Abstract
Object
The aim of this study was to determine whether patients with neurofibromatosis Type 2 (NF2) who have intact ipsilateral cochlear nerves can have open-set speech discrimination following cochlear implantation.
Methods
Records of 7 patients with documented NF2 were reviewed to determine speech discrimination outcomes following cochlear implantation. Outcomes were measured using consonant-nucleus-consonant words and phonemes; Hearing in Noise Test sentences in quiet; and City University of New York sentences in quiet and in noise.
Results
Preoperatively, none of the patients had open-set speech discrimination. Five of the 7 patients had previously undergone excision of ipsilateral vestibular schwannoma (VS). One of the patients who received a cochlear implant had received radiation therapy for ipsilateral VS, and another was undergoing observation for a small ipsilateral VS. Following cochlear implantation, 4 of 7 patients with NF2 had open-set speech discrimination following cochlear implantation during extended follow-up (15–120 months). Two of the 3 patients without open-set speech understanding had a prolonged period between ipsilateral VS resection and cochlear implantation (120 and 132 months), and had cochlear ossification at the time of implantation. The other patient without open-set speech understanding had good contralateral hearing at the time of cochlear implantation. Despite these findings, 6 of the 7 patients were daily users of their cochlear implants, and the seventh is an occasional user, indicating that all of the patients subjectively gained some benefit from their implants.
Conclusions
Cochlear implantation can provide long-term auditory rehabilitation, with open-set speech discrimination for patients with NF2 who have intact ipsilateral cochlear nerves. Factors that can affect implant performance include the following: 1) a prolonged time between VS resection and implantation; and 2) cochlear ossification.
Keywords: neurofibromatosis Type 2, cochlear implantation, cochlear implant, auditory brainstem implant, vestibular schwannoma
Neurofibromatosis Type 2 is a rare hereditary disease resulting from mutations in the merlin/schwannomin gene.27 This disease is characterized by the development of bilateral VSs in 90%–95% of affected individuals.4 Auditory rehabilitation becomes a significant aspect in the care of these patients as their tumor burden increases. Complete bilateral hearing loss is a frequent consequence of tumor growth and/or the management of VS that ultimately causes compression and dysfunction of the cochlear nerve or devascularization of the cochlea. Currently, hearing preservation is achieved following initial management intervention in less than 45% of patients with NF2,18 and the tumors may evolve over time, adversely affecting treatments that were initially effective for hearing preservation.
Three options are available for auditory rehabilitation in patients with NF2: 1) hearing aids; 2) ABIs; and 3) cochlear implants. Each of these devices has advantages, disadvantages, and limitations in the treatment of hearing loss in general and for patients with NF2 in particular.
As auditory sensitivity begins to decline, hearing can be augmented using traditional amplification (with hearing aids). These devices are currently available in multiple forms and strengths, and can amplify sounds entering the external auditory canal up to 70 dB of gain. Although digital hearing aid technology provides some control over the degree of gain of an auditory stimulus, it is less effective in cases in which the speech discrimination (or sound clarity) has been compromised. Speech discrimination that is worse than expected for the degree of hearing loss can be a common audiometric sign of the presence of a VS. For maximal amplification, hearing aids require an intact external auditory canal, tympanic membrane, and ossicular chain. Occasionally, these external structures can be affected by NF2 disease (for instance, by the presence of a neuroma within the external auditory canal), thus limiting the efficacy of hearing aids in these situations. Hearing aids require the presence of at least some inner hair cells as well as an intact cochlear nerve. Tumor resection leading to removal of one or more of these structures will prohibit hearing aids from transmitting sounds to the central auditory system. Patients with unilateral hearing losses can be treated with aids that route sound information to the contralateral ear. These types of aids include CROS, biCROS hearing aids (for individuals with some hearing loss in the “good” ear), and bone-anchored hearing aids.
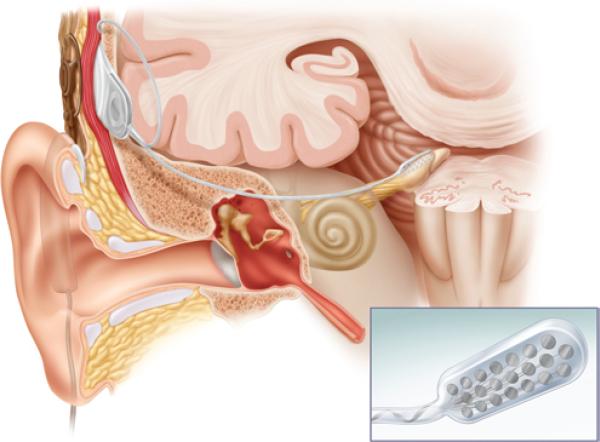
Auditory brainstem implants have been used to restore auditory capability in patients with NF2, with limited success.8 First implanted in 1979, ABIs are approved by the FDA for patients with NF2 who are ≥ 12 years old, and who are unable to benefit from hearing aids or cochlear implants. The ABIs are composed of external and internal devices (Fig. 1). The external device contains a microphone and speech processor, and can either be worn solely at ear level or can include a belt-level speech processor. The internal device is composed of a receiver/stimulator and electrode array. An ABI is typically placed following VS removal via either a translabyrinthine or RS approach (when cochlear nerve integrity is not preserved). Following tumor removal, landmarks for the lateral recess of the fourth ventricle at the foramen of Luschka (seventh, ninth, and stump of the eighth cranial nerves, choroid plexus, and tenia of the lateral recess) are used to identify the site of placement of the electrode paddle adjacent to the cochlear nucleus. Further adjustment of the site of the ABI electrode paddle is achieved by stimulating through the electrode array (or probe) and optimizing measured auditory evoked potentials intraoperatively. Simultaneously, the site of electrode placement is chosen to minimize stimulation of seventh and ninth cranial nerves. The receiver/stimulator is placed beneath the periosteum posterior to the auricle, incision, and craniotomy defect.
