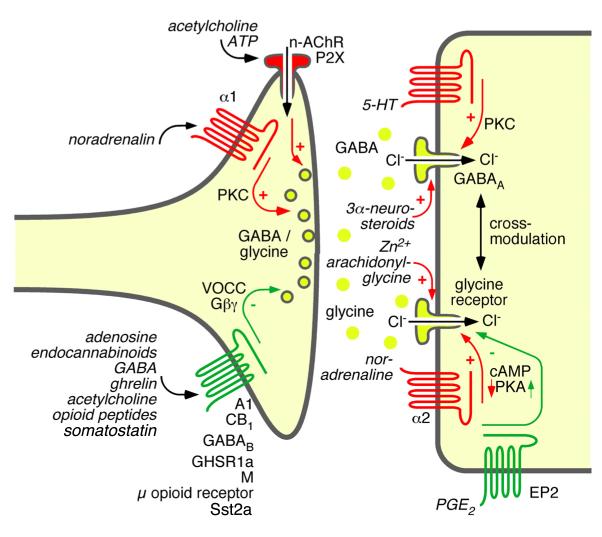
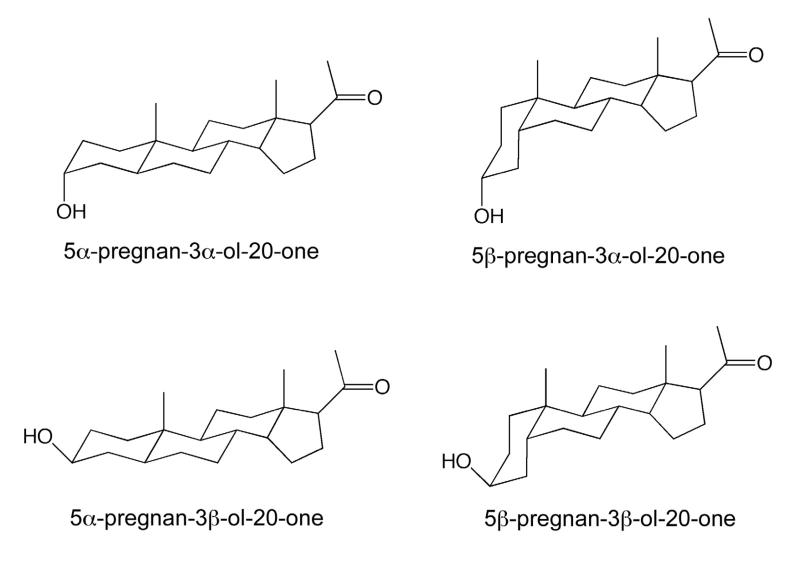
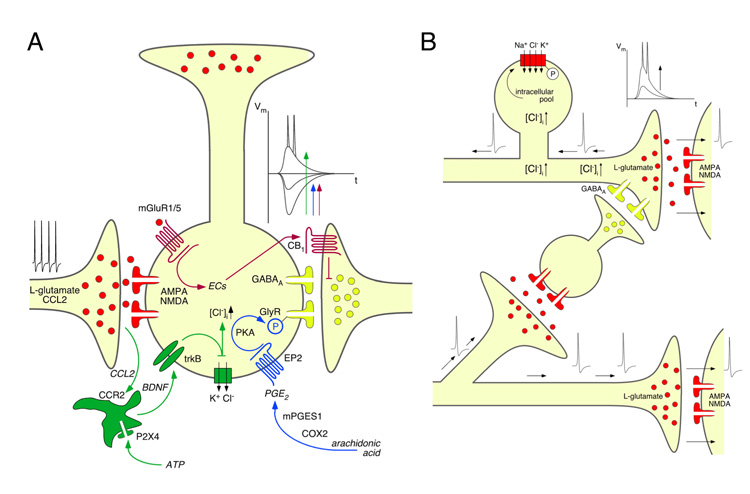
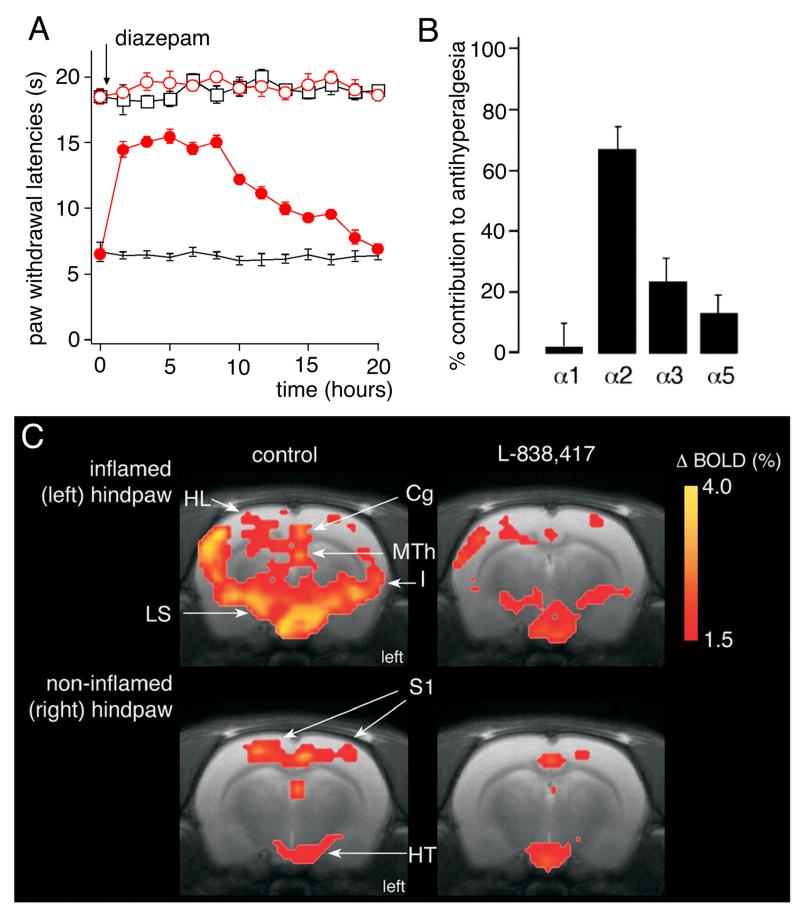
Abstract
The two amino acids γ-amino butyric acid (GABA) and glycine mediate fast inhibitory neurotransmission in different CNS areas and serve pivotal roles in the spinal sensory processing. Under healthy conditions, they limit the excitability of spinal terminals of primary sensory nerve fibers and of intrinsic dorsal horn neurons through pre- and postsynaptic mechanisms, and thereby facilitate the spatial and temporal discrimination of sensory stimuli. Removal of fast inhibition not only reduces the fidelity of normal sensory processing but also provokes symptoms very much reminiscent of pathological and chronic pain syndromes. This review summarizes our knowledge of the molecular bases of spinal inhibitory neurotransmission and its organization in dorsal horn sensory circuits. Particular emphasis is placed on the role and mechanisms of spinal inhibitory malfunction in inflammatory and neuropathic chronic pain syndromes.
I. Introduction
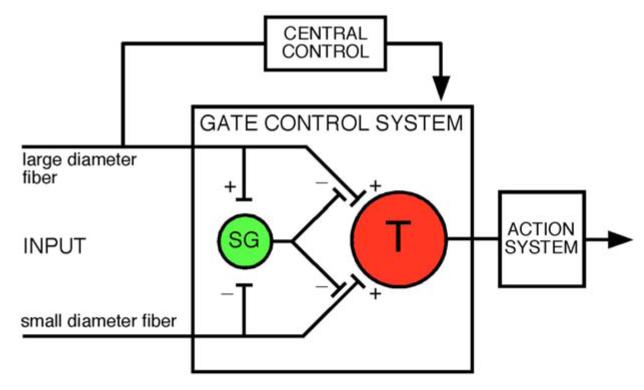
Proper processing of sensory information in the CNS depends critically on inhibitory synaptic transmission. The contribution of GABAergic and glycinergic neurons to this process is probably best studied in the retina where the neuronal circuits underlying lateral inhibition and feed-forward and feed-back inhibition have extensively been characterized as important mechanisms contributing to contrast enhancement and to increased spatial and temporal resolution. In the case of the somatosensory system, a similar computation occurs first at the level of the spinal dorsal horn (or in the trigeminal nucleus, the analogue structure in the brainstem). At these sites, somatosensory processing involves the precise interaction of GABAergic and glycinergic interneurons with other dorsal horn neurons and with the spinal terminals of primary sensory fibers through postsynaptic and presynaptic mechanisms. The function of inhibitory dorsal horn neurons however extends far beyond the physiological processing of somatosensory stimuli and has important implications also for the generation and maintenance of chronic pain states. An important role in nociceptive processing and in pain has been proposed more than 45 years ago by Melzack and Wall (248) in the gate control theory of pain (Figure 1). In the original model, signals arriving in the spinal dorsal horn from high threshold nociceptors and from low threshold mechanosensitive fibers were proposed to interact with local inhibitory interneurons to open or close the “pain gate”. Although some of the proposed synaptic connections were later shown to be incorrect, the pivotal role of inhibitory dorsal horn neurons in the spinal control of nociceptive signal propagation became firmly established especially when the introduction of selective blockers of GABAergic and glycinergic inhibition allowed direct proof of the contribution of the two fast inhibitory neurotransmitters to dorsal horn pain control. Today we know not only the structural, molecular, and neurochemical bases of this inhibition, but also that a loss of GABAergic and glycinergic synaptic transmission is an underlying mechanism of neuropathic and inflammatory pain. Work from several laboratories has discovered key elements of maladaptive plasticity in inhibitory dorsal horn circuits during different pathological pain states. Recent drug development programs have started to use this knowledge to develop new strategies aiming to restore proper synaptic inhibition in the spinal dorsal horn. Current basic research is focusing upon the precise components of neuronal circuits underlying spinal inhibitory pain control.
Figure 1.
Gate control theory of pain (modified from ref. 248). This model proposed that inhibitory interneurons (yellow) located in the substantia gelatinosa (SG) would determine whether nociceptive input from the periphery would be relayed through the spinal transmission system (red, T) to higher CNS areas where pain would be consciously perceived.
II. Molecular composition of fast inhibitory neurotransmitter receptors, synthesis, storage and re-uptake of GABA and glycine
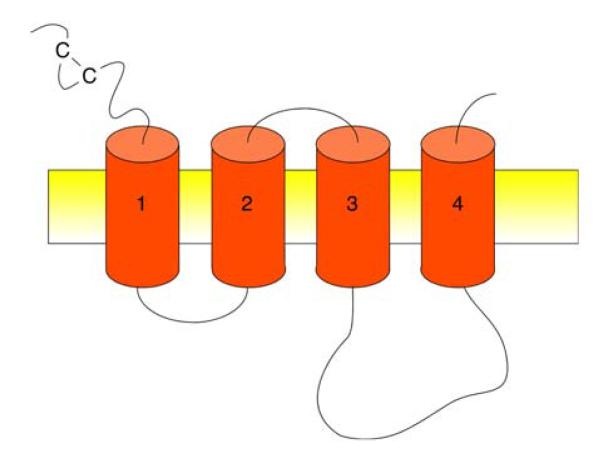
GABAA and glycine receptors belong to the cys loop superfamily of ligand-gated ion channels, which also includes nicotinic acetylcholine receptors and ionotropic serotonin (5-HT3) receptors (Figure 2). Members of this family are distinguished by the presence of an N-terminal extracellular domain containing a disulfide bridge between two cysteine residues. Both GABAA and inhibitory (strychnine-sensitive) glycine receptors are chloride permeable, pentameric, transmitter-gated ion channels with four transmembrane domains per subunit.
Figure 2.
Membrane topology of cys loop ion channels as proposed by Karlin and Akabas (186)
A. GABAA receptors
The molecular architecture of GABAA receptors has been the subject of extensive research for several decades and has been comprehensively reviewed elsewhere (e.g. ref. 29). Here, we briefly summarize the molecular composition of GABAA receptors. Most of the data discussed here are based on experiments performed in rodent tissue or receptors unless stated otherwise.
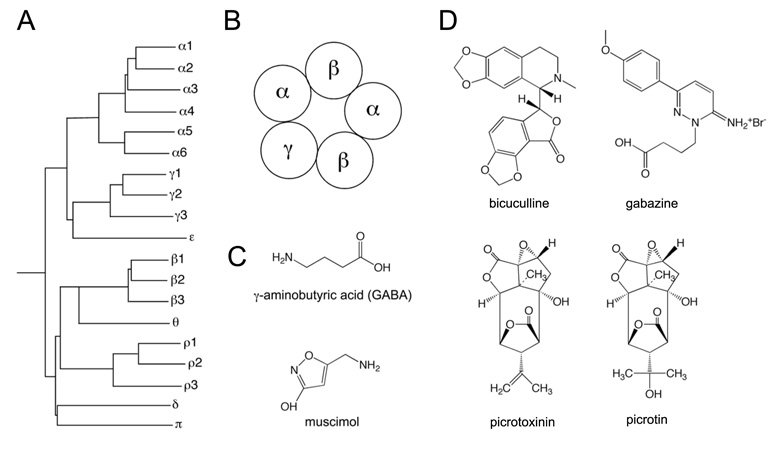
Mammalian GABAA receptors are assembled from a repertoire of 19 subunits designated: α1-α6, β1-β3, γ1-γ3, δ, ε, π, θ and ρ1-ρ3 (283) (Figure 3). “Additional” subunits, i.e. a β4 subunit and a γ4 subunit, have been described in chicken (31, 141). These subunits correspond to the mammalian θ and ε subunits, which are conversely absent in birds (346). If one were to apply an unrestricted combinatorial approach, these 19 subunits gave rise to thousands of subunit combinations. In reality however it is likely that no more than 50 different subunit combinations exist in relevant amounts (283). Despite this, GABAA receptors remain the most diverse family of neurotransmitter receptors in the mammalian nervous system. The majority of these receptors contain two α subunits, two β subunits and one γ subunit. They are typically clustered in membrane spots opposing GABAergic boutons, and activated by GABA released from presynaptic terminals. They have a lower affinity for GABA than the extrasynaptic receptors discussed below and mediate phasic inhibition. In the brain, most GABAA receptor isoforms are composed of α1, β2, and γ2 subunits. In the spinal cord, α2 and α3 are more abundant than α1 subunits (48), and β2 is replaced in the majority of spinal GABAA receptors by β3 (211, 396). The “wheel” arrangement of α, β and γ subunits in these channel complexes (32, 33) is shown in Figure 3B. The physiological activator GABA binds to an interface formed by the α and β subunits, which occurs twice in a typical GABAA receptor. In addition to the physiological activator GABA, many GABAA receptors bind endogenous neuromodulators, such as neurosteroids, and modulatory drugs, including benzodiazepines, barbiturates, alcohols, and anesthetics. The benzodiazepine binding site is generated by the γ2 subunit and by one neighboring α subunit (256). Receptors containing γ1 or γ3 subunits are also able to bind benzodiazepine-site agonists but with strongly reduced affinity (38). Only receptors containing at least one α1, α2, α3 or α5 subunit are potentiated by benzodiazepine-site agonists whereas α4 and α6 subunits are resistant to potentiation by classical benzodiazepines (257). Channel complexes containing α1/γ2 binding sites have been previously termed type I benzodiazepine receptors, whereas those possessing α2/γ2, α3/γ2, or α5/γ2 binding sites correspond to type II benzodiazepine receptors. Apart from contributing to the benzodiazepine binding site, the γ2 subunit is also required for the synaptic clustering of major GABAA receptor subtypes (102).
