax/Bcl-2 ratio in the corpus luteum (52), respectively. In our studies, PGE2 was detected in lower levels in COX-2-deficient livers as compared with WT controls after I/R injury.
One of the most striking effects observed in COX-2−/− deficient mice was the marked decrease in Ly-6G neutrophil infiltration and MPO activity after liver I/R injury. Neutrophils are considered to be critical mediators in acute inflammatory liver injury (53), and MPO has emerged as an enzyme critically involved in the pathogenesis of inflammatory diseases (54). In general, leukocyte transmigration across endothelial and ECM barriers results from a complex series of mechanisms that include expression of cell-activating chemokines, adhesive interactions, and focal matrix degradation events. CXCL2, a cytokine-induced neutrophil chemoattractant (39), was selectively down-regulated in the COX-2−/− livers after I/R, providing an indication that this chemokine may participate in neutrophil activation and recruitment in this model. Neutrophil activation can also be mediated by prostanoid metabolites. For example, TXA2, a potent deteriorating factor in I/R injury, has been shown to activate neutrophils and to mediate their H2O2 production after ischemia (21). We have recently shown that MMP-9 mediates the transmigration of activated neutrophils across fibronectin (29), a key ECM protein expressed very early by endothelial cells in liver I/R injury (31). Others have shown that COX-2 inhibition in vitro is able to block ECM-induced MMP-9 by RAW264.7 macrophage-like cells (55), likely by targeting PGE2 receptors in these cells (56). In our study, COX-2−/− mice had reduced numbers of MMP-9-positive neutrophils infiltrating the livers after I/R injury; thus, it is reasonable to postulate that COX-2, through selective prostanoid–receptor interactions, may also regulate MMP-9 expression by these cells. Further experimentation is required to test this hypothesis, as there are at least five primary active prostanoid metabolites (PGD2, PGE2, PGF2, PGI2, and TXA2) and five major groups of receptors identified (DP, EP, FP, IP, and TP) (57).
T cells are considered to participate on neutrophil recruitment in liver I/R injury (40), and Th1 cells may represent the best candidates in this process (58). COX-2 deficiency did not disrupt T lymphocyte infiltration in liver I/R injury; however, the inability of COX-2−/− mice to elaborate COX-2 products favored a Th2-type response in these mice. COX-2−/− livers after I/R injury showed significantly decreased levels of IL-2 and IL-12, with the latter being a cytokine known to have a central role in Th1 effector cell differentiation (42). Moreover, these livers also expressed enhanced levels of the anti-inflammatory cytokine IL-10, shifting the balance in favor of a Th2 response in these mice. Furthermore, the addition of the COX-2 inhibitor NS-398, or meloxicam (not shown), to anti-CD3-activated murine splenocytes depressed IL-2 and up-regulated IL-10 expression by these cells. It has been reported that COX-2 inhibition with NS-398 markedly depresses IL-2 release by anti-CD3/CD28-activated human T lymphocytes (43). COX-2 has been shown to play a prominent role in regulating Th1 and Th2 type responses in other pathological conditions. COX-2 inhibitors, which ameliorate experimental autoimmune encephalomyelitis and autoimmune myocarditis, inhibit IL-12 signaling (59), suppress IL-2 expression, and increase IL-10 levels by T lymphocytes (60, 61), in a similar fashion to liver I/R injury. It has also been shown that prostanoids, such as TXA2, which was detected in postischemic livers and is linked to hepatic damage (62), are capable of potentiating the function of naive and primed alloreactive immune T cell populations crucial to the rejection of renal transplants (63). Furthermore, PGE2 stimulates the production of IL-12 by human dendritic cells in a dose-dependent manner (64), and it inhibits Th2 cytokine secretion by Con A-stimulated murine spleen cells (65). Conversely, it has also been suggested that PGE2 can inhibit the production of the Th1 cytokines IL-2 and IFN-γ (66). The ability of a certain prostanoid to affect T cell functions may depend on the engagement of different G proteincoupled cell-surface receptors (10). For example, when using mice lacking PGE2 (EP) receptors, it has been shown that deletion of the EP1, EP2, or EP3 receptors does not affect the generation of collagen Ab-induced arthritis, whereas EP4 receptor-deficiency reduces inflammation and decreases the incidence and severity of disease (67). Studies using mice deficient in individual prostanoid receptors may clarify the function of distinct COX-2 products in T cell-dependent responses in liver I/R injury.