Fig. 1.
Illustration of an ABI. The device consists of an external microphone and processor, which connects to an internalized receiver and electrode array through the scalp. The electrode paddle, shown in greater detail in the inset, is placed within the lateral recess by the cochlear nucleus. Illustration provided courtesy of Cochlear™ Americas, ©2009 Cochlear Americas.
Ideally, devices used for auditory rehabilitation would give the patient who receives the implant the ability to understand speech without visual cues (open-set speech perception). Placement of ABIs typically results in environmental sound awareness without open-set speech perception, and these devices mainly serve to enhance lip reading. Rarely, ABIs may be indicated for patients who do not have NF2 (for instance, following bilateral cochlear nerve trauma), and in those individuals open-set speech perception may be achieved.5 Speech perception outcomes following ABI placement tend to be worse in patients with NF2 compared with those without tumors, and are rarely adequate for speech understanding in social or professional situations.28 The insertion of an ABI requires a craniotomy for placement, with the resulting risks, surgical sequelae, and costs of that procedure. Nonauditory stimulation from the ABI electrode can yield a plethora of effects, including twitching and tingling sensations in the arms, legs, and throat, facial nerve stimulation, dizziness, and aural fullness.6,17 Although future improvements in ABI technology may yield improved outcomes, alternative strategies to restore hearing such as cochlear implantation can potentially provide significant advantages.
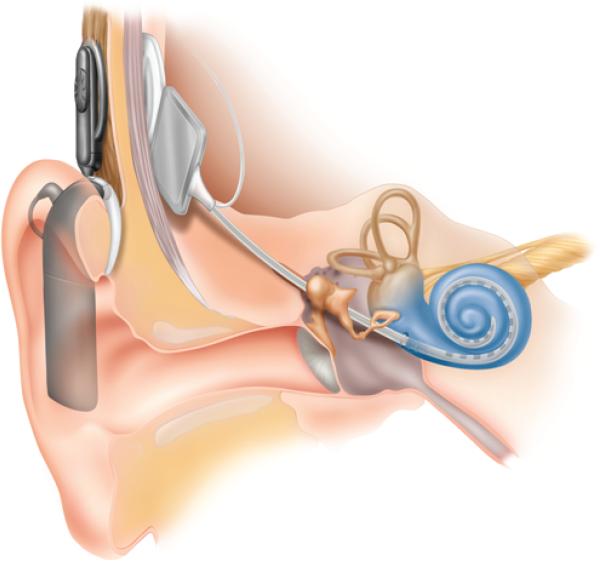
Similar to ABIs, cochlear implants are devices with analogous external and internal components (Fig. 2). The greatest difference is in the site of electrode array placement and the surgical procedure necessary to gain access to that site. Following cortical mastoidectomy, the cochlear implant's electrode is inserted into the cochlea through a small cochleostomy, and the receiver is placed in a well drilled in the bone posterior to the mastoid. Cochlear implants bypass the external auditory canal, tympanic membrane, ossicles, and inner-ear hair cells, and directly stimulate the cochlear nerve within the cochlea. Because cochlear implants are placed within a defined bone structure and can use the linear tonotopic frequency representation present within the cochlea, these devices have a theoretical advantage over ABIs for hearing rehabilitation. Generally, auditory results of cochlear implants have proven to be superior to those from ABIs, typically resulting in open-set speech recognition, the ability to converse by telephone, and improved social and professional functioning.21 Several case reports and case series from a variety of centers have reported open-set speech recognition in patients with NF2 who have received cochlear implants.21 These devices require an intact and functional cochlear nerve to rehabilitate hearing, so they are not appropriate to remediate hearing in patients with NF2 in whom the cochlear nerve has been removed during tumor resection.
Fig. 2.
Illustration of the cochlear implant. The device consists of an external microphone and processor, which connects to an internalized receiver and electrode array through the scalp. The linear electrode is placed within the cochlea. Illustration provided courtesy of Cochlear™ Americas, ©2009 Cochlear Americas.
In this study we review speech outcomes in a group of patients with NF2 who have received cochlear implants at our center between 1992 and 2010. We provide an analysis of auditory outcomes, and discuss factors associated with improved outcomes and treatment failure.