Figure 3.
GABAA receptor subunits and ligands (A) Dendrogram of mammalian GABAA receptors (modified from ref. 30). (B) wheel arrangement of the five subunits of a typical GABAA receptor containing α, β and γ subunits seen from the extracellular side. Data based on (32, 33). (C) Chemical structures of GABA and of the GABAA receptor agonist muscimol. (D) Chemical structures of GABAA receptor blockers.
A subset of GABAA receptors, which possess the δ or ε subunit in place of the γ subunit, are benzodiazepine-insensitive and are exclusively located at extrasynaptic sites. They typically exhibit a higher affinity for GABA than γ2 subunit containing receptors and mediate tonic inhibitory currents. These channels exhibit a highly restricted distribution within the CNS. The δ subunit is most abundant in the cerebellum but is also found in several forebrain areas including the dentate gyrus, the neostriatum, and certain cortical layers. The ε subunit is found in the spinal cord (287), the hypothalamus and several other hindbrain areas (260). The π and θ subunit are the least well characterized GABAA receptor subunits. Expression of the θ subunit overlaps with the ε subunit in several CNS areas (287), while the π subunit is generally restricted to peripheral tissues such as lung, thymus, prostate, uterus (147), pancreas (51), and respiratory epithelia (67).
Bicuculline is the most commonly used GABAA receptor antagonist. It blocks all ionotropic GABA receptors, with the exception of those containing ρ subunits, but also inhibits certain potassium channels (96, 193). Gabazine is another GABAA receptor antagonist, which has been reported to elicit preferential block of synaptic GABAA receptors (26, 235). A corresponding subunit specificity is not known.
The ρ subunits are probably the most peculiar GABA receptor subunits as they are the only ones capable of forming homopentameric channel assemblies. Furthermore, GABA receptors composed entirely of ρ subunits are relatively insensitive to bicuculline (and diazepam). These pharmacological characteristics match those of previously described bicuculline and baclofen insensitive GABA-evoked currents whose underlying receptors have been termed GABAC (93). The current IUPHAR nomenclature, recommends that this term be replaced by GABAA0r. In this case, the “0” denotes the absence of typical GABAA receptor pharmacology while the “r” indicates the exclusive arrangement of ρ subunits (282). The ρ subunits are most prevalent in the retina although ρ2 exhibits wide-spread expression throughout the brain (101, 392) and ρ1 is expressed in the spinal cord (423).
It should be noted that GABAA receptors may serve functions in the CNS which go beyond inhibitory neurotransmission. Such additional processes include adult hippocampal neurogenesis which is impaired in mice carrying deficits in γ2 subunit containing GABAA receptors (97). At present, evidence for adult neurogenesis in the spinal cord is lacking. Functional GABAA receptors are also expressed by spinal astrocytes (160, 288). Astrocytes do participate (indirectly) in sensory processing and do contribute to the generation of chronic pain states (reviewed in ref. 118). However a role of glial GABAA receptors in these processes is unknown.
B. Strychnine-sensitive glycine receptors
In addition to GABA, glycine is a second fast inhibitory neurotransmitter in the spinal cord, brainstem and a few other selected areas of the CNS including the retina. It activates a plasma membrane chloride channel that is selectively blocked by strychnine, an alkaloid from the Indian plant Strychnos nux vomica. It distinguishes inhibitory glycine receptors not only from GABA receptors but also from excitatory N-methyl-d-aspartate (NMDA) receptors, which also possess a glycine binding site. At these excitatory receptors, glycine (8, 39, 183) and d-serine (265) serve as endogenous co-agonists and are required, together with the principal excitatory neurotransmitter L-glutamate, for full channel activation.
Interestingly, the distribution of glycinergic terminals and postsynaptic glycine receptors does not correlate well at supraspinal levels. At several sites, most strikingly in the hippocampus, strychnine-sensitive glycine receptors are abundant while glycinergic terminals are very sparse. It is possible that other agonists such as taurine or β-alanine function as endogenous activators of glycine receptors at these sites (263, 401).
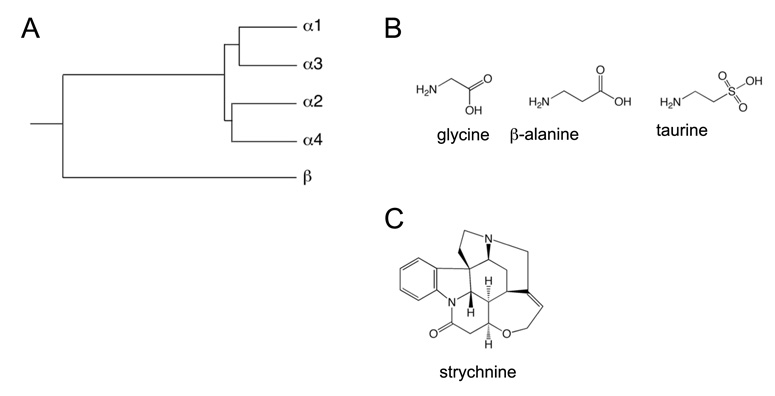
The subunit composition of strychnine-sensitive glycine receptors shows considerably less heterogeneity than that of GABAA receptors. Like GABAA receptors, glycine receptors are heteropentameric transmitter-gated cys-loop ion channels. However, unlike GABAA receptors, the repertoire of subunits that glycine receptors can draw from is limited to four α subunits, designated α1-α4, and one β subunit (Figure 4). In rodents, all five genes encode functional channel subunits, however, in humans the α4 subunit gene is a pseudogene due to the presence of a premature stop codon (346).
Figure 4.
Inhibitory (strychnine-sensitive) glycine receptor subunits and ligands. (A) Dendrogram of mammalian inhibitory glycine receptors. (B) Chemical structures of glycine and of other putative endogenous glycine receptor agonists β-alanine and taurine. (C) chemical structure of the glycine receptor antagonist strychnine.
Glycine receptor α subunits are capable of forming functional glycine-gated homomeric ion channels, but in the adult nervous system most inhibitory glycine receptors are heteromeric receptors formed by α and β subunits (207). Until recently, it was thought that heteromeric glycine receptors consisted of three α subunits and two β subunits. The α subunits were thought to provide the binding sites for glycine and strychnine whereas the primary function of the β subunits was thought to be the anchoring the receptor complex to the postsynaptic membrane via the scaffolding protein gephyrin (291, 334). However, recent evidence suggests that the β subunits also participate in the formation of the glycine binding site and that glycine receptors are composed of two α and three β subunits (132).
In most parts of the immature CNS, glycine receptors are probably homomeric α2 receptors, which become later replaced by α/β heteromers (357). In the adult nervous system, the α1 subunit is the most prevalent, while the α3 subunit is expressed in a spatially restricted manner (238). In certain areas, such as the retina, the α2 subunit continues to be expressed into adulthood (146).
Besides strychnine, picrotoxin, a mixture of picrotin and picrotoxinin, is sometimes used to pharmacologically characterize inhibitory glycine receptors. Picrotoxin cannot be used to distinguish between glycine and GABAA receptors but it can be used to separate homomeric glycine receptors, composed entirely of α subunits, from heteromeric receptors, containing both α and β subunits. This is due to the preferential block of glycine receptors lacking β subunits at low concentrations of the drug (304).
C. Synthesis, storage, and re-uptake of GABA and glycine
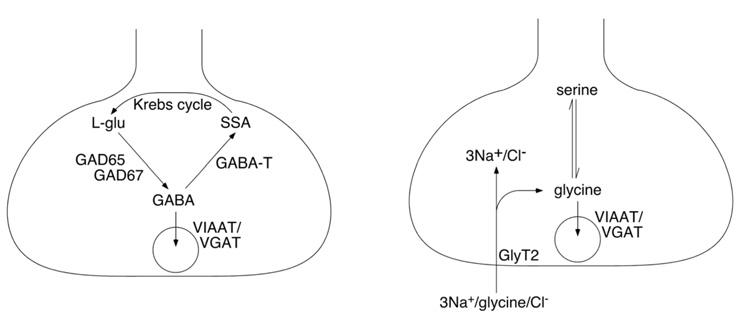
GABA is synthesized in GABAergic neurons from glutamic acid by the enzyme glutamic acid decarboxylase (GAD). Two isoforms of this protein have been identified, GAD65 and GAD67, which are encoded by the genes gad2 and gad1, respectively. Once synthesized, GABA is loaded into presynaptic storage vesicles via the vesicular GABA transporter (VGAT, gene slc32a1; ref. 245) also called vesicular inhibitory amino acid transporter (VIAAT; ref. 329). VGAT/VIAAT is also responsible for glycine uptake into synaptic vesicles (Figure 5A). Combined expression of GAD65 or GAD67 with VGAT/VIAAT is likely to be sufficient to make a neuron GABAergic. GAD65 and GAD67 are frequently used as marker proteins or marker genes for GABAergic neurons (e.g. ref. 359).
Figure 5.
Key elements of GABAergic (A) and glycinergic (B) presynaptic terminals. Abbreviations: GABA-T, GABA transaminase; SSA, succinic semialdehyde.
After synaptic release, GABA is taken up by plasma membrane transporters. To date, four GABA transporters have been cloned: GAT1 (slc6a1), GAT2 (slc6a13), GAT3 (slc6a11) and BGT, for betain-GABA transporter (slc6a12) (for a recent review see ref. 103). The specific contribution of these transporters to the termination of GABAergic inhibitory postsynaptic currents (IPSCs), recycling of GABA, or to the control of ambient extracellular GABA concentrations has not yet been resolved. However, experiments using GAT1 deficient mice show increases in the amplitude of tonic GABAA receptor-mediated currents in the hippocampus (178), cerebral cortex (50), and cerebellum (74), as well as prolonged evoked GABAergic inhibitory postsynaptic currents (IPSCs) in cortical neurons (50).
Glycine, the other fast inhibitory neurotransmitter, is transported into the presynaptic vesicles by the same vesicular amino acid transporter VGAT/VIAAT. However, while GABA is specifically synthesized in GABAergic neurons, glycine is a ubiquitous proteinogenic amino acid, which raises the question why are not all GAD and VGAT/VIAAT positive neurons also glycinergic. VGAT/VIAAT has however a rather low affinity for glycine (in the range of 25 mM; ref. 245), which renders glycine uptake into presynaptic vesicles very inefficient unless glycine is enriched intracellularly through specific mechanisms. This specific accumulation is accomplished through the expression of the plasma membrane glycine transporter GlyT2 in glycinergic neurons (Figure 5B). The co-expression of GlyT2 and VGAT/VIAAT renders neurons glycinergic (18). In most parts of the CNS, with the possible exception of retinal amacrine cells, expression of GlyT2 is also a necessary prerequisite for glycinergic neurotransmission. GlyT2 protein and its encoding gene slc6a5 are therefore reliable markers for glycinergic neurons (302, 419).
The GlyT2 protein is predominantly located in the axon terminals of glycinergic neurons (351) and hence in glycinergic termination areas (414). The GlyT2 mRNA is found in the spinal cord, brainstem and cerebellum and parts of CNS grey matter where the somata of glycinergic neurons are abundant (229, 415). Mice deficient in GlyT2 exhibit a hyperekplexic phenotype characterized by an exaggerated startle response, tremor, and elevated muscle tone (125), and therefore show a hypoglycinergic phenotype consistent with the requirement of GlyT2 for the loading of glycine into presynaptic terminals. This deficit results in death of GlyT2 knock-out mice about 10 days after birth.
Unlike expression of GlyT2, expression of the second plasma membrane glycine transporter (GlyT1; gene slc6a9) is not restricted to glycinergic neurons or glycinergic innervation territories. Instead it is expressed widely throughout the CNS including in forebrain regions such as the hippocampus and the olfactory bulb (415), the thalamus, and the cerebellum (419). It has been suggested that GlyT1 is only expressed in glia cells (4, 414, 415), however recent work has clearly established that GlyT1 is also expressed in neurons of different CNS areas including of the spinal cord (85, 104, 409). Neuronal GlyT1 appears to be enriched at pre- and postsynaptic sites at glutamatergic synapses (85), where it may regulate the ambient concentration of glycine at NMDA receptors (409). Gene deletion studies suggest a role of GlyT1 in both inhibitory glycinergic and excitatory NMDA receptor-mediated neurotransmission. It has been shown that GlyT1 contributes to the termination of glycinergic IPSCs in hypoglossal motoneurons through uptake of glycine after synaptic release (124). Accordingly, GlyT1 knock-out mice show reduced muscle tone and altered respiratory rhythms and die shortly after birth (124). Evidence for the involvement of GlyT1 in NMDA receptor activation comes from studies performed in hemizygous GlyT1+/− mice. These mice exhibit increased NMDA receptor activation in the hippocampus and perform better in learning and memory tasks (376).