Interestingly, the levels of TNF-α and iNOS, two major inflammatory mediators of hepatic injury, including liver I/R injury (31, 46, 48), that are mostly released by activated macrophages (45, 47), were equally highly expressed in COX-2−/− and WT livers during the first hours of liver I/R injury. It has been shown that iNOS specifically binds to COX-2 and that NOS inhibition decreases prostaglandin formation (44). In the present study, we show that the up-regulations of iNOS and TNF-α were independent of COX-2 expression in the early hours of I/R injury. The fact that the expressions of TNF-α and iNOS were virtually unaffected in the COX-2-deficient livers at 6 h after I/R injury may explain in part the focal signs of necrosis still observed in these livers. Indeed, at 24 h after I/R reperfusion, well-preserved COX-2−/− livers showed virtually no signs of necrosis and expressed negligible levels of TNF-α and iNOS, contrasting with the respective WT controls, which were characterized by high levels of TNF-α and iNOS expression and by extensive necrosis.
The findings of the present study support that COX-2 has an active role in liver I/R injury. Moreover, COX-2 deficiency favored a Th2-type cytokine response, disrupted MMP-9-positive neutrophil infiltration, impaired late macrophage activation, and reduced injury in livers after I/R insult. Therefore, our work supports the concept that further studies aimed at identifying specific COX-2-derived prostanoid pathways may lead to potential valuable therapies in liver I/R injury.
Footnotes
This work was supported in part by the National Institutes of Health Grant R01 AIO57832 (to A.J.C.).
Abbreviations used in this paper: I/R, ischemia/reperfusion; COX, cyclooxygenase; ECM, extracellular matrix; iNOS, inducible NO synthase; KO, knockout; MPO, myeloperoxidase; ND, nondetectable; PGI2, prostacyclin; sGOT, serum glutamic-oxaloacetic transaminase; sGPT, serum glutamic-pyruvic transaminase; TXA2, thromboxane A2; WT, wild type; gr, group.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 3.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- 4.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem. Biophys. Res. Commun. 1989;165:888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- 6.Smith WL, DeWitt DL. Prostaglandin endoperoxide H synthases-1 and-2. Adv. Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 7.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Isakson P. Distribution of COX-1 and COX-2 in normal and inflamed tissues. Adv. Exp. Med. Biol. 1997;400A:167–170. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- 8.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–175. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 9.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62:929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am. J. Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AS, Chan HL, Leung NW, Liew CT, To KF, Lai PB, Sung JJ. Expression of cyclooxygenase-2 in chronic hepatitis B and the effects of anti-viral therapy. Aliment. Pharmacol. Ther. 2002;16:251–260. doi: 10.1046/j.1365-2036.2002.01163.x. [DOI] [PubMed] [Google Scholar]
- 16.Manning DS, Sheehan KM, Byrne MF, Kay EW, Murray FE. Cyclooxygenase-2 expression in chronic hepatitis C and the effect of interferon α treatment. J. Gastroenterol. Hepatol. 2007;22:1633–1637. doi: 10.1111/j.1440-1746.2007.04869.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AS, Chan HL, Leung WK, To KF, Go MY, Chan JY, Liew CT, Sung JJ. Expression of HBx and COX-2 in chronic hepatitis B, cirrhosis, and hepatocellular carcinoma: implication of HBx in upregulation of COX-2. Mod. Pathol. 2004;17:1169–1179. doi: 10.1038/modpathol.3800196. [DOI] [PubMed] [Google Scholar]
- 18.Claria J, Kent JD, Lopez-Parra M, Escolar G, Ruiz-Del-Arbol L, Gines P, Jimenez W, Vucelic B, Arroyo V. Effects of celecoxib and naproxen on renal function in nonazotemic patients with cirrhosis and ascites. Hepatology. 2005;41:579–587. doi: 10.1002/hep.20595. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, Rocken C, Malfertheiner P, Farrell GC. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43:134–143. doi: 10.1002/hep.20994. [DOI] [PubMed] [Google Scholar]