Methods
Patient Population
We reviewed the records of 7 patients with NF2 who received cochlear implants at our center between 1992 and 2010. All patients had bilateral VS, and they typically had multiple other tumors characteristics of NF2. Five of the patients had undergone surgical removal of the ipsilateral VS prior to cochlear implant placement, via either an RS (3 patients) or an MCF (2 patients) approach. In 2 of these cases (both with the RS approach), hearing preservation was not achieved intraoperatively, whereas in the other 3 cases (2 MCF, 1 RS), hearing was lost after a period of time postsurgery (18 months, 18 months, and 7 years, respectively). For the 2 patients who were not treated surgically for tumor in the ipsilateral ear, one received stereotactic radiation therapy for her ipsilateral VS, and the other was observed for a very small VS (Table 1). A full audiometric test battery, as described below, was performed in all patients both pre- and postoperatively. Each patient had a profound SNHL in the implant-treated ear preoperatively. None of the patients had significant preoperative hearing benefit from hearing aid use in the ipsilateral ear. Additionally, preoperative promontory stimulation was performed in 6 of these patients. The mean age at cochlear implantation was 35 ± 15.6 years; 3 patients were men and 4 were women.
TABLE 1.
Summary of characteristics and preoperative ipsilateral speech understanding in 7 patients with NF2 who received cochlear implants*
| Case No. | Age (yrs) at Op, Sex | Ipsilat VS Size (cm) | Management of Ipsilat VS | Time From Intervention to Implant | CNCw/CNCp Score (%) | CUNYq/HINTq Score (%) |
|---|---|---|---|---|---|---|
| 1 | 30, M | 1.5 | RS | 4 mos | 0/0 | 0/0 |
| 2 | 22, M | 2.5 | MCF | 11 yrs | 0/0 | 0/0 |
| 3 | 53, F | 0.1 | obs | NA | 0/0 | 0/0 |
| 4 | 60, F | 2.5 | GKS | 10 yrs | 0/0 | 0/0 |
| 5 | 27, M | 1.9 | RS | 6 mos | 2/12 | 0/0 |
| 6 | 38, F | 1.5 | MCF | 17 mos | 0/0 | 0/0 |
| 7 | 19, F | >2 | RS | 10 yrs | 0/0 | 0/0 |
GKS = Gamma Knife surgery; Implant = Implantation; NA = not applicable; obs = observation.
This study was a review of existing clinical data with patient identifiers removed. Following collection, the data were de-identified and analyzed in a blinded fashion. This study was covered by Institutional Review Board Protocol No. 11281.
Preoperative Audiometric Assessment
Preoperatively, audiometric assessment of hearing in these 7 patients with NF2 included pure tone thresholds, SRTs or SDTs, word recognition score, CNCw and CNCp, and sentence testing. All audiometric testing was performed with the patients wearing their hearing aids. Pure tone thresholds are the lowest intensity of sound at which a patient can accurately detect the presence of an auditory stimulus 50% of the time, and are measured in decibels of hearing loss at individual frequencies from 250 to 8000 Hz. The SRT is defined as the lowest intensity of sound at which the patient can accurately identify 50% of the 2-syllable words that are presented from a standard English list (spondees). When the SRT could not be determined due to poor sound clarity or poor hearing thresholds, the SDTs were often measured. The SDT is the lowest intensity of sound at which the patient can hear but not identify speech.
Word recognition scores were determined using a list of 50 single-syllable phonemically balanced words, which were presented either at 30–35 dB above the patient's SRT or at a most comfortable listening level. Results for word recognition were recorded as percent correct. The CNC test determined patient recognition of single-syllable words using 10 lists of 50 monosyllabic words that were scored as the percentage of CNCw and CNCp correctly repeated by the patient.
The sentence tests included HINTq and HINTn, and CUNYq and CUNYn. The HINT test consists of 25 10-sentence lists that were scored as the percentage of words correctly repeated after hearing the sentence in both quiet and noise. The CUNY sentence test includes 72 lists of 12 sentences that vary in length from 3 to 14 words, and was scored as the percentage of words correctly repeated after hearing the sentence in both listening conditions.
Promontory Stimulation Protocol
Following application of topical anesthetic to the tympanic membrane of an awake patient, a transtympanic needle electrode was placed onto the cochlear promontory near the round window niche. The promontory was electrically stimulated with a promontory stimulator unit (Cochlear Americas), which delivers a constant current stimulus. Current was varied in amplitude from 0 to 500 μA and in frequency from 50 to 1600 Hz during testing. Hearing thresholds and dynamic range of hearing resulting from electrical stimuli were assessed for all patients with NF2 who received cochlear implants in this study. Other testing included the temporal difference limen and gap detection. Six of the 7 patients with implants had positive results on promontory stimulation; the seventh was not tested (Table 2).
TABLE 2.