GlyT1 and GlyT2 also differ in the stoichiometry of ion transport. This difference is likely to have significant implications on their function. GlyT1 has a stoichiometry of 2 Na+/Cl−/glycine (all transported in the same direction), while GlyT2 has a stoichiometry of 3 Na+/Cl−/glycine (324). Consequently, GlyT2 always transports glycine inwardly whereas GlyT1 may change direction and secrete glycine under conditions of low extracellular glycine, high intracellular Na+, or depolarization (324). One might thus speculate that GlyT1 could supply glycine to NMDA receptors under certain conditions.
While the gene deletion studies discussed above provided evidence for very distinct functions of GlyT1 and GlyT2, studies employing pharmacological inhibitors have produced less dichotomous results. For example, electrophysiological experiments in lamina X of the rat spinal cord using Org 24598 and Org 25543, to block GlyT1 and GlyT2 respectively, showed that both transporters shape the decay phase of evoked IPSCs, induce tonic glycine receptor currents, and facilitate NMDA receptor activation (49).
III. Laminar organization of the spinal cord
In this chapter we briefly summarize the anatomical organization of the spinal grey matter and the innervation pattern of the spinal cord by sensory fibers (for a more comprehensive overview of this topic see ref. 364).
Fibers conveying sensory information from peripheral tissues to the spinal cord originate from neurons located in the dorsal root ganglia (DRGs), which are situated adjacent to the spinal cord on either side. These neurons send their axons both to the peripheral tissue and to the spinal dorsal horn, which they enter through the dorsal roots. The afferent fibers are usually classified according to their conduction velocity, diameter, extent of myelination and by their responsiveness to sensory stimuli of a different nature (thermal, mechanical, chemical) or intensity (noxious or innocuous). Aα and Aβ fibers have the largest diameter, are thickly myelinated, and conduct action potentials with the highest velocity. The majority of these neurons are activated by low intensity (innocuous) mechanical stimuli and do not encode stimulus intensity at least not in the noxious range. Aδ fibers possess axons with smaller diameters, conduct more slowly, are thinly myelinated, and respond to noxious thermal and intense mechanical stimuli. C fibers are the thinnest fibers, are unmyelinated, and have the lowest conduction velocity. The vast majority of C fibers are activated solely by noxious thermal or mechanical stimuli, however, some subsets also encode innocuous thermal (cool or warm) information or are activated by low intensity mechanical stimuli. C fiber nociceptors are also notable for their sensitivity to the transient receptor potential vanilloid 1 (TRPV1) ion channel agonist, capsaicin, the pungent compound found in hot peppers. Nociceptive C fibers can be further subdivided into peptidergic and non-peptidergic classes. Peptidergic C fibers express calcitonin gene related peptide (CGRP) and, in most cases, the neuropeptide Substance P while non-peptidergic C fibers bind the Griffonia simplicifolia isolectin B4. It has recently been suggested that behavioral responses to noxious heat are exclusively mediated by TRPV1 positive peptidergic nociceptors whereas responses to noxious mechanical stimuli are governed solely by non-peptidergic nociceptors expressing the sensory neuron-specific G protein-coupled receptor mrgprd (61, 332). However, this matter remains controversial and awaits further confirmation (3). Recently, a distinct population of C fibers with low mechanical activation thresholds has been described which is characterized by the expression of the low abundance type 3 vesicular glutamate transporter (VGluT3; gene slc17A8). These fibers appear to play a major role in the generation mechanical allodynia following inflammation, nerve injury, or trauma. Their termination area is lamina I and the innermost layer of lamina II (339). It should be added that in general, all three fiber classes include both nociceptors and low threshold mechanoreceptors although to very different degrees.
On a gross scale, the spinal cord can be divided into a dorsal horn, the sensory part, and a ventral horn, mainly harboring motor control circuits. The superficial dorsal horn is mainly innervated by nociceptive fibers, whereas fibers from low threshold mechanoreceptors are largely lacking from this area. By contrast, the deep dorsal horn is innervated mainly by low threshold mechanoreceptors. The vast majority of neurons throughout the dorsal horn respond to both noxious and innocuous stimuli. They are therefore called wide dynamic range neurons. Projection neurons in lamina I are an exception. Under physiological conditions they are only excited by noxious stimuli. The excitation of superficial dorsal horn neurons by innocuous stimulation can be explain by the extension of their dendritic trees into the deep dorsal horn or by polysynaptic connections formed by interneurons connecting the deep with the superficial dorsal horn.
According to Rexed (316), the grey matter can be further subdivided into 10 laminae (Figure 6A). The original work was initially carried out in the cat but the laminar organization is also found in rats and mice. Lamina I, also known as the marginal zone, is the thinnest outermost layer of the dorsal horn and is only a few cell diameters thick. It contains segmental excitatory and inhibitory interneurons and projection neurons responsible for conveying information from the spinal cord to supraspinal levels including the lateral parabrachial area, the periaqueductal grey and the thalamus. Estimates of the number of projection neurons range between 5% and more than 9% of all lamina I neurons in the lumbar segments of the rat (349, 413). Additional projection areas have been discovered more recently (120) and studies using retrograde labeling may hence have missed some projection neurons (11). Projection neurons in lamina I receive monosynaptic input from Aδ and C fiber nociceptors (86, 169), as well as input from excitatory and inhibitory segmental interneurons (86, 293, 308) and from descending serotonergic fiber tracts (298). Lamina I projection neurons are normally not activated by non-nociceptive input and are therefore sometimes referred to as nociceptive-specific. Many, but not all of the lamina I projection neurons express the neurokinin 1 (NK1) receptor activated by Substance P (242, 293, 365, 367). Ablation of NK1 receptor-positive lamina I neurons using Substance P-conjugated saporin has demonstrated that these neurons serve a pivotal role in acute and chronic hyperalgesia (240, 275). It should be added that NK1 receptor expression is not entirely specific for lamina I projections neurons as some interneurons also express NK1 receptors albeit in smaller amounts (10). Lamina II is located directly below lamina I and is sometimes referred to as the substantia gelatinosa due to its transparent appearance. This is a consequence of the absence of innervation by myelinated fibers. In electrophysiological studies, lamina I and lamina II are often collectively termed the ‘superficial dorsal horn’ (occasionally together with lamina III). Lamina II is densely innervated by both peptidergic and non-peptidergic C fibers. Peptidergic C fibers terminate predominately at the outer region of lamina II (lamina IIo), while non-peptidergic fibers terminate at the inner region (lamina IIi) close to the border with lamina III. Lamina II mainly contains glutamatergic excitatory interneurons and GABAergic inhibitory interneurons. The cell bodies of glycinergic neurons are less frequent in lamina II. A specific subtype of excitatory glutamatergic neurons, which express protein kinase Cγ, is located at the border of lamina IIi and lamina III (237, 264, 294).
Figure 6.
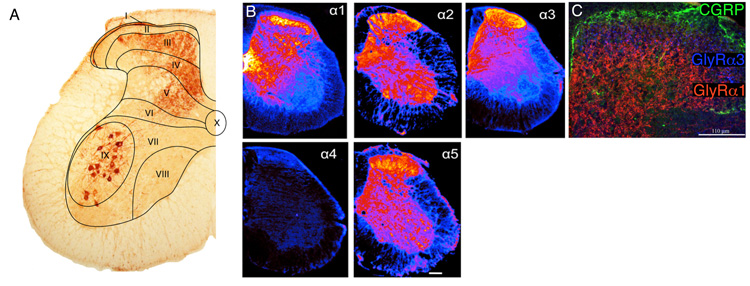
Laminar organization of the spinal cord and distribution of inhibitory neurotransmitter receptors. (A) Spinal laminae illustrated in a coronal section of the lumbar spinal cord taken from a mouse whose sciatic nerve has been injected with cholera toxin B subunit in order to label axons and terminals of myelinated sensory nerve fibers and motoneurons (curtsey of Dres. Jolly Paul and Jean-Marc Fritschy). (B) Distribution of GABAA receptor subunits is shown as pseudocolor images. Highest density, yellow, low density, blue (modified from ref. (417). (C) Distribution of glycine receptor subunits GlyRα1 and GlyRα3 in the spinal dorsal horn (modified from ref. 140). Counterstaining against calcitonin gene related peptide, which marks lamina II outer.
The deeper laminae (III and VI) are innervated mainly by myelinated Aβ and Aδ fibers (Figure 6A) but also receive significant input from C fiber nociceptors (88, 262). Projection neurons located in the deep dorsal horn typically respond to both nociceptive and non-nociceptive input and therefore belong to the class of wide-dynamic range (WDR) neurons. Inhibitory interneurons in this region of the dorsal horn utilize both GABA and glycine in most cases. Laminae VII and VIII cover the area of the ventral horn not populated by motoneurons (Figure 6A). These laminae contain, among others, commissural interneurons projecting to the contralateral ventral horn. Lamina IX contains motoneurons innervating skeletal muscle. Lamina X, also known as area X, covers the grey matter surrounding the central canal and is also involved in sensory function. Area X receives input from C fibers innervating the viscera and contains neurons which project to the brain stem and thalamus.
IV. Laminar distribution of GABAA and glycine receptors in the spinal dorsal horn
The distribution of GABAA and glycine receptors within the CNS was studied extensively during the late 1980s and early 1990s, when the different subunit genes were cloned (116, 210, 396, 397). Most of the results from this period remain valid today. Here we briefly review the expression pattern of these receptors in the spinal cord and discuss them in the context of more recent observations.
A. GABAA Receptors
The expression pattern of the major GABAA receptor isoforms in the spinal cord has been studied at the protein and mRNA level mainly in mice and rats. The protein distribution in the rat has been analyzed in detail by Bohlhalter et al. (48). This study showed that the α3, β2/3 (β2 and β3 could not be distinguished with the antibody used in this study), and γ2 subunits exhibit a uniform distribution throughout the various laminae in the adult rat spinal cord. Other subunits exhibit a more lamina-specific localization. α2 subunits are most abundant in the superficial dorsal horn and in motoneurons. The α1 and α5 subunits are most densely expressed in laminae III-VIII, while the superficial dorsal horn (lamina I/II) is largely devoid of these subunits. A virtually identical pattern has also been recently described in the mouse (ref. 196 and Figure 6B). The distribution of GABAA receptors has also been assessed in the human hindbrain and most rostral segments of the cervical spinal cord (386). The results of this study are mostly in agreement with the data obtained from rodents with one possible exception. The authors describe strong expression of α1 subunits in lamina II of the spinal cord (Table 3 in ref. 386). However, closer inspection of the data (see Figure 5, Panel A and B, ref. 386) indicates that the area of α1 immunoreactivity is more likely to be located in lamina III rather than lamina II.
Several other studies have addressed the distribution of GABAA receptor subunits using in situ hybridization both in adult rats (290, 396) or during development (211, 232). These studies have largely focused on the four benzodiazepine-sensitive α subunits (α1, α2, α3, and α5), the β1-3 subunits and the γ1-3 subunits. In the adult spinal cord, α2 and α3 were the most abundant α subunit mRNAs. Spinal GABAA receptors hence resemble mainly type II benzodiazepine receptors. The α2 subunit mRNA was particularly concentrated in ventral horn motoneurons, while the α3 subunit mRNA was expressed to an equal degree in both ventral and dorsal horns (290, 396). In situ hybridization also showed that, in contrast to the brain, the β3 subunit is much more abundant than the β2 subunit in the spinal cord (211, 396).
Strong in situ hybridization signals for α2, β3 and γ2 subunits are also observed in DRG neurons of adult rats (232, 290). The α2 subunit mRNA is strongly expressed in large diameter DRG neurons and to a lesser degree in small diameter cells (290). These observations correlate well with electrophysiological studies which have found that large diameter capsaicin-insensitive DRG neurons exhibit bigger GABAergic membrane currents than small diameter capsaicin-sensitive cells (393). Since most morphological studies have failed to detect GABAA receptor protein in the soma of DRG neurons, it is likely that most of the protein is transported into the spinal terminals of these cells (48, 290). Indeed, the subunit pattern in the termination area of primary sensory fibers in the dorsal horn largely mirrors the expression of subunit mRNA in DRG neurons. A recent study using confocal microscopy to evaluate the co-localization of GABAA receptor subunits with markers for different classes of afferent sensory fiber has revealed that α2 (and α3) subunits are expressed on dorsal horn axons and/or axon terminals of nociceptive (CGRP- and IB4-positive) and non-nociceptive afferents (i.e. those positive for the vesicular glutamate transporter VGluT1) (398). However, recent electrophysiological experiments indicate that a significant portion of the dorsal horn α2 subunits is still located on intrinsic dorsal horn neurons (196).
Other groups have addressed the issue of GABAA receptor subunit expression in cultured embryonic and adult human DRGs using reverse-transcriptase PCR (RT-PCR) (234). The results of this study confirmed that the α2 and β3 subunits were the most consistently expressed subunits both in embryonic and adult DRG neurons. Additional subunits detected in adult human DRG neurons included α3, α5, γ3, ε, θ, ρ1, and β2. ρ1 GABA receptor subunit protein is largely concentrated in the superficial layers of the mouse dorsal horn and also found in the cell bodies of most mouse DRG neurons (423).