- 20.Moore C, Shen XD, Fondevila C, Gao F, Coito AJ. Blockade of fibronectin-α4β1 adhesive interactions down-regulates cyclooxygenase-2 inducible nitric oxide synthase and prolongs recipient survival in a 24-hour model of cold hepatic ischemia-reperfusion injury. Transplant. Proc. 2005;37:1682–1683. doi: 10.1016/j.transproceed.2005.03.146. [DOI] [PubMed] [Google Scholar]
- 21.Takeyoshi I, Sunose Y, Iwazaki S, Tsutsumi H, Aiba M, Kasahara M, Ohwada S, Matsumoto K, Morishita Y. The effect of a selective cyclooxygenase-2 inhibitor in extended liver resection with ischemia in dogs. J. Surg. Res. 2001;100:25–31. doi: 10.1006/jsre.2001.6211. [DOI] [PubMed] [Google Scholar]
- 22.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatol. Res. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Takeyoshi I, Kurabayashi M, Matsumoto K, Morishita Y. The effects of a cyclooxygenase-2 inhibitor, FK3311, on total hepatic ischemia-reperfusion injury of the rat. Hepatogastroenterology. 2007;54:522–526. [PubMed] [Google Scholar]
- 24.Katagiri H, Ito Y, Ishii K, Hayashi I, Suematsu M, Yamashina S, Murata T, Narumiya S, Kakita A, Majima M. Role of thromboxane derived from COX-1 and-2 in hepatic microcirculatory dysfunction during endotoxemia in mice. Hepatology. 2004;39:139–150. doi: 10.1002/hep.20000. [DOI] [PubMed] [Google Scholar]
- 25.Leach M, Hamilton LC, Olbrich A, Wray GM, Thiemermann C. Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br. J. Pharmacol. 1998;124:586–592. doi: 10.1038/sj.bjp.0701869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I2 and E2 mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45:159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 27.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase α2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1: role of H2O2 and cyclooxygenase-2. J. Biol. Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 28.Salinas G, Rangasetty UC, Uretsky BF, Birnbaum Y. The cyclooxygenase-2 (COX-2) story: it’s time to explain, not inflame. J. Cardiovasc. Pharmacol. Ther. 2007;12:98–111. doi: 10.1177/1074248407301172. [DOI] [PubMed] [Google Scholar]
- 29.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2007 doi: 10.1002/hep.21922. In press. [DOI] [PubMed] [Google Scholar]
- 30.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1 H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib) J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 31.Amersi F, Shen XD, Moore C, Melinek J, Busuttil RW, Kupiec-Weglinski JW, Coito AJ. Fibronectin-α4β1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am. J. Pathol. 2003;162:1229–1239. doi: 10.1016/s0002-9440(10)63919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coito AJ, Binder J, Brown LF, de Sousa M, Van De WL, Kupiec-Weglinski JW. Anti-TNF-α treatment down-regulates the expression of fibronectin and decreases cellular infiltration of cardiac allografts in rats. J. Immunol. 1995;154:2949–2958. [PubMed] [Google Scholar]
- 33.Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-α4β1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am. J. Pathol. 2007;170:567–577. doi: 10.2353/ajpath.2007.060456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warford-Woolgar L, Peng CY, Shuhyta J, Wakefield A, Sankaran D, Ogborn M, Aukema HM. Selectivity of cyclooxygenase isoform activity and prostanoid production in normal and diseased Han:SPRD-cy rat kidneys. Am. J. Physiol. 2006;290:F897–F904. doi: 10.1152/ajprenal.00332.2005. [DOI] [PubMed] [Google Scholar]
- 35.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 36.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-γ in response to pulmonary infection with Nocardia asteroides . J. Leukocyte Biol. 2002;72:373–381. [PubMed] [Google Scholar]
- 37.Ho MK, Springer TA. Mac-1 antigen: quantitative expression in macrophage populations and tissues, and immunofluorescent localization in spleen. J. Immunol. 1982;128:2281–2286. [PubMed] [Google Scholar]