Speech outcomes following cochlear implantation in 7 patients with NF2*
| Case No. | Device | Side | Ipsilat Deafness (mos) | Prom Stim | CNCw/CNCp Score (%) | CUNYq/HINTq/CUNYn Score (%) | Length of FU (mos) |
|---|---|---|---|---|---|---|---|
| 1 | Nucleus 22 | lt | 3 | + | 50/69 | 97/89/39 | 120 |
| 2 | Nucleus Freedom CA | lt | 132 | + | 0/0 | 0/0/0 | 12 |
| 3 | Nucleus 24M | lt | <12 | + | 34/53 | 93/96/82 | 24 |
| 4 | Nucleus 24 RCA | lt | 12 | + | 68/87 | 92/58/90 | 36 |
| 5 | Nucleus 512 | lt | 6 | + | 0/0 | 0/0/0 | 2 |
| 6 | Nucleus Freedom CA | lt | 17 | + | † | † | 15 |
| 7 | Nucleus 24M | rt | >36 | ND | 0/0 | 0/0/0 | 16 |
FU = follow-up; ND = not done; Prom Stim = promontory stimulation; + = positive.
Postimplantation testing for this patient was performed at another center, and the standard test battery was not performed. For this patient, the postoperative pure tone average was 20 dB, and word recognition scores were 80% without visual cues (also known as open-set speech recognition) and 92% with visual cues.
Surgical Technique
All patients with NF2 satisfied current criteria for diagnosis of the disorder,10 and had a severe-to-profound SNHL in the implanted ear, with poor speech understanding in the best-aided condition. Preoperative hearing levels are shown in Table 1. Placement of cochlear implants was performed after induction of general endotracheal tube anesthesia without prolonged administration of paralytics. Intraoperative facial nerve monitoring was performed using a Medtronic Nerve Integrity Monitor (NIM model 1 or model 2). Following skin incision, a small mastoidectomy and facial recess were drilled. A well was drilled posterior to the mastoidectomy site for the speech processor, and the well and mastoid cavity small connection was drilled to contain the implant electrode array. A small cochleostomy was drilled into the cochlear promontory, and the tip of the electrode array was inserted into the site. Following placement of the cochlear implant, an implant-specific software program was used to perform intraoperative interrogation of the device in all cases. Intraoperative cochlear implant testing included measuring impedances, which are used to evaluate the cochlear implant's integrity, and neural response telemetry, which noninvasively records spiral ganglion neural responses to electrical signals sent through the cochlear implant. Proper placement of the cochlear implant electrode was confirmed intraoperatively with a lateral skull x-ray study.
Assessment of Speech Outcomes
After cochlear implantation, speech outcomes were assessed using CUNYq and CUNYn, HINT, and CNCw and CNCp tests. Typically, patients were tested at 3-month intervals following cochlear implant placement for 2 years, and subsequently at yearly visits. One patient (Case 6) received audiometric follow-up at another center. Her speech outcomes were measured using word recognition scores with and without visual cues according to the protocol at that center. Follow-up duration averaged 32 months (median 16 months, range 2 months–10 years). One of the patients has been discussed in a previous report; the follow-up data and clinical course for this patient are updated in this report.15
Statistical Methods
Data were compiled and analyzed using Microsoft Excel. The threshold for statistical significance was set as p ≤ 0.05, and was assessed using the Student t-test. Linear regression analysis was also performed. An R2 value < 0.5 was considered insignificant.
Results
Our group of patients with NF2 and bilateral VSs who received cochlear implants consisted of 3 men and 4 women. The average age at implantation was 36 years, with a range of 19–60 years (Table 1). Of the 7 patients with NF2 who underwent cochlear implantation, 5 were treated with surgery for tumor in the ipsilateral ear by either RS or MCF approaches. One patient was observed for a very small ipsilateral VS, and another was treated with stereotactic radiation for her ipsilateral tumor. Six of the 7 patients had positive results on promontory stimulation prior to cochlear implantation; in the seventh, promontory stimulation was not performed. One patient initially had negative results on promontory stimulation at 4 weeks after tumor resection, but results of repeat testing at 8 weeks were positive.
For the entire group of patients, the duration of ipsilateral deafness ranged from 3 to 132 months (average 31.14 months; Table 2). Patients received the most advanced Cochlear Corporation device available at the time of their implantation. Within this group of patients, 1 has a Nucleus 512 device; 1 received a Nucleus 22; 2 have Nucleus 24M devices; 1 was implanted with a Nucleus 24 RCA; and 2 received a Nucleus Freedom CA. Follow-up ranged from 2 to 120 months, with a mean follow-up of 32 months and a median of 16 months.
Four of 7 patients had open-set speech recognition at the latest recorded follow-up visit. Six of the 7 patients continue to use the cochlear implant daily (Cases 1–6), and the seventh is an “occasional user.” Continued use of the cochlear implant provides additional evidence of the usefulness of the device in these patients.