A few studies have addressed developmental regulation of GABAA receptor subunits. In rat DRG neurons, a shift occurs from α3 and α5 subunits towards higher expression of α2 subunits (232). In the rat spinal cord, mRNAs encoding the α4, γ1, γ3 and δ subunits are expressed in a spatially discrete manner during development (232), while α6 mRNA is absent from the spinal cord and DRGs throughout development (211, 232).
B. Inhibitory glycine receptors
Whereas GABAA receptors are expressed throughout the mammalian CNS, glycine receptors show a more restricted distribution. A high density of glycine receptors are found in both the ventral and the dorsal horn of the spinal cord, in various nuclei of the brain stem, including the trigeminal nucleus, and the cerebellum. As mentioned previously, immature glycine receptors generally assemble as α2 homomeric channels, however, by adulthood most glycine receptors comprise α1/β heteromers. Channel complexes containing the α3 subunit are found in the spinal cord and also in hippocampus. In the spinal cord, α3 subunits are concentrated in the superficial layers of the dorsal horn where nociceptive primary afferent fibers terminate (Figure 6C) (140).
The scaffolding protein gephyrin is frequently used as a postsynaptic marker of inhibitory synapses in the CNS including the spinal dorsal horn (373). Gephyrin was initially discovered by co-immunoprecipitation with glycine receptors (42, 291, 334) but has since also been found in GABAergic postsynaptic structures lacking glycine receptors (370). It is involved in the clustering of both glycine and GABAA receptors (58, 102, 198, 331).
V. Distribution of presynaptic elements of GABAergic and glycinergic neurotransmission in the spinal dorsal horn
The spinal dorsal horn receives inhibitory GABAergic and glycinergic input from local interneurons and through fiber tracts descending from supraspinal areas. The distribution of local inhibitory interneurons has been studied at the transmitter level, using antibodies raised against GABA and glycine, and at the mRNA and protein level using GAD65, GAD67 and GlyT2 as marker proteins. More recently transgenic mice expressing EGFP under the transcriptional control of the aforementioned genes became frequently used and very valuable tools.
Immunohistological staining of GAD65, GAD67 and GlyT2 has provided information about the regions innervated by GABAergic and glycinergic terminals, since these proteins are preferentially located in presynaptic boutons (28, 247, 351). These studies have shown that GABAergic terminals are found throughout the spinal grey matter. In an effort to determine the relative abundance of GAD65 and GAD67 in the spinal cord, Mackie et. al. (233) demonstrated that the majority of boutons in the dorsal horn exhibit immunoreactivity to both isoforms. However, certain boutons exhibited stronger staining either for GAD65 or GAD67. At sensory-motor synapses in the ventral horn GAD65 is exclusively associated with terminals presynaptic to primary afferents. GAD67 is associated in addition with GABAergic terminals that form synapses with dendrites and somata (41). Many of the GABAergic boutons also express GlyT2 in addition to GAD, with no difference in association with either GAD65 or GAD67 (233).
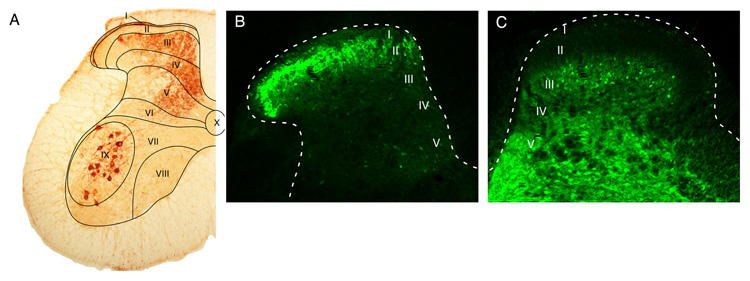
The localization of GABAergic neuronal cell bodies became for the first time possible, when it was discovered that GAD proteins become retained in the cell body by the treatment of animals with the colchicine, a blocker of axoplasmic transport. This approach has revealed that GABAergic neurons are distributed throughout the spinal grey matter (27, 168). Later, antibodies raised against GABA and glycine became available which allowed the detection of these amino acids in the terminals as well as the somata and dendrites of GABAergic and glycinergic neurons. The latter approach enabled the reliable identification of GABAergic (155, 353, 366) and glycinergic cell bodies (284, 300) without the need for colchicine pretreatment. Importantly, glycine immunoreactivity is restricted to glycinergic neurons, despite the fact that it is a ubiquitous proteinogenic amino acid. It is likely that the concentration of glycine in non-glycinergic cells is too low to produce significant staining. In general, these studies demonstrate an enrichment of GABAergic somata in the superficial layers (I-III) of the dorsal horn. These findings have since been confirmed by in situ hybridization experiments (231, 348) and studies employing EGFP reporter mice (359) (Figure 7B).
Figure 7.
Distribution of GABAergic and glycinergic neurons in the dorsal horn. (A) Dorsal horn laminae (same as figure 6A). (B) Distribution of GABAergic neurons visualized as EGFP expression driven by the GAD67 promoter, and (C) distribution of glycinergic neurons visualized through EGFP expression driven by the GlyT2 promoter.
Glycine immunoreactive neurons were found throughout the spinal grey matter although they are concentrated in the deeper laminae of the dorsal horn (lamina III and deeper) (361). Comparative analyses of GABA-positive and glycine-positive neurons revealed that approximately 30-50% of superficial dorsal horn neurons are GABAergic and about half of these are also immunoreactive for glycine (350, 369, 372). These observations have largely been confirmed by in-situ hybridization studies (162) and in mice expressing EGFP in glycinergic neurons (419, 420) (Figure 7C).
VI. Corelease of GABA and glycine in the spinal dorsal horn
As already discussed in Chapters III and IV, elements of GABAergic and glycinergic neurotransmission exhibit an overlapping distribution in the spinal cord. Most inhibitory postsynaptic responses recorded in the spinal cord exhibit in fact two kinetically distinct components: a glycinergic, strychnine-sensitive component with fast decay kinetics and a GABAergic, bicuculline-sensitive component with slower kinetics (24, 412). These observations indicate that many dorsal horn neurons receive both GABAergic and glycinergic synaptic input. The nature of this mixed input goes beyond the simple targeting of the same neuron by GABAergic and glycinergic synapses. Ample evidence, obtained using a variety of technical approaches, indicates that GABA and glycine are co-released from the same presynaptic vesicles (47, 77, 106, 372, 373). Jonas et al. (184) provided the first direct demonstration following the analysis of unitary synaptic currents in spinal motoneurons. Similar results have since been obtained from lamina I dorsal horn neurons (71) and in neonatal area X neurons (340). In some neurons, such as those in lamina I, a dual component was not apparent at rest but could be unmasked following application of flunitrazepam, a benzodiazepine site agonist which increases activation of GABAA receptors (71). Based on the findings discussed in Chapter III, the underlying molecular requirement for co-release of both transmitters is most likely to be the co-expression of at least one isoform of GAD with the neuronal glycine transporter GlyT2 and VGAT/VIAAT.
Although the co-release of glycine and GABA is now well established, the physiological function is clear. Co-release of GABA and glycine from the same presynaptic vesicle does not necessarily mean that both transmitters contribute to postsynaptic inhibition. Initial studies performed at room temperature did not provide a compelling answer since at unphysiologically low temperatures neurotransmitter transporter are not fully active and transmitter molecules may hence diffuse out of the synaptic cleft to activate extrasynaptic receptors. It was, therefore, important to investigate whether co-transmission occurs at (near) physiological temperature. In the case of the dorsal horn, this was done in a recent study by analyzing the kinetics of mIPSCs recorded at 35°C (252). Under these conditions, approximately 10% of mIPSCs continued to show kinetic properties consistent with co-activation of GABAA and glycine receptors. There is evidence to suggest that functional mixed GABAergic/glycinergic unitary events are more frequent during early postnatal development than in adulthood (172). Work by Chery and De Koninck (70, 71) has suggested that, at least in lamina I in adult rats, glycine serves as the major fast inhibitory neurotransmitter. Further evidence supporting a dominant role for glycine in phasic and tonic inhibition of superficial dorsal horn neurons has also been reported by Michell et al. (252), but see also Chapter XI. These authors found that neurons receiving a dominant glycinergic input were more abundant than those receiving stronger GABAergic input. In addition, the latter study detected a tonically active glycinergic conductance but no baseline current mediated by GABAA receptors. In adults, mixed events could however still be unmasked using benzodiazepine agonists (192) suggesting that the developmental specialization occurs at the level of the postsynapse rather than at the presynapse. However, regional differences in the mechanism may still exist (271).
Given that glycine apparently mediates the bulk of fast synaptic inhibition, the question arises what function is served by the co-released GABA. Chery and De Koninck (70, 71) suggested that GABA primarily acts via extrasynaptic GABAA and via GABAB receptors. The function of the GABAergic component may be to provide a feedback signal to the presynaptic release machinery. Indeed, at inhibitory synapses in lamina I, where co-release results solely in glycinergic postsynaptic responses, application of GABAB receptor antagonists increases glycinergic IPSCs. This suggests that the primary function of co-released GABA may provide a negative feedback signal to the presynaptic terminal (70). Other studies have provided evidence for other forms of cross-talk between the two transmitter systems. Yevenes et al. (411) have shown that activation of GABAB receptors through G protein βγ subunits potentiates glycine receptor currents in spinal cord neurons. Studies carried out in the medial nucleus of the trapezoid body (MNTB) of the auditory system revealed that the co-release of GABA dramatically shortens the kinetics of glycine receptor currents (225). In recombinantly expressed glycine receptors, the extremely fast kinetics of glycinergic IPSCs in this cell type could only be replicated when GABA was co-applied together with glycine. There is also evidence for an interaction in the opposite direction. Activation of glycine receptors in lamina X cells decreased the amplitude and accelerated the rate of desensitization of GABA-induced currents through activation of phosphatase 2B (215).
VII. Morphologically defined subtypes of dorsal horn interneurons
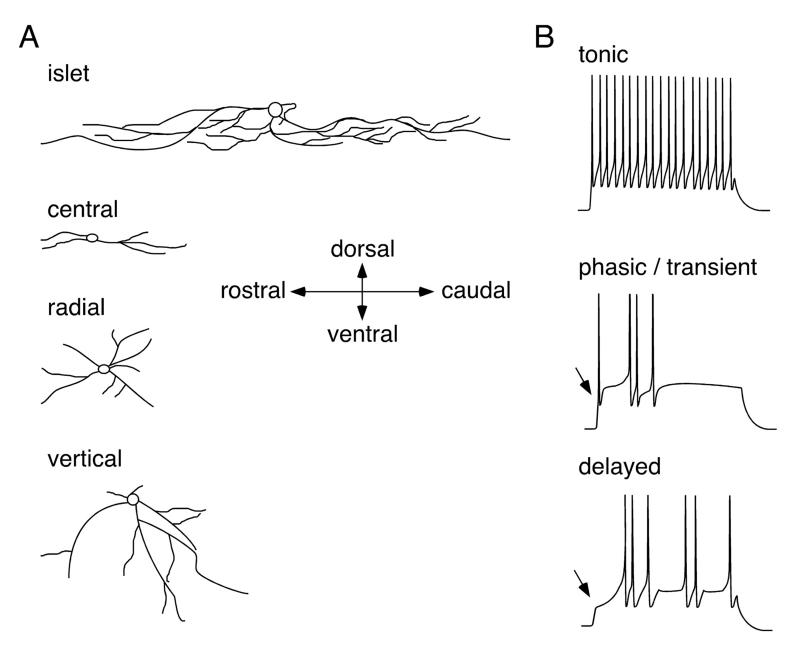
Inhibitory dorsal horn neurons exhibit morphological and biophysical properties, which can be used to distinguish them from other types of neurons with a reasonable degree of reliability (Figure 8). Several recent studies have identified four neuronal cell types in lamina II based on the morphology of their dendritic trees (131, 150, 303). These cell types are termed islet, central, radial, and vertical neurons (150, 406). A number of publications define additional cell types such as antenna (243) or medial-lateral cells (131). Some of these cell types, in particular islet cells, also exhibit biophysical characteristics, such as firing patterns, and physiological features including excitation by certain subclasses of primary afferent fibers (compare Figure 9), which distinguish them from other cell types. However, in the other cell types morphological characteristics correlate less well with functional properties. Furthermore, a significant number of dorsal horn neurons remain unclassified due to their incongruous morphology. For the purpose of this review we will focus on islet, central, radial, and vertical cells.
Figure 8.
Subtypes of dorsal horn interneurons defined by the morphology of their dendritic trees (A) and their firing patterns (B). An islet cell-like morphology and tonic action potential firing are good predictors of an inhibitory (GABAergic or glycinergic) phenotype.
A. Islet cells
Islet cells were first described by Gobel in 1975 in the substantia gelatinosa of the cat trigeminal nucleus (123). Their somata are found mainly in lamina IIi, but cells with similar morphology are also found in lamina III. The dendritic trees of islet cells predominantly extend in a rostrocaudal direction (~450 μm in hamsters) with smaller extensions (~60 μm) in the mediolateral and dorsoventral directions (131). Their axons are restricted to lamina IIi. The vast majority of islet cells are GABAergic, i.e. their activation elicits monosynaptic bicuculline-sensitive IPSCs in postsynaptic cells (226, 243). Accordingly, they are labeled by antisera raised against GABA or glycine (301). Islet cells exhibit a depolarized resting membrane potential of about −48 mV and display a tonic firing pattern, i.e. repeated action potential firing at relatively constant intervals throughout the duration of the depolarization (131). Virtually all islet cells receive monosynaptic input from comparatively large diameter, fast conducting C fibers. This C fiber input is of larger amplitude than that of other superficial dorsal horn neurons.
B. Central cells
The cell bodies of central cells are found in both lamina IIi and IIo. Their dendritic trees lie mainly in lamina IIi, where they project in the rostrocaudal direction but do not extend as far as those of islet cells (~200-300 μm). However, the mediolateral and dorsoventral dimensions of their dendritic trees are comparable to those of islet cells. Unlike islet cells, central neurons can be either inhibitory or excitatory. Inhibitory (GABAergic) central cells exhibit a tonic firing pattern (131), whereas excitatory (glutamatergic) ones fire transiently. The latter can be further subdivided into those exhibiting a fast inactivating A type potassium current and those lacking this type of current (131).