- 38.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 39.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 40.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J. Clin. Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 42.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 43.Iniguez MA, Punzon C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J. Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- 44.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 45.Nathan C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr Role of tumor necrosis factor-α in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J. Clin. Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102:1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 48.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J. Clin. Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 50.Krebs JF, Armstrong RC, Srinivasan A, Aja T, Wong AM, Aboy A, Sayers R, Pham B, Vu T, Hoang K, et al. Activation of membrane-associated procaspase-3 is regulated by Bcl-2. J. Cell Biol. 1999;144:915–926. doi: 10.1083/jcb.144.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2006;103:11790–11795. doi: 10.1073/pnas.0604400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav VK, Lakshmi G, Medhamurthy R. Prostaglandin F2α-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis: involvement of caspase-activated DNase. J. Biol. Chem. 2005;280:10357–10367. doi: 10.1074/jbc.M409596200. [DOI] [PubMed] [Google Scholar]
- 53.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 54.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan KM, Howe LR, Falcone DJ. Extracellular matrix-induced cyclooxygenase-2 regulates macrophage proteinase expression. J. Biol. Chem. 2004;279:22039–22046. doi: 10.1074/jbc.M312735200. [DOI] [PubMed] [Google Scholar]
- 56.Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, Dannenberg AJ, Falcone DJ. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J. Biol. Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- 57.Pierce KL, Gil DW, Woodward DF, Regan JW. Cloning of human prostanoid receptors. Trends Pharmacol. Sci. 1995;16:253–256. doi: 10.1016/s0165-6147(00)89035-5. [DOI] [PubMed] [Google Scholar]
- 58.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 59.Muthian G, Raikwar HP, Johnson C, Rajasingh J, Kalgutkar A, Marnett LJ, Bright JJ. COX-2 inhibitors modulate IL-12 signaling through JAK-STAT pathway leading to Th1 response in experimental allergic encephalomyelitis. J. Clin. Immunol. 2006;26:73–85. doi: 10.1007/s10875-006-8787-y. [DOI] [PubMed] [Google Scholar]
- 60.Ni J, Shu YY, Zhu YN, Fu YF, Tang W, Zhong XG, Wang H, Yang YF, Ren J, Wang MW, Zuo JP. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-γ and IL-10 production by inhibiting T-bet expression. J. Neuroimmunol. 2007;186:94–103. doi: 10.1016/j.jneuroim.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Tanaka H, Isobe M. A cyclooxygenase-2 inhibitor alters Th1/Th2 cytokine balance and suppresses autoimmune myocarditis in rats. J. Mol. Cell. Cardiol. 2006;40:688–695. doi: 10.1016/j.yjmcc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama Y, Toth B, Kitchens WC, Schwacha MG, Bland KI, Chaudry IH. Role of thromboxane in producing portal hypertension following trauma-hemorrhage. Am. J. Physiol. 2003;285:G1293–G1299. doi: 10.1152/ajpgi.00268.2003. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz P, Rey L, Spurney R, Coffman T, Viciana A. Thromboxane augmentation of alloreactive T cell function. Transplantation. 1992;54:498–505. doi: 10.1097/00007890-199209000-00021. [DOI] [PubMed] [Google Scholar]
- 64.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor α cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J. Exp. Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker CW, Huber MG, Godt SM. Modulation of IL-4 production in murine spleen cells by prostaglandins. Cell. Immunol. 1995;160:278–285. doi: 10.1016/0008-8749(95)80039-l. [DOI] [PubMed] [Google Scholar]
- 66.Kaur K, Harris SG, Padilla J, Graf BA, Phipps RP. Prostaglandin E2 as a modulator of lymphocyte mediated inflammatory and humoral responses. Adv. Exp. Med. Biol. 1999;469:409–412. doi: 10.1007/978-1-4615-4793-8_59. [DOI] [PubMed] [Google Scholar]
- 67.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]