Two of the 3 patients without open-set speech recognition had labyrinthitis ossificans. These patients’ cochlear implants were placed following an extended period of time (120 and 132 months; Cases 7 and 2, respectively) after resection of the ipsilateral VS. The third patient with no open-set speech recognition (Case 5) had retained good hearing in the contralateral ear (contralateral CUNYq, 100%; contralateral CUNYn, 88%; contralateral HINTq, 99%). These results corroborate previous reports that some of the main determinants for poor speech outcomes in patients with NF2 who have received a cochlear implant are preserved hearing in the contralateral ear,20 as well as reports that labyrinthitis ossificans is associated with worse speech outcomes, as discussed below.
Although cochlear implants are often not considered optimal devices for long-term auditory rehabilitation of patients with NF2, data in the literature on cochlear implant performance in this group of patients often do not include long-term follow-up speech outcomes data. Within our cohort of 7 patients with NF2 who received cochlear implants, we had an average of 32 months follow-up (range 2 months–10 years postoperatively, median 16 months) after cochlear implantation. Within this cohort, 4 of 7 patients maintained their open-set speech recognition for up to 10 years postimplantation (range 15–120 months). No significant decrease in the performance of cochlear implants over time was noted.
When analyzed using linear regression methods, no correlation between the age of the patient or the tumor size prior to resection and functional hearing outcomes was observed.
Discussion
Most patients with NF2 will almost invariably lose hearing bilaterally.11 The ability to rehabilitate functional hearing in these patients results in significant improvement in quality of life.19 Currently, there are 3 options for auditory rehabilitation for patients with NF2: hearing aids, ABIs, and cochlear implants. Each of these options has significant advantages and disadvantages for auditory rehabilitation of the patient with NF2, and ideally may represent a continuum of treatment for these individuals. Traditional hearing aids can provide substantial benefit to patients with NF2 who have increased hearing thresholds but maintain good word recognition scores.
Ultimately, most patients with NF2 lose word intelligibility disproportionately compared with pure tone hearing thresholds, and require implanted devices for hearing remediation. Auditory rehabilitation in these individuals often has relied on ABIs, which have the potential to recover some degree of auditory perception, and can enhance lip-reading ability. Because ABIs bypass the external, middle, and inner ear and directly stimulate the cochlear nucleus, they remain the sole means of hearing rehabilitation postoperatively for patients with NF2 who no longer have intact cochlea or cochlear nerves.
The first use of a cochlear implant for auditory rehabilitation in a patient with NF2 was reported in 1992.15 This patient had SNHL and had a cochlear implant placed after unsuccessful hearing preservation surgery. Follow-up evaluation demonstrated open-set hearing following cochlear implantation, validating this procedure as a potential additional therapy to restore hearing in patients with NF2. Since this initial study, the cases of 32 patients with NF2 who have received cochlear implants have been reported in the literature.1,2,7,12,15,16,20–23,29,30,32,34 Our study demonstrates open-set speech recognition in 4 of 7 patients with NF2 who had cochlear implants. We acknowledge that our results represent a retrospective assessment of patients who were available for follow-up at our institution. Patients served as their own controls. Low numbers reduce the strength of our inferences. Furthermore, selection bias and confounding variables cannot be eliminated in retrospective reviews such as this. However, our study agrees with the findings of previous case reports and small studies of similar patients. In these previous studies, 24 of 32 patients with NF2 who have received cochlear implants have had good auditory outcomes, as measured by speech understanding on open-set hearing tests. These results demonstrate that cochlear implants have the potential to improve auditory outcomes in patients with NF2 and allow open-set speech recognition.
Eight of 32 previously reported patients with NF2 receiving cochlear implants had poor speech outcomes. Factors associated with poor speech outcomes in these patients include no hearing sensation generated during promontory stimulation testing,23,34 and good residual hearing in the contralateral ear.20 One of the 3 patients in our series with poor speech understanding outcomes following cochlear implantation had good residual hearing in the contralateral ear.
We would also add the following 2 conditions that may impact speech outcomes after cochlear implants: cochlear ossification and prolonged time between RS or MCF resection of the ipsilateral VSs and cochlear implantation (or duration of deafness). These factors have been noted previously to impact speech understanding outcomes in the general cochlear implant literature,9,26 but have not been specifically noted in reported cases of cochlear implantation for patients with NF2. Two of 3 patients in our series (Cases 7 and 2) who did not have open-set speech recognition following cochlear implant placement received implants 120 and 132 months after ipsilateral tumor resection, respectively. Interestingly, 1 of the 2 patients (Case 7) initially had hearing preservations following her RS resection, and lost hearing over time. These patients also had cochlear ossification, which was noted intraoperatively at the time of cochlear implant placement. Neither patient had a history of clinical meningitis. Cochlear ossification can occur following microsurgical resection of VS via either a translabyrinthine or a failed hearing preservation approach (RS or MCF), and is well known in the general cochlear implantation literature as a negative factor impacting performance of the devices in patients without NF2.9,26 In the case of cochlear nerve–sparing translabyrinthine resection of VS in patients with NF2, cochlear implant placement immediately or within a few months of resection has been recommended due to the high probability that cochlear ossification will occur within a few months of tumor excision via this approach.1–3 Certainly in the 2 patients in our series, earlier cochlear implant placement may have decreased the likelihood of cochlear ossification and improved post-implant open-set speech recognition.