The morphological and electrophysiological properties of GABAergic central cells have also been studied in transgenic mice expressing GFP under the prion protein (prp) promoter. In these mice, GFP is specifically expressed in tonically firing GABAergic neurons in lamina IIi of the dorsal horn (136, 137). Dorsal root stimulation evokes monosynaptic excitatory input in tonic GABAergic central cells through relatively fast conducting C fibers and possibly also through Aδ fibers (131).
C. Radial and vertical cells
Radial cells are so named because their dendrites “radiate” in all directions. It is likely that previously described “star shaped” cells (44) and “stellate” cells (335) in the rat and human dorsal horn respectively, are also radial cells. Upon a depolarizing current injection, radial neurons fire action potentials only after a short delay during which the membrane potential slowly depolarizes (131). Most radial neurons are glutamatergic (408), however, GABAergic cells have also been reported (243).
Vertical cells resemble the partially stalked cells previously described by Gobel (123). Most of these neurons are located in lamina IIo. Their dendritic trees extend either ventrally or dorsally but not in both directions at the same time. The majority of vertical neurons, like radial cells, are excitatory, although exceptions to this rule have also been reported (227, 408). In mice expressing GFP under the control of the GAD67 (gad1) promoter, four out of 29 GFP labeled neurons exhibit vertical cell morphology (150). Since these mice express GAD67-GFP as a conventional transgene (281), it is possible that this is due to ectopic GFP expression in some cells. However, Maxwell et al. (243) also described a GABAergic phenotype in two out of six randomly selected lamina II neurons exhibiting vertical cell morphology.
D. Glycinergic neurons
The physiological properties of glycinergic neurons have been analyzed using bacterial artificial chromosome (BAC) transgenic mice expressing EGFP under the transcriptional control of the GlyT2 promoter (419). The discrete pattern of EGFP expression in these mice allows glycinergic cells to be distinguished from other types of dorsal horn neuron. Glycinergic neurons in the superficial dorsal horn show a slightly depolarized membrane potential compared to non-glycinergic cells and a slightly higher membrane input resistance. The majority of these cells display a tonic firing pattern, but single spiking activity and phasic and delayed firing patterns are also apparent. At least some glycinergic cells in lamina III show an islet cell like morphology (301).
E. Outlook
The studies discussed above indicate that islet cell morphology and tonic firing patterns are reasonable predictors of an inhibitory phenotype among lamina II neurons. However, non-islet cells and cells with non-tonic firing patterns can also be GABAergic (150). In fact, it is very likely that neither dendritic tree morphology nor firing pattern are fully satisfying as predictors of the function of inhibitory dorsal horn interneurons.
Additional criteria including the expression of specific transcription factors (discussed in the following chapter), neuropeptide content and the presence of additional transmitters, enzymes or calcium binding proteins will have to be considered in addition. Many GABAergic spinal cord neurons co-express peptide transmitters such as neuropeptide Y (299, 325), galanin (345), enkephalin, or thyreotropin-releasing hormone (113). In addition, many GABAergic and combined GABAergic/glycinergic neurons also express parvalbumin or NADPH diaphorase / nitric oxide synthase (NOS). Some NOS-positive neurons, specifically those which lack glycine immunoreactivity, also express choline acetyl transferase (350, 363). Finally, inhibitory interneurons in lamina I and II do not contain somatostatin or neurotensin (368), whereas some cells in lamina III do express these neuropeptides (307). These markers may become increasingly relevant in the future, particularly since they are genetically encoded and thereby provide means to specifically interfere with interneuron functions through genetic manipulation.
It is likely that recently developed techniques will lead to the discovery of new marker proteins and to more sophisticated interneuron classifications. New technologies already enable the isolation of mRNA from defined cell types with improved fidelity. Fluorescence-activated cell sorting (FACS) of EGFP tagged neuronal subtypes or the BAC TRAP technique (92, 148) allow the retrieval of translated mRNA even from neuronal subtypes showing a scattered distribution and being intermingled with other cell types. Correlation of gene expression with neuronal function should be greatly facilitated by the recent advent of novel techniques allowing the expression of proteins suitable the activation, silencing or ablation of neurons in a cell type-specific manner. Such innovative approaches include among others optogenetics (9) and the expression of diphtheria toxin under the control of cell type-specific promoters (3).
VIII. Transcription factors determining the specification of dorsal horn inhibitory interneurons
A better understanding of transcription factor expression in dorsal horn interneurons is likely to help establish a more complete classification system for the various neuronal populations. It will also provide the basis for developing tools capable of genetically manipulating these cells.
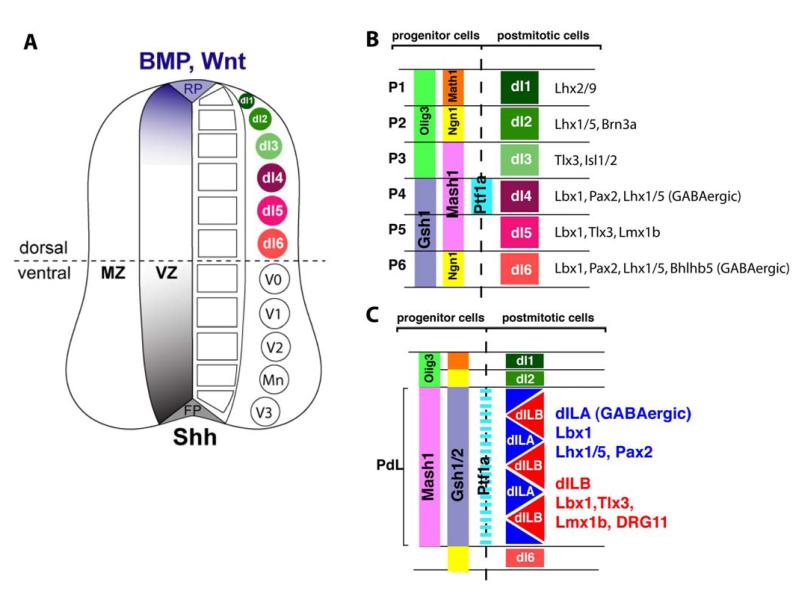
In the mouse, dorsal horn interneurons are born between E10.5 and E14. Those born during the early phase of neurogenesis (E10.5 - E11.5) settle in the deep dorsal horn, whereas those born during the late phase (E11.5 - E14) comprise the upper layers of the dorsal horn (308) reviewed in (60, 126, 153). During this period, six types of interneuron (dI1-6) are generated from spatially distinct progenitor domains (129, 267) (Figure 9AB). The three uppermost neuronal types, generated in the alar plate (dI1-3), depend on morphogen signals from the roof plate (212). In contrast, the three ventral alar plate populations appear to be generated independently from dorsal or ventral morphogen signals (129, 267). The majority of dorsal interneurons are generated during the second phase of neurogenesis (267, 394). Two main types of neuron (dILA and dILB) are generated from a large progenitor domain expressing a seemingly uniform transcription factor code (Figure 9C).
Figure 9.
Generation of spinal interneuron diversity (A) The neural tube is patterned by morphogen gradients secreted from the floor and the roof plate (FP and RP, respectively). Morphogen activity, such as sonic hedgehog (Shh) activity from the FP or Wnt and Bone morphogenic protein (BMP) activity from the RP, lead to the concentration dependent activation or repression of various transcription factors, and thereby to the generation of distinct progenitor domains. Within the ventricular zone (VZ) of the ventral neural tube five distinct progenitor domains are formed. Neurons which arise from the VZ populate the mantle zone (MZ). Each progenitor domain gives rise to a different type of ventral neuron. Therefore five types of neurons are generated in the ventral spinal cord (V3, Mn, V2, V1, and V0). In the dorsal spinal cord six types of interneurons (dI1-6) are generated from six different progenitor domains. Only the three dorsal most populations (dI1-3) are dependent on morphogen signals from the RP, like BMPs or Wnts. The three ventral most interneuron populations (dI4-dI6) are also generated in the absence of a dorsal signaling center. (B and C) A transcription factor code for dorsal spinal interneuron specification. (B) During the early phase of neurogenesis six types of dorsal interneurones (dI1-6) arise from six distinct progenitor domains (P1-6). Individual progenitor domains (P1-P6) express a unique combination of transcription factors thereby establishing the identity of the respective interneuron population. Newborn dorsal interneurons also express a unique set of transcription factors required for the further specification of their identity. (C) During the late phase of neurogenesis mainly two types of late born interneurones (dILA and dILB) arise from a broad progenitor domain (PdL) expressing a seemingly uniform transcription factor code (e.g. Mash1 and Gsh1/2). This suggests the involvement of additional mechanisms than combinatorial expression of transcription factors to generate neuronal diversity. The two late born neuron populations are distinguished by the expression of a different set of transcription factors subsequently determining their identity.
The six early born and two late born interneuron populations, can be distinguished because a transcription factor code specific for each subtype has been identified (127, 129, 212, 267). Furthermore, Cheng et al. (68, 69) demonstrated that the neurotransmitter content of dorsal horn interneurons correlates with the expression of the paired domain transcription factor Pax2 and homeodomain transcription factor Tlx3. Pax2-positive neurons co-express molecular markers for GABAergic neurons, including GAD65, GAD67 and VIAAT, whereas Tlx3-positive neurons co-express genes required for a glutamatergic phenotype (e.g.VGluT2). The use of Tlx3 and Pax2 as molecular markers for glutamatergic or GABAergic fate respectively enables the identification of GABAergic populations generated at different times within the developing dorsal spinal cord, namely, early born Pax2-positive, GABAergic dI4 neurons and late born Pax2-positive, GABAergic, dILA neurons (68). Another transcription factor, the Ladybird homolog Lbx1, is expressed in both GABAergic and glutamatergic neurons. Interestingly deletion of the Lbx1 gene leads to a fate change from GABAergic to glutamatergic neurons suggesting that Lbx1 is a postmitotic selector gene for GABAergic fate (69). Conversely, deletion of the postmitotically expressed transcription factor Tlx3 and its homolog Tlx1 lead to a fate change of glutamatergic neurons into GABAergic neurons, thus establishing Tlx3 as a postmitotic selector gene for glutamatergic fate (68). Furthermore, co-deletion of Tlx3 and Lbx1 reestablishes the glutamatergic fate. This suggests that early postmitotic expression of Lbx1 ensures a basal GABAergic differentiation state and that Tlx3 and Tlx1 act to oppose Lbx1 in order to establish the glutamatergic fate (69). Another transcription factor, Ptf1a, a basic helix loop helix transcription factor, has also been shown to be essential for GABAergic fate determination (122, 158). Ptf1a acts as part of a trimeric complex, together with RBPj and an E-protein, to suppress Tlx3 thereby allowing Lbx1 to promote GABAergic differentiation (157).
A. GABAergic fate decisions in dorsal spinal progenitor cells
Neuronal identity is first specified in neural progenitor cells. Early dI4 GABAergic neurons are generated from a distinct progenitor domain expressing a unique combination of transcription factors including Ptf1a, Mash1 and Gsh1/2 which thereby determining the identity of dI4 neurons (122, 152). In contrast, late born dILA GABAergic neurons are generated from the same progenitor pool as late born dILB glutamatergic neurons. Work from the labs of Birchmeier and Goulding has shown that the bHLH transcription factor Mash1, which is expressed in neural progenitors of dILA and dILB neurons, is required for specification of late born GABAergic neurons but not glutamatergic dILB neurons (255, 394). It has also been indicated that GABAergic dILA neurons are generated from asymmetric divisions and are dependent on Notch signaling. This suggests that asymmetric distribution of Notch activity is involved in determining the fate of late born GABAergic neurons.
B. Defining GABAergic subpopulations
The two different GABAergic subpopulations, dI4 and dILA, are likely to be comprised of additional neuronal subpopulations. For example, the expression of certain neuropeptide markers is restricted to specific subsets of dorsal horn interneurons. Bröhl et al. (54) and Huang et al. (164) have shown that the expression of neuropeptides including nociceptin, galanin, NPY and enkephalin depends on Ptf1a or Lbx1. This suggests that the expression of these neuropeptides may require transcription factors which act downstream of the selector genes Ptf1a or Lbx1 with respect to their role in specifying dorsal horn GABAergic interneurons. Furthermore, the results indicate that a subsequent combinatorial expression of bHLH and Lim, HD transcription factor leads to the sub-specification of GABAergic interneurons.
It is hoped that studies such as these will ultimately result in the identification of transcription factors involved in the determination of the different morphologically and functionally defined neuronal subpopulations described in the previous chapter. This gain in knowledge will not only promote our understanding of spinal cord development but should also lead to the generation of novel tools allowing the genetic manipulation of specific interneuron populations in vivo. Examples of the great potential of transcription factor-dependent cre expression in spinal interneuron populations are Pax2-cre (279) and Ptf1a-cre (188) mice. In the spinal cord, Pax2 and Ptf1a are expressed by the inhibitory interneurons either of the entire spinal cord (Pax2) (68)or of the dorsal horn only (Ptf1a) (122, 153). They thus allow specific gene deletion in this cell population (289, 323). Another elegant example involves a small subpopulation of dorsal horn interneurons that depend on the transcription factor Bhlhb5 which control itch processing dorsal horn circuits (323) (see also chapter XIV G). Bhlhb5 is an atonal related basic helix loop helix transcription factor which is expressed in early born dI6 neurons and in a subset of late born dorsal horn interneurons consisting of inhibitory as well as excitatory interneurons (220, 323). Interneurons that express Bhlhb5 have been demonstrated to control itch processing in dorsal horn circuits.
IX. Excitatory drive onto inhibitory dorsal horn neurons
Inhibitory interneurons in the dorsal horn are activated by primary afferent sensory nerve fibers and by fiber tracts descending from supraspinal areas. Electron microscopy studies in the monkey (59) and rat (361) demonstrate that all three classes of sensory fibers (Aβ, Aδ and C fibers) contact dendrites of inhibitory neurons in the spinal dorsal horn. Glycinergic (or mixed GABAergic/glycinergic) neurons are preferentially targeted by thickly myelinated low threshold fibers (301, 361, 390), whereas purely GABAergic neurons are preferentially contacted by thinly myelinated and unmyelinated fibers (12). This differential innervation is also reflected in the somewhat different distribution of GABAergic and glycinergic cells with glycinergic neurons being concentrated more in the deeper dorsal horn layers (compare Chapter V). In vivo patch-clamp recordings in the rat have provided corresponding functional data. GABAergic and glycinergic IPSCs could be evoked by innocuous mechanical stimulation (274) and subsequent work by a number of other groups has shown that the majority of GABAergic superficial dorsal horn neurons receive mono- and polysynaptic excitatory input from C and Aδ afferent nerve fibers (131, 137, 226, 227, 406, 408) (Table 1). The presence of C fiber input in GABAergic neurons does not necessarily mean that these neurons are excited by noxious stimuli. It has rather been demonstrated that the C fibers that excite islet cells are different from typical nociceptive C fibers specifically in their conduction velocities which are significantly higher (131). These cells might correspond to a particular subclass of C fibers with a low activation threshold, which has been described in microneurographic single fiber recording experiments in humans (40, 380). Psychophysical experiments suggest that these fibers convey pleasant touch sensations (221).
Table 1.
Primary afferent input onto subtypes of dorsal horn inhibitory interneurons
| cell type | EPSCs | IPSCs | reference | ||
|---|---|---|---|---|---|
| monosynaptic | polysynaptic | all polysynaptic | neurochemistry of IPSCs |
||
| islet cells | C fibers | Aδ and C fibers | Aδ fibers | GABA>mixed>glycine | (131, 226, 406, 422) |
| central cells | C fibers | Aδ and C fibers | Aδ and C fibers | GABA | (131, 137, 226, 227, 406, 422) |
| radial cells | Aδ and C fibers | Aδ and C fibers | Aδ and C fibers | GABA>mixed | (131, 406) |
| vertical cells | Aδ and C fibers | Aδ and C fibers | Aδ and C fibers | n.d. | (131, 227, 406, 422) |
n.d., not determined
As discussed in Chapter VII, the vast majority of inhibitory lamina II interneurons evoke pure GABAergic IPSCs in their postsynaptic target neurons (130, 226, 243). The presence of a strong glycinergic IPSC component elicited in vivo by light touch stimulation (274) indicates that additional (glycinergic or mixed GABAergic/glycinergic) interneurons must also become activated by low threshold primary afferent fibers. Interestingly, mixed GABAergic/glycinergic neurons in lamina III that show an islet cell-like morphology receive synaptic input from low threshold myelinated primary afferent fibers (301) and could thus be responsible for the IPSCs recorded after light touch stimulation by Narikawa et al. (274).
A second source of excitatory drive to inhibitory dorsal horn neurons originates from supraspinal sites that send (nor-)adrenergic and serotonergic fibers to the spinal dorsal horn. These fiber tracts have received significant attention as source of endogenous pain control (107). Both noradrenaline and serotonin have specific effects on defined dorsal horn neuron populations (228). In addition to inhibiting excitatory neurons and terminals, noradrenergic and sertonergic fibers excite GABAergic and glycinergic interneurons. Noradrenaline depolarizes EGFP labeled GABAergic neurons by activating α1 adrenoceptors (119), while serotonin increases the frequency of GABAergic mIPSCs and evoked inward currents by activating 5-HT3 receptors (2).
In addition to serotonergic and noradrenergic fibers, a high number of GABAergic and glycinergic fibers descend from supraspinal sites and innervate the dorsal horn. A direct inhibitory innervation (i.e. via monosynaptic connections) of dorsal horn neurons from the RVM has been demonstrated using in vivo patch-clamp recordings (187). Morphological evidence for the existence of GABAergic and glycinergic fibers descending from the rostral ventromedial medulla (RVM) comes from studies by Antal et al. (17). The glycinergic innervation is also evident in reporter mice expressing EGFP in glycinergic neurons (419). In the spinal cord, descending GABAergic and glycinergic projections mainly target presumed excitatory neurons (17).
X. Inhibitory neurons in the dorsal horn neuronal circuits: classical postsynaptic inhibition
Over the last few decades neuroanatomists and electrophysiologists have established a very precise blueprint for neuronal circuits in several CNS areas including the hippocampus and the cerebellum. Unfortunately, this is not the case in the dorsal horn of the spinal cord. Progress in this area has been impeded in part because of the diversity of neurons in this area but also as a result of the inherent difficulties associated with the identification of neuronal subtypes in “living” unstained slice preparations. In this section we shall summarize what is currently known about dorsal horn circuits.
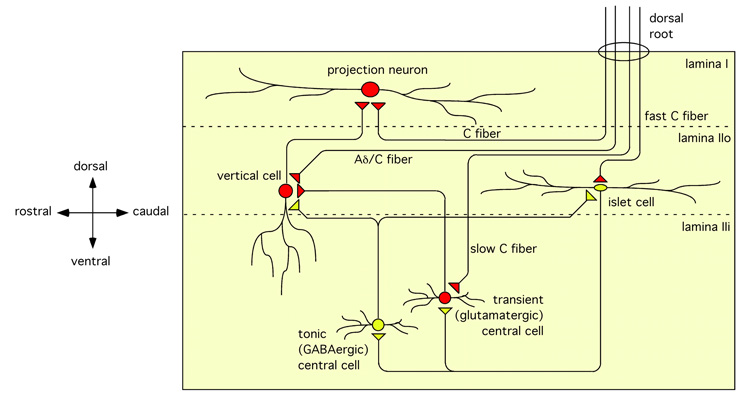
In an effort to delineate neuronal circuits in the rat spinal cord Lu and Perl (226, 227) performed simultaneous whole-cell recordings from a priori un-identified neurons in lamina I and II of the rat. In the first of two studies the authors identified 28 pairs of synaptically connected lamina II neurons from a total of 248 simultaneous whole-cell recordings (226). Of these, 15 were connected via inhibitory synapses. Each recorded neuron was classified according to its depolarization-induced action potential firing pattern and the morphology of its dendritic tree (see also Chapter VII). A commonly occurring synaptic arrangement consisted of a presynaptic tonically firing GABAergic islet cell and a postsynaptic central cell. Only one glycinergic connection was observed and no mixed GABAergic/glycinergic connections were detected. The predominance of GABAergic versus glycinergic connections correlates well with the relative scarcity of glycinergic neurons in lamina II (see also Chapter V). In the same set of experiments, the authors also stimulated the dorsal root. They observed that both types of neuron receive monosynaptic input from afferent C fibers. However, the “presynaptic” GABAergic islet cell received input with a shorter latency than the “postsynaptic” central cell. These findings suggest that islet cells are innervated by relatively fast conducting C fibers, whereas GABAergic neurons belonging to the central tonically firing type were contacted by thinner, slowly conducting C fibers. Expression of c-fos, a marker for neuronal activation, in response to formalin injections suggests that the input from these slowly conducting C fibers is nociceptive in nature (137).
In a second study (227), the authors analyzed monosynaptic excitatory connections in the same region of the dorsal horn. They identified 27 such connections out of more than 400 simultaneously recorded pairs of neurons. These included monosynaptic connections between transiently firing central cells in lamina IIi and vertical cells in lamina IIo and from these cells to cells in lamina I. A percentage of these lamina I cells were projection neurons. All three cell types receive monosynaptic input from primary sensory fibers. More specifically, lamina I neurons and central cells receive input from C fibers whereas vertical cells are excited by input from Aδ fibers (Figure 10).
Figure 10.
Synaptic connections in the superficial dorsal horn. Excitatory and inhibitory terminals are depicted as red or yellow triangles, respectively. Diagram based on data from the groups of Perl (226, 227, 422) and Yoshimura (406).
Many of Lu and Perl’s observations have since been substantiated by others. Yasaka et al. (406) identified four morphologically distinct classes of dorsal horn neuron that receive synaptic input from primary afferent fibers. They confirmed that islet cells receive monosynaptic excitatory input from large diameter C fibers, whereas primary afferent evoked GABAergic input was elicited through Aδ fiber stimulation. This GABAergic input is likely to originate from neurons other than islet cells and is consistent with the findings of Lu and Perl who did not find reciprocal connections between islet cells (226, 422). Yasaka and coworkers also confirmed that central cells receive monosynaptic input exclusively from C fiber afferents, while their polysynaptic GABAergic input is likely to be triggered by both C and Aδ fibers. Radial and vertical cells receive both monosynaptic primary afferent input and polysynaptic inhibitory input from C and Aδ fibers. Radial cells were found to receive glycinergic input after primary afferent stimulation, whereas primary afferent evoked inhibitory input to islet, central, and vertical cells was exclusively GABAergic.
Most recently, Zheng et al. (422) successfully mapped synaptic connections in the superficial dorsal horn using transgenic mice expressing EGFP in central cells driven by the prion protein (prp) promoter (381). The authors identified GABAergic connections between EGFP-positive central cells and both islet cells and vertical cells, and between islet cells and EGFP-positive central cells. In the latter case even reciprocal inhibitory connections were found between central and islet cells. Inhibitory connections were also found between islet and transient central cells.
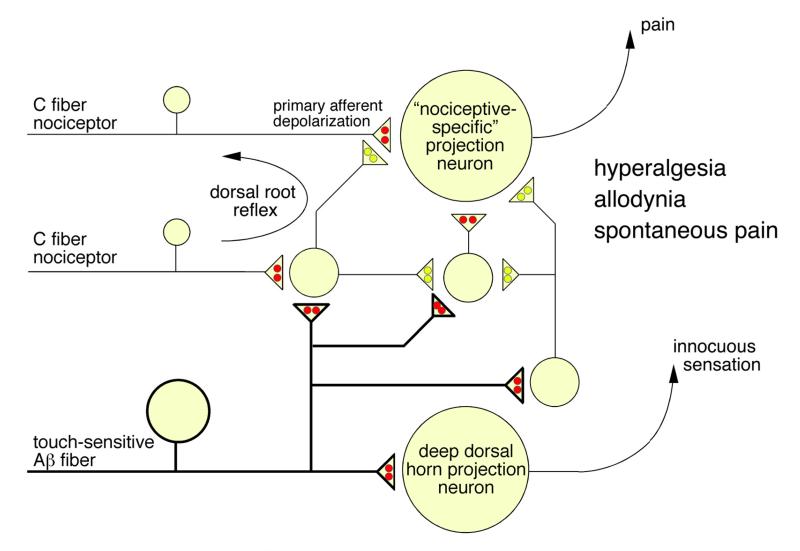
XI. Synaptic targets of inhibitory neurons in dorsal horn neuronal circuits - primary afferent depolarization, presynaptic inhibition, and dorsal root reflexes
Dorsal horn GABAA receptors are found on the somata and dendrites of intrinsic dorsal horn neurons, where they mediate classical postsynaptic inhibition, and at presynaptic sites on the spinal terminals of primary afferent sensory nerve fibers. In the following section we address the organization and function of this presynaptic inhibition.
A. Structural arrangement of primary afferent presynaptic inhibition
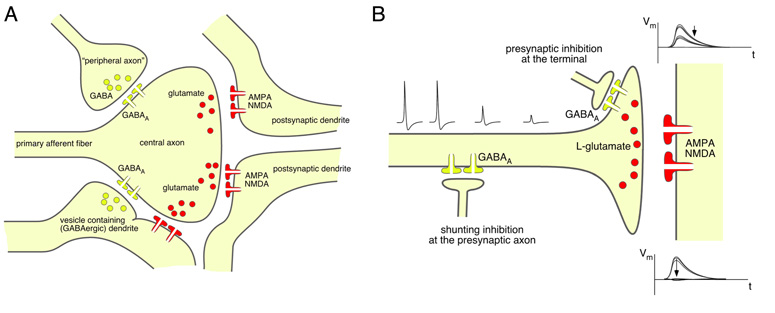
In the spinal dorsal horn, primary afferent presynaptic inhibition occurs either in form of rather simple axo-axonic synapses mainly in the case of Aβ fiber terminals (244), or in form of complex synaptic arrangements called synaptic gluomeruli. These glomeruli are located in the superficial dorsal horn and comprise interneuron axon terminals and postsynaptic dendrites that surround the central primary afferent fiber terminal (Figure 11A). At least four such elements must be present in a glomerulus in addition to the central axon (318). The vast majority of glomeruli contain peripheral axons that originate from GABAergic interneurons while the dendrites postsynaptic to the central axon belong to glutamatergic excitatory neurons (391). Two major types of synaptic glomeruli have been described in the rat dorsal horn. Type I glomeruli possess an unmyelinated primary afferent axon at their center (362). These axons are non-peptidergic and fluoride-resistant acid phosphatase (FRAP)-positive (319). According to more recent classifications these axons correspond to non-peptidergic IB4-positive C fibers. Type I glomeruli are mainly found at the center of lamina II and typically contain one to two peripheral axon terminals. Peptidergic axons (identified by their immunoreactivity against Substance P in rats, ref. 320, or CGRP in monkeys, ref. 13) rarely terminate in synaptic glomeruli. In the few cases where this does occur, they also form type I glomeruli, although even under these circumstances they seldom contain axo-axonic synapses onto the central axon. A recent study in the rat trigeminal nucleus caudalis found no GABAergic axo-axonic synapses onto peptidergic TRPV1-positive axons as defined by the presence of more than 5 peptide containing (dense) vesicles (410). The central axon of type II glomeruli is myelinated and originates from (low threshold) down hair (D-hair) receptors (315) or from high threshold Aδ mechanoreceptors (12, 315). Type II glomeruli are concentrated at the inner region of lamina II and the adjacent part of lamina III. They usually contain several inhibitory axo-axonic synapses.