Typically, reported cases of cochlear ossification (also known as labyrinthitis ossificans) follow bacterial meningitis, or rarely other causes of suppurative labyrinthitis. Neither of our patients with cochlear ossification had a history of meningitis, as mentioned above. In patients with NF2 and ipsilateral tumor resection, longer duration of deafness and labyrinthitis ossificans may not be independent factors. Although duration of deafness is difficult to determine in many of the cases reported in the literature, longer duration of deafness prior to placement of the cochlear implant in patients with NF2 does not appear to be necessarily associated with worse postimplant outcomes. In fact, there are some reports of patients with NF2 obtaining open-set speech recognition after a deafness-to-implant duration of more than 5–8 years following resection via MCF or RS approaches.12,20,21
Good contralateral hearing levels have also been associated with poor speech outcomes, as reported by Lustig et al.,20 and as found in general for patients without NF2.33 Results from our patient population corroborate these findings; 1 of the 3 patients with poor speech outcomes had good residual hearing in the contralateral ear. These results are consistent with those found in cochlear implant recipients without NF2, suggesting that it can be difficult to integrate sensory information from a cochlear implant in the presence of a functional contralateral cochlea and eighth nerve.2,20,33
Of note, all but 1 of the patients with NF2 reported in this study, including 2 of the 3 with poor speech outcomes, had positive results on promontory stimulation. Use of this test in potential cochlear implant candidates with NF2 has been recommended, and 2 studies reported that patients with NF2 who had no hearing thresholds on promontory stimulation had poor speech outcomes.23,34 Thus, the findings of this study suggest that although promontory stimulation may be a necessary condition for open-set speech recognition in patients with NF2 following cochlear implantation, it is not sufficient in and of itself to indicate that a given patient will have open-set speech recognition following implantation. Additionally, we found that although 1 of our patients had negative results on promontory stimulation at 4 weeks after ipsilateral tumor resection, he ultimately had a positive promontory stimulation at 8 weeks postoperatively. The phenomenon of initial false-negative promontory stimulation following VS resection had been observed previously in similar patients at other institutions.2,12,21 Thus, caution must be exercised in the use of promontory stimulation in the evaluation of the candidacy of patients with NF2 for cochlear implantation, as well as in its prognostic interpretation regarding speech outcomes following cochlear implantation.
As time progresses, patients with NF2 continue to grow tumors within their internal auditory canals, either from incompletely resected VSs or de novo tumor formation on the facial and cochlear nerves. This may impact long-term auditory rehabilitation over extended periods of time (> 10–20 years) following cochlear implantation. One of our most successfully treated patients (Case 1), who received the implant in 1989 and had excellent speech outcomes and open-set speech recognition documented for 12 years, contacted us recently to inquire about an ABI. Although he has not yet been seen in our center and has not had formal audiometric testing, he reports that he now has a 3-cm tumor on the side of the cochlear implant and very little speech understanding. Thus, in some patients cochlear implants may serve as a valuable interim step in auditory rehabilitation between hearing aid use and ABI.
Use of cochlear implants for remediation of severe-to-profound hearing loss in patients with NF2 whose VS is managed without open surgical intervention can yield significant hearing benefits. Although only 1 patient in our series was treated with radiotherapy, she was successfully rehabilitated with cochlear implantation for her delayed profound SNHL. Open-set speech recognition following cochlear implantation in 6 patients with NF2 has been noted in previous series.20,31,32 Long-term follow-up of patients treated with radiosurgery has found hearing preservation rates of approximately 50% at 3 years;36 thus, hearing remediation in 50% of these patients may ultimately include cochlear implantation.
Conclusions
Patients with NF2 provide management challenges in terms of hearing rehabilitation as well as treatment of tumors. In this series of 7 patients with NF2, we found that cochlear implants offer superior auditory outcomes in patients with retained cochlear nerves and positive results on promontory stimulation testing. Factors associated with worse postimplant speech understanding outcomes include long duration (> 3 years) of ipsilateral hearing loss following tumor resection, and good contralateral hearing levels. Patients with a longer period of time between resection and cochlear implantation had labyrinthitis ossificans, which negatively impacts speech outcomes following cochlear implantation in recipients with and without NF2.13,14 Promising new techniques using monitoring systems for cochlear nerve action potentials may allow real-time monitoring for preservation of cochlear nerve function, and may expand the potential for the use of cochlear implants in patients with NF2.23
Only 1 patient in this series was treated with radiotherapy prior to receiving an ipsilateral cochlear implant. This patient has done well and has open-set speech recognition (CUNYq, 92%; CUNYn, 90%). Similar results have been previously reported for patients who had received stereotactic or Gamma Knife radiotherapy,20,31,32 suggesting that cochlear implants can provide significant auditory rehabilitation for patients following radiotherapy and ipsilateral hearing loss for VS.