Figure 11.
Synaptic glomeruli and presynaptic inhibition. (A) Schematic drawing of a synaptic glomerulum in the dorsal horn formed around the central axon of a primary afferent fiber and containing four peripheral elements, two “classical” postsynaptic dendrites originating from a glutamatergic neuron, one “peripheral” GABAergic axon terminal forming an axo-axonic synapse, and a vesicle containing “presynaptic” dendrite. (B) Possible arrangement of GABAergic innervation of primary sensory fibers and terminals in the spinal dorsal horn. Presynaptic inhibition at the primary afferent sensory terminal through axo-axonic synapse formed between GABAergic interneurons and a primary afferent terminal. The existence of such connections is well established for low threshold primary sensory axon terminals. Although physiological evidence clearly supports the existence of a GABAergic innervation of peptidergic C fibers, axo-axonic synapses have not been unambiguously described in peptidergic nociceptors, and GABA might merely act as a volume transmitter on these fibers. In this latter case, inhibition may primarily occur through activation of a shunting conductance for example at the axon shaft and subsequent impairment of action potential propagation. Insets show possible consequences of both arrangements for the postsynaptic signal evoked by primary afferent stimulation.
Some evidence suggests that two different populations of inhibitory interneurons contact unmyelinated and myelinated primary afferent fibers (41). The majority of peripheral terminals in type I synaptic glomeruli are purely GABAergic (362), while those of D-hair receptor type II glomeruli are mainly mixed GABAergic/glycinergic (391). The glycine released in type II glomeruli is nevertheless unlikely to contribute to inhibition of the central axon. DRG neurons do not exhibit glycinergic membrane currents (5) and morphological studies did not detect glycine receptors on axon terminals (254).
B. Physiological basis of presynaptic inhibition and primary afferent depolarization
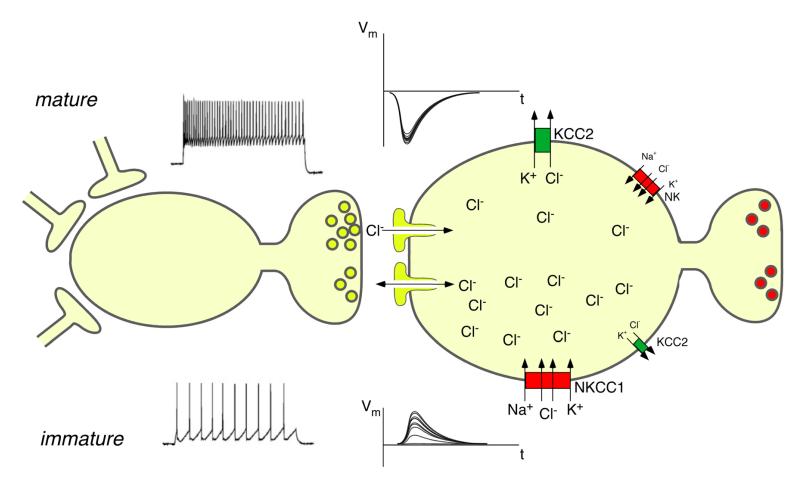
Primary sensory neurons, including primary nociceptors, exhibit a pattern of chloride transporter expression which is different from that of central neurons. The sodium/potassium/chloride transporter NKCC1 is expressed at high levels whereas the potassium chloride co-exporter KCC2 is expressed either at low levels or, in some cases, completely absent (185, 306). Because NKCC1 transports chloride ions from the extracellular space into the cytoplasm (45), intracellular chloride concentration in primary sensory neurons remains above electrochemical equilibrium into adulthood (322). As a consequence, activation of GABAA receptors on primary sensory neurons induces depolarization (termed primary afferent depolarization [PAD]) rather than hyperpolarization. Genetic deletion of NKCC1 abolishes depolarizing chloride currents from the somata of primary sensory neurons emphasizing the importance of chloride currents for PAD (356). Under certain conditions, glutamate (313) and potassium (149, 181) also contribute to PAD but the GABAergic (bicuculline-sensitive) component is usually dominating (98, 258, 277). PAD inhibits rather than facilitates transmitter release from the primary afferent terminal. Different explanations have been proposed to explain this phenomenon (204). PAD may lead to the inactivation of voltage-gated Ca2+ channels on primary afferent terminals and may thus reduce presynaptic Ca2+ influx and transmitter release. Alternatively, it may interfere with action potential propagation into the terminal through either voltage-dependent inactivation of Na+ channels or through activation of a shunting conductance.
C. Physiological functions and consequences of primary afferent depolarization in the spinal dorsal horn
As outlined in A, there is clear evidence showing that the spinal terminals of low threshold Aβ and Aδ fibers and of some nociceptors undergo presynaptic inhibition by inhibitory interneurons. This presynaptic inhibition permits the unique possibility to differentially regulate the excitability of different central branches of the same primary afferent sensory fiber. Although it is very likely that presynaptic inhibition plays a major role in the processing of sensory information, the precise contribution of presynaptic versus postsynaptic inhibition is difficult to define at present due to the inherent difficulties to selectively interfere with only one of the two components. However, the immediate effects of spinal application of the GABA or of the GABAA receptor agonist bicuculline on receptive field sizes of dorsal horn neurons (424) and on temporal adaptation to repeated sensory stimulation (89) are at least consistent with a contribution of presynaptic GABAergic inhibition to the processing of cutaneous sensory stimuli.
As discussed previously morphological evidence for a relevant GABAergic innervation is much weaker in the case of the majority of primary nociceptor terminals, especially for peptidergic nociceptors. Whether or not presynaptic inhibition by GABAergic interneurons is relevant for nociceptive transmission is therefore controversial. Supporting evidence comes from physiological experiments, which have demonstrated the presence of dorsal root reflexes in capsaicin-sensitive primary afferent axons (219) and the blockade by intrathecal bicuculline of peripheral flare responses which depend on the release of CGRP from the peripheral terminals of peptidergic nociceptors (218). In the absence of a direct innervation by axo-axonic synapses of the majority of primary nociceptors, GABA could still act as a volume transmitter (328). In this case, GABAA receptors along the intraspinal segment of the primary afferent axon could be activated by ambient GABA to cause voltage-dependent inactivation of Na+ channels or activation of a shunting conductance (Figure 11B). Both would prevent the invasion of the presynaptic terminal by axonal action potentials. Direct experimental proof for either of these possibilities is lacking, in part due to the intrinsic difficulties associated with recording from spinal primary afferent axon terminals.
A very recent study investigated sns-α2 knock-out mice in which GABAA receptor α2 subunits were specifically ablated from primary nociceptors and investigated subsequent changes in presynaptic inhibition and dorsal root potentials (DRPs) (398). DRPs are local field potentials generated by GABAergic interneurons and occurring in one dorsal root after electrical stimulation of another dorsal root in a neighboring segment. While there was no change in baseline synaptic transmission in sns-α2-deficient mice, diazepam facilitated DRPs in these mice much less than in wild-type mice. In addition, the inhibitory action of muscimol on synaptic transmission between primary high threshold afferents and second order neurons was potentiated by diazepam much less than in wild-type mice verifying that GABAergic PAD and presynaptic inhibition occur indeed in nociceptors.
When occurring in nociceptor terminals, PAD and presynaptic inhibition should reduce pain. In fact, part of the anti-hyperalgesic action of intrathecally injected diazepam (compare Chapter XV) occurs through an enhancement of presynaptic inhibition as demonstrated in experiments using the sns-α2-deficient mice described above (398). However, in nociceptors PAD cannot only cause presynaptic inhibition, but may under certain conditions also give rise to so-called dorsal root reflexes. These are action potentials elicited in primary sensory fiber terminals by stimulation of a second afferent fiber via an interconnected GABAergic interneuron (compare also Figure 15B). They occur when PAD reaches the threshold of action potentials. These action potentials may then propagate both in an orthodromic (central) and antidromic (centrifugal) direction. The centrally propagating action potential is thought to reinforce pain sensation, while the peripheral action potential, in case of peptidergic nociceptors, contributes to neurogenic inflammation, vasodilatation and plasma extravasation through the release of CGRP and Substance P. For a possible contribution of dorsal root reflexes to certain pain states see Chapter XIV F.
XII. Changes in dorsal horn synaptic inhibition during development
Nociceptive behavior and spinal nociceptive processing in young rodents differ significantly from that in adults. Immature nociception in newborn rats is characterized by lower withdrawal reflex thresholds in response to thermal, mechanical and chemical stimuli (105, 179, 241) and reduced guarding in response to noxious stimuli (385). Although these behavioral findings could be explained by immature motor coordination, electrophysiological studies have provided direct evidence in favor of underdeveloped sensory processing. Dorsal horn neurons of immature rats have larger receptive fields (111, 374), exhibit prolonged after-discharges following sensory stimulation (110), and display increased c-fos expression in response to innocuous stimuli (176). These differences are most pronounced in newborn animals and gradually diminish during postnatal development until they disappear in the third postnatal week. Many facets of nociception in the immature organism resemble those observed in adult animals following pharmacological blockade of GABAergic or glycinergic inhibition in the dorsal horn (compare chapter XIV). These parallels have led pain researchers to speculate that undeveloped synaptic inhibition is the underlying difference. Hypotheses proposed over the years include (i) a depolarizing (instead of hyperpolarizing) action of GABA and glycine in the early postnatal dorsal horn, (ii) lower chloride extrusion capacity of dorsal horn neurons, (iii) less reliable GABAergic transmission and (iv) diminished intrinsic excitability of GABAergic neurons (summarized in Figure 12). These hypotheses have been addressed in a series of elegant studies by Fitzgerald and others, and are summarized below. For a more detailed review see also (109).
Figure 12.
Possible “deficits” in inhibitory synaptic control in the immature. Upper and lower part of the figure depict proposed characteristics of the mature and immature synaptic inhibition, respectively. Weaker excitatory drive onto inhibitory interneurons, higher intracellular chloride concentration, lower chloride extrusion capacity, lower membrane excitability, and less reliable transmitter release have been proposed for the immature GABAergic neuron.
A. Depolarizing action of GABA and glycine in the spinal dorsal horn
The effect of GABAergic and glycinergic neurotransmission on neuronal excitability depends critically on the anion gradient of the postsynaptic cell. It is well known that this chloride gradient undergoes an embryonic maturation process extending into postnatal development (for a review see ref. 37). Shortly after birth, intracellular neuronal chloride concentrations are still high enough to render anion channel opening depolarizing rather than hyperpolarizing. The developmental maturation of the chloride gradient and of the hyperpolarizing action of GABA and glycine occurs through down-regulation of NKCC1 expression and increased expression of KCC2 (352).
The depolarizing actions of GABA have been thoroughly studied in many areas of the immature CNS. In the cortex and hippocampus they extend into the second postnatal week, on average, and are sometimes large enough to trigger action potentials (37). In the dorsal horn, a depolarizing or even excitatory action of GABA could contribute to increased nociceptive excitability in young animals. However, the time course for maturation of the neuronal chloride gradient does not correlate well with developmental changes in nociceptive behavior. In fact, in dorsal horn neurons, GABA starts to exhibit a hyperpolarizing effect during the first few days of the first postnatal week, significantly earlier than in most forebrain areas (24, 80).
B. Lower chloride extrusion capacity
The experiments yielding the data discussed above were performed in spinal cord slices and thus under these conditions where neuronal activity is considerably lower than in intact animals. Although intracellular chloride may already reach adult levels in resting cells by this time, it is possible that chloride extrusion mechanisms may still be insufficient to maintain this low level during higher neuronal activity. Cordero-Erausquin et al. (80) found that the chloride extrusion capacity of postnatal (juvenile) lamina I dorsal horn neurons was less than that of adult neurons up to an age of three weeks. As a result, prolonged application of GABA onto dorsal horn neurons causes responses to be hyperpolarizing at the beginning of the application, but becoming depolarizing within tens of milliseconds. Furthermore, by the end of the second postnatal week 35-40% of neurons still exhibit GABA-induced, depolarization-mediated increases in intracellular Ca2+ despite the presence of an initial hyperpolarizing action. By comparison these observations were absent in experiments carried out in spinal cords taken from healthy adult rats.
Although those experiments were convincing at the cellular level, in vivo recordings from single dorsal horn neurons provided direct evidence for an inhibitory action of GABA already by P3. Local application of gabazine, a selective GABAA receptor blocker, consistently increased the size of receptive field and action potential firing in response to low intensity or high intensity mechanical stimulation (52). In a parallel study, the same group (144) did report a lowering of nociceptive thresholds in P3 rats following application of gabazine, however, this facilitation was lost in spinalized animals and therefore attributed to supraspinal circuits.
C. Less reliable release of inhibitory transmitters
The absence of an excitatory action of GABA during the first postnatal days and weeks does not necessarily preclude a critical contribution of an immature GABAergic system to altered nociception in the young animals. Weaker GABAergic or glycinergic inhibition caused by fewer or less active inhibitory neurons, or fewer synapses or receptors might be alternative factors, and more subtle differences including temporally or spatially less well coordinated inhibitory inputs to postsynaptic cells might already be sufficient to explain the behavioral and cellular phenotypes of young animals described above.
Indeed, amplitudes of spontaneous and evoked GABAergic IPSCs are smaller in P3 rats than at P14 (24) or P21 (171). In addition, evoked IPSCs at P3 and P10 show a higher coefficient of variation (171) consistent with a presynaptic difference resulting from, for example, fewer synaptic contacts, fewer active zones, or a smaller pool of available presynaptic vesicles. The same study also found that the speed of recovery of GABAergic IPSCs, after trains of stimulation, increased with age suggesting that there are differences in the short-term plasticity of GABAergic synapses.