As progress toward a chemotherapeutic option for management of NF2 is made,24,25,35 many patients with this disease may require auditory rehabilitation for existing or progressive hearing deficits. In this population, cochlear implantation would allow for rehabilitation of severe-to-profound hearing deficits that do not respond to conventional amplification, with the possibility of open-set speech perception following device placement. Thus, as medical treatments for NF2 evolve, there will be an in creasing role for cochlear implantation in hearing rehabilitation.
Acknowledgments
This work was presented as a lecture at the Children's Tumor Foundation's Neurofibromatosis Type 2 Meeting: The State of the Art, held in Las Vegas, Nevada, on May 3, 2010.
Abbreviations used in this paper
- ABI
auditory brainstem implant
- CNC
consonant-nucleus-consonant
- CNCp, CNCw
CNC phonemes and words
- CROS
contralateral routing of sound devices
- CUNY
City University of New York
- CUNYn, CUNYq
CUNY sentences in noise and in quiet
- HINT
Hearing in Noise Test
- HINTn, HINTq
HINT performed in noise and in quiet
- MCF
middle cranial fossa
- NF2
neurofibromatosis Type 2
- RS
retrosigmoid
- SDT
speech detection threshold
- SNHL
sensorineural hearing loss
- SRT
speech reception threshold
- VS
vestibular schwannoma
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Roehm, Waltzman. Acquisition of data: all authors. Analysis and interpretation of data: Roehm, Mallen-St. Clair, Waltzman. Drafting the article: Roehm, Mallen-St. Clair, Jethanamest, Waltzman. Critically revising the article: Roehm, Golfinos, Shapiro, Waltzman, Roland. Statistical analysis: Roehm, Mallen-St. Clair. Administrative/technical/material support: Roehm, Shapiro. Study supervision: Roehm.
This work was presented as a lecture at the Children's Tumor Foundation's Neurofibromatosis Type 2 Meeting: The State of the Art, held in Las Vegas, Nevada, on May 3, 2010.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Ahsan S, Telischi F, Hodges A, Balkany T. Cochlear implantation concurrent with translabyrinthine acoustic neuroma re-section. Laryngoscope. 2003;113:472–474. doi: 10.1097/00005537-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma). Otol Neurotol. 2005;26:205–210. doi: 10.1097/00129492-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Arriaga MA, Marks S. Simultaneous cochlear implantation and acoustic neuroma resection: imaging considerations, technique, and functional outcome. Otolaryngol Head Neck Surg. 1995;112:325–328. doi: 10.1016/s0194-5998(95)70257-1. [DOI] [PubMed] [Google Scholar]
- 4.Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- 6.Colletti V, Shannon RV, Carner M, Veronese S, Colletti L. Complications in auditory brainstem implant surgery in adults and children. Otol Neurotol. 2010;31:558–564. doi: 10.1097/MAO.0b013e3181db7055. [DOI] [PubMed] [Google Scholar]
- 7.Denia A, Arístegui M. Cochlear implantation after translabyrinthine acoustic tumour removal: preliminary results. Cochlear Implants Int. 2005;6(Suppl 1):20–24. doi: 10.1179/cim.2005.6.Supplement-1.20. [DOI] [PubMed] [Google Scholar]
- 8.Di Nardo W, Fetoni A, Buldrini S, Di Girolamo S. Auditory brainstem and cochlear implants: functional results obtained after one year of rehabilitation. Eur Arch Otorhinolaryngol. 2001;258:5–8. doi: 10.1007/pl00007518. [DOI] [PubMed] [Google Scholar]
- 9.El-Kashlan HK, Ashbaugh C, Zwolan T, Telian SA. Cochlear implantation in prelingually deaf children with ossified cochleae. Otol Neurotol. 2003;24:596–600. doi: 10.1097/00129492-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Evans DG. Neurofibromatosis 2. In: Pagon RA, Bird TC, Dolan CR, et al., editors. GeneReviews. University of Washington, Seattle; Seattle: 1998. [June 3, 2011]. ( http://www.ncbi.nlm.nih.gov/books/NBK1201/) [Google Scholar]
- 11.Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. doi: 10.1186/1750-1172-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham J, Lynch C, Weber B, Stollwerck L, Wei J, Brookes G. The magnetless Clarion cochlear implant in a patient with neurofibromatosis 2. J Laryngol Otol. 1999;113:458–463. doi: 10.1017/s0022215100144214. [DOI] [PubMed] [Google Scholar]
- 13.Green JD, Jr, Marion MS, Hinojosa R. Labyrinthitis ossificans: histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg. 1991;104:320–326. doi: 10.1177/019459989110400306. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa R, Green JD, Jr, Marion MS. Ganglion cell populations in labyrinthitis ossificans. Am J Otol. 1991;12(Suppl):3–7. 18–21. [PubMed] [Google Scholar]
- 15.Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol. 1992;13:525–528. [PubMed] [Google Scholar]
- 16.Hulka GF, Bernard EJ, Pillsbury HC. Cochlear implantation in a patient after removal of an acoustic neuroma. The implications of magnetic resonance imaging with gadolinium on patient management. Arch Otolaryngol Head Neck Surg. 1995;121:465–468. doi: 10.1001/archotol.1995.01890040083014. [DOI] [PubMed] [Google Scholar]
- 17.Kanowitz SJ, Shapiro WH, Golfinos JG, Cohen NL, Roland JT., Jr Auditory brainstem implantation in patients with neurofibromatosis type 2. Laryngoscope. 2004;114:2135–2146. doi: 10.1097/01.mlg.0000149447.52888.f6. [DOI] [PubMed] [Google Scholar]
- 18.Kaylie DM, Horgan MJ, Delashaw JB, McMenomey SO. A meta-analysis comparing outcomes of microsurgery and gamma knife radiosurgery. Laryngoscope. 2000;110:1850–1856. doi: 10.1097/00005537-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Kondziolka D, Lunsford LD, Flickinger JC. Acoustic neuromas. Curr Treat Options Neurol. 2002;4:157–165. doi: 10.1007/s11940-002-0024-2. [DOI] [PubMed] [Google Scholar]
- 20.Lustig LR, Yeagle J, Driscoll CL, Blevins N, Francis H, Niparko JK. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006;27:512–518. doi: 10.1097/01.mao.0000217351.86925.51. [DOI] [PubMed] [Google Scholar]
- 21.Neff BA, Wiet RM, Lasak JM, Cohen NL, Pillsbury HC, Ramsden RT, et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117:1069–1072. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- 22.Nölle C, Todt I, Basta D, Unterberg A, Mautner VF, Ernst A. Cochlear implantation after acoustic tumour resection in neurofibromatosis type 2: impact of intra- and postoperative neural response telemetry monitoring. ORL J Otorhinolaryngol Relat Spec. 2003;65:230–234. doi: 10.1159/000073122. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo E, Guida M, Flanagan S, Lauda L, Fois P, Sanna M. CNAP to predict functional cochlear nerve preservation in NF-2: cochlear implant or auditory brainstem implant. Skull Base. 2008;18:281–287. doi: 10.1055/s-2008-1043753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG., II Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotkin SR, Singh MA, O'Donnell CC, Harris GJ, McClatchey AI, Halpin C. Audiologic and radiographic response of NF2-related vestibular schwannoma to erlotinib therapy. Nat Clin Pract Oncol. 2008;5:487–491. doi: 10.1038/ncponc1157. [DOI] [PubMed] [Google Scholar]
- 26.Rauch SD, Herrmann BS, Davis LA, Nadol JB., Jr Nucleus 22 cochlear implantation results in postmeningitic deafness. Laryngoscope. 1997;107:1606–1609. doi: 10.1097/00005537-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brack-mann DE. Auditory brainstem implants. Neurotherapeutics. 2008;5:128–136. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temple RH, Axon PR, Ramsden RT, Keles N, Deger K, Yücel E. Auditory rehabilitation in neurofibromatosis type 2: a case for cochlear implantation. J Laryngol Otol. 1999;113:161–163. doi: 10.1017/s0022215100143452. [DOI] [PubMed] [Google Scholar]
- 30.Tono T, Ushisako Y, Morimitsu T. Cochlear implantation in an intralabyrinthine acoustic neuroma patient after resection of an intracanalicular tumour. J Laryngol Otol. 1996;110:570–573. doi: 10.1017/s0022215100134292. [DOI] [PubMed] [Google Scholar]
- 31.Tran Ba Huy P, Kania R, Frachet B, Poncet C, Legac MS. Auditory rehabilitation with cochlear implantation in patients with neurofibromatosis type 2. Acta Otolaryngol. 2009;129:971–975. doi: 10.1080/00016480802510202. [DOI] [PubMed] [Google Scholar]
- 32.Trotter MI, Briggs RJ. Cochlear implantation in neurofibromatosis type 2 after radiation therapy. Otol Neurotol. 2010;31:216–219. doi: 10.1097/MAO.0b013e3181c348e7. [DOI] [PubMed] [Google Scholar]
- 33.UK Cochlear Implant Study Group Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults III: prospective evaluation of an actuarial approach to defining a criterion. Ear Hear. 2004;25:361–374. doi: 10.1097/01.aud.0000134551.13162.88. [DOI] [PubMed] [Google Scholar]
- 34.Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: cochlear versus auditory brainstem implantation. Audiol Neurootol. 2008;13:273–280. doi: 10.1159/000115437. [DOI] [PubMed] [Google Scholar]
- 35.Wong HK, Lahdenranta J, Kamoun WS, Chan AW, McClatchey AI, Plotkin SR, et al. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res. 2010;70:3483–3493. doi: 10.1158/0008-5472.CAN-09-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang I, Sughrue ME, Han SJ, Aranda D, Pitts LH, Cheung SW, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. Clinical article. J Neurosurg. 2010;112:851–859. doi: 10.3171/2009.8.JNS0985. [DOI] [PubMed] [Google Scholar]