D. Intrinsic firing properties of dorsal horn neurons
It has also been proposed that immature GABAergic neurons may exhibit reduced intrinsic excitability. Baccei and Fitzgerald (25) characterized the intrinsic firing properties of neurons in the superficial dorsal horn of the rat at three developmental stages (P3, P10 and P21). The authors found no differences in the distribution of firing patterns, firing frequencies, frequency adaptation and threshold of firing. The most frequently observed firing pattern, at all three developmental stages, was tonic firing, which is also characteristic of adult GABAergic neurons. Consequently, developmental changes in the firing properties of GABAergic neurons can be excluded, although it is important to note that no attempts have yet been made to specifically select GABAergic neurons for recording.
E. Fidelity of GABAergic transmission
Even if cell-autonomous functions such as chloride gradient, chloride extrusion capacity, transmitter release and firing properties function already properly in young mice, differences may still exist in the organization of GABAergic connections. Bremner and Fitzgerald (53) provided evidence that the structural organization of inhibitory connections in the developing spinal cord is different from the adult. The authors assessed cutaneous inhibitory receptive fields at different stages of postnatal development. Inhibitory receptive fields are areas of skin that, following mechanical stimulation, can inhibit the response of a dorsal horn neuron to stimulation of its “excitatory” field. Inhibitory receptive fields in P3 rat dorsal horn neurons were more diffuse than in adults. In addition, inhibitory fields located on the side contralateral to the recorded neuron could be activated by low-intensity stimulation. In adult rats a more intense pinch stimuli is required in order to achieve the same result. Alternative explanations for these observations, such as the issue of whether inhibitory GABAergic neurons receive less synaptic drive at younger ages, have yet to be fully addressed. Furthermore, the specific contribution of glycinergic transmission to dorsal horn function during the first days of postnatal development remains unresolved (24).
F. Possible implications for pain in neonates
It should be emphasized that lower nociceptive thresholds in immature dorsal horn do not necessarily mean that neonates are more susceptible to hyperalgesia or chronic pain. In adult humans, peripheral nerve damage resulting from plexus lesions can cause severe neuropathic pain, however, newborn infants are unaffected (14). A similar situation exists in rats where peripheral nerve damage does not lead to long-lasting neuropathic pain when it occurs before three weeks of age (163). Other examples include differences in the development of secondary hyperalgesia resulting from intense C fiber input into the dorsal horn. In adults, intense C fiber stimulation leads to microglia-dependent central sensitization, however, neither pain sensitization nor microglial activation is seen in neonates (145). It is important to point out that differences in GABAergic inhibition during development do not necessarily favor a shift towards stronger inhibition in the adult. For example, the decay of GABAergic IPSCs is significantly prolonged in young animals. This is due to the constitutive postnatal production of GABAA receptor facilitating neurosteroids, which disappear shortly after birth (ref. 191; see also Chapter XIII A). With respect to the net charge transfer of GABAergic IPSCs, the longer duration of GABAergic IPSCs compensates for their smaller amplitude. Whether or not this influences pain behaviors at the level of the whole animal is not known.
XIII. Endogenous modulators of GABAergic and glycinergic transmission in the spinal dorsal horn
Inhibitory synaptic transmission in the dorsal horn is regulated by a variety of neuromodulatory compounds at both presynaptic and postsynaptic sites (Figure 13, Table 2 and Table 3). These include monamines such as noradrenaline and serotonin (5-hydroxytryptamine, 5-HT), various neuropeptides, purines, lipid mediators such as the neurosteroids, prostaglandins and cannabinoids, acetylcholine, and GABA itself.
Figure 13.
Chemical structures of neurosteroids active at GABAA receptors.
Table 2.
Presynaptic modulators of inhibitory synaptic transmission in the dorsal horn
| target of modulation |
modulator | receptor | effect | reference |
|---|---|---|---|---|
| GABA / glycine |
acetylcholine | nicotinic | facilitation | (194, 358) |
| adenosine | A1 | inhibition | (166, 404) | |
| ATP | P2X3 | facilitation | (167, 175, 317) | |
| cannabinoids | CB1 | Inhibition | (177, 289) | |
| GABA | GABAB | inhibition | (70, 174) | |
| nocistatin | ?? | inhibition | (8, 418) | |
| noradrenaline | α1 | facilitation | (20, 23) | |
| opioids | μ | inhibition | (130, but see also ref. 199) | |
| somatostatin | sst2a | facilitation | (407) | |
| GABA | acetylcholine | muscarinic | facilitation | (22) |
| ghrelin | GHSR1 | facilitation | (384) |
Presynaptic in this context refers to an action on the “presynaptic” inhibitory neuron. This is not restricted to the presynaptic terminal but includes also actions on the somata or dendrites or the presynaptic neuron.
Table 3.
Postsynaptic modulators of inhibitory synaptic transmission in the dorsal horn
| Target of modulation |
modulator | effect | mechanism | site | reference |
|---|---|---|---|---|---|
| GABAA | neurosteroids serotonin |
potentiation potentiation |
direct allosteric effect PKC |
entire dorsal horn lamina X superficial dorsal horn |
(36, 191, 253) (213, 403) |
|
| |||||
| glycine | noradrenaline (α2) |
potentiation | cAMP ↓, inhibition of PKA |
lamina X | (272) |
| serotonin | potentiation | PKC | lamina X | (402) | |
| prostaglandin E2 (EP2) |
inhibition | cAMP ↑ , PKA- dependent inhibition of GlyRα3 |
superficial dorsal horn |
(6, 7, 140, 314) | |
| GABA | potentiation | GABAB | (411) | ||
A. Neurosteroids
Neurosteroids (Figure 14) are synthesized from cholesterol and are structurally related to steroid hormones. They produce fast changes in neuronal excitability by interacting directly with ion channels and are therefore mechanistically distinct from classical steroid hormones, which are slow acting and mediate their effects through changes in gene expression. Neurosteroids are locally produced in the central nervous system and are likely to act in a spatially restricted (paracrine) manner. An important step in the synthesis of these compounds involves the uptake of cholesterol into mitochondria. This process is mediated by the 18 kDa translocator protein TSPO, formerly known as peripheral benzodiazepine receptor (286).
Figure 14.
Endogenous pre- and postsynaptic modulators of inhibitory synaptic transmission in the dorsal horn.
The endogenous neurosteroids 3α-reduced tetrahydroprogesterones and 3α-reduced tetrahydrocorticosterones positively modulate or activate all major isoforms of GABAA receptors through allosteric sites (36, 253). The C3α hydroxyl group (“3α-ol”) on the A ring and the C20 ketone moiety on the D ring are particularly important for mediating the potentiating effect of these molecules on GABAA receptors (139). In addition, 5α-pregnan-3α-ol-20-one (3α,5α-tetrahydroprogesterone; 3α,5α-THPROG; allopregnanolone) and 5β-pregnan-3α-ol-20-one (3α,5β-THPROG; pregnanolone) are positive allosteric modulators at concentrations <100 nM and direct activators at higher concentrations. In contrast, 3β,5β-THPROG (iso-pregnanolone) and 3β,5α-THPROG (iso-allopregnanolone) are inactive at GABAA receptors, because they lack the 3α hydroxyl group. Glycine receptors are neither directly activated nor potentiated by 3α,5α-THPROG (252) or 3α,5β-THPROG (180). 3α,5β-THPROG even produces slight but significantly inhibition of glycine receptor currents in cultured spinal cord neurons (114, 180).
The actions of endogenous neurosteroids on dorsal horn GABAA receptors and their contribution to nociceptive processing and pain have been studied extensively by the groups of Schlichter and Poisbeau. In the dorsal horn, pregnanolone acts specifically during development and in inflammatory pain states. During early postnatal development endogenous neurosteroids shape GABAergic mIPSCs by prolonging their decay. In slices taken from infant rats (≤ 23 days old) blockade of 18 kDa TSPO with PK11195 or of 5α reductase with finasteride significantly shortens GABA mIPSCs in lamina II neurons. Alternatively, activation of 18 kDa TSPO following extended incubation with diazepam (applied in the presence flumazenil to avoid direct potentiation of GABAA receptors) prolongs the decay of mIPSCs (191). An acceleration in the decay of GABAergic mIPSCs during postnatal development echoes the gradual decrease in the production of endogenous neurosteroids. This process also contributes to the progressive fading of the GABAergic component of mixed glycinergic/GABAergic mIPSCs. Endogenous neurosteroid production appears to subside at a varying rate in different regions of the dorsal horn. For example, in laminae III-IV synthesis has ceased by day P8 but remains maximal in lamina II until P15 at which point it gradually decreases until it reaches zero at day P21 (173). This differential, lamina-dependent decline may explain the different mIPSC kinetics in lamina II versus laminae III/IV during early postnatal development (172).
Constitutive neurosteroid production can be restored pharmacologically once it has subsided by incubating spinal cord slices with different pregnanolone precursors or through combined incubation with diazepam and flumazenil. The latter finding indicates that the enzymatic machinery required for neurosteroid synthesis is still present. In fact, the limiting factor for neurosteroid production appears to be the transport of cholesterol across the mitochondrial membrane (173). Importantly, there are also natural stimuli that can restore neurosteroid production. Peripheral inflammation can lead to a re-emergence of neurosteroid production both in the superficial dorsal horn and in its deeper laminae (173, 292). In response to a peripheral inflammatory stimulus, such as the injection of carrageenan under the plantar surface of the left hind paw, GABAergic mIPSCs become progressively slower and mixed GABAergic/glycinergic mIPSCs reappear. At the behavioral level, increased GABAergic inhibition by endogenous neurosteroids produces reduced thermal hyperalgesia but has no effect on mechanical allodynia (292).
When administered exogenously through intrathecal injection into the spinal canal, the action of different pregnanolone isomers is more complex. 3α,5α-THPROG (allopregnanolone) reduces thermal hyperalgesia and mechanical allodynia in rodent models of inflammatory pain, consistent with the effects of endogenous neurosteroids described above. However 3α,5β-THPROG (pregnanolone) is also able to reduce mechanical allodynia but has no effect on thermal hyperalgesia (65). The authors have postulated that this difference may be linked to the inhibitory effect of 3α,5β-THPROG on glycine receptor function (180), which according to their data facilitates thermal hyperalgesia more than mechanical hyperalgesia.
B. Monamines
The monamines noradrenaline and serotonin have both been shown to interfere with synaptic transmission in the spinal dorsal horn. Noradrenaline reduces the release of glutamate from primary afferent terminals in the substantia gelatinosa (189, 285) and facilitates glycine and GABA release in the dorsal horn (20, 23). The inhibition of glutamate release is mediated by α2 adrenoceptors and probably underlies the analgesic effects of the α2 adrenoceptor agonist clonidine and related compounds. The facilitation of GABA and glycine release is mediated by the activation of α1 adrenoceptors on inhibitory interneurons and terminals (119). Other groups have also proposed a postsynaptic action of noradrenaline on glycine receptors in rat sacral commissural neurons (lamina X), where glycinergic membrane currents are potentiated by noradrenaline acting on α2 adrenoceptors (272). Activation of α2 adrenoceptors results in a decrease in cAMP and inhibition of protein kinase A (PKA). Pretreatment with pertussis toxin prevents the potentiation of glycinergic currents suggesting that this effect is due to a reversal of PKA-dependent inhibition. Interestingly, this process is reminiscent of the inhibition of GlyRα3 by prostaglandin E2 (PGE2) and PKA as discussed in Chapter IV D.
Serotonin potentiates GABAA receptor-mediated responses through G-protein-coupled 5-HT receptors on neurons in the superficial dorsal horn (213) and in lamina X (403) resulting in activation of protein kinase C. A similar effect also occurs with glycine receptors (402). In the isolated spinal cord, serotonin reduces the excitatory postsynaptic potentials in dorsal horn neurons triggered by electrical stimulation of dorsal roots of adjacent segments (342) and thus reduces the intersegmental propagation of sensory signals in the spinal cord.
C. Purines
ATP facilitates the release of glycine (175, 317), GABA (167) and glutamate (133, 214) through the activation of P2X receptors in the spinal cord. Since ATP is co-released with GABA from many dorsal horn interneurons (182), it is likely to serve as an endogenous regulator of dorsal horn neurotransmission. The ATP metabolite adenosine inhibits glycine and GABA release (166, 404) and also reduces synaptic transmission between primary afferent C- and Aδ-fibers (208). Both effects are consistent with the strong expression of A1 adenosine receptors within the inner part of lamina I (338). Inhibition of excitatory transmission between primary nociceptive afferents and dorsal horn neurons probably underlies the well-documented antinociceptive effects of adenosine.
D. Neuropeptides
Endogenous and synthetic opioid peptides exert analgesic effects by inhibiting glutamate release from primary afferent terminals (199, 217) and disinhibiting descending antinociceptive tracts through reduced GABA release. Whether or not opioids also interfere with inhibitory transmission at the spinal cord or trigeminal level remains controversial. Grudt and Henderson (130) reported an inhibition of glycine and GABA release by the selective μ opioid receptor agonist Tyr-D-Ala-Gly-N-methyl-Phe-Gly-ol (DAMGO) in the rat trigeminal nucleus caudalis. However, Kohno et al. (199) found no such effect in the substantia gelatinosa of the spinal dorsal horn. If, under certain conditions, opioids or opioid metabolites reduce inhibitory control in the spinal cord, it is tempting to speculate that this may contribute to opioid-induced hyperalgesia (15).
Somatostatin is a neuropeptide produced by primary afferent neurons and dorsal horn interneurons (307). In the dorsal horn, it activates somatostatin (sst) 2a receptors, which are mainly located on inhibitory neurons (371). Activation of these receptors produces outward currents in inhibitory neurons (408) and thus inhibits their activation. Therefore, the pronociceptive effects of somatostatin in the spinal cord (341) are likely to result from a disinhibition of dorsal horn circuits.
Nocistatin (NST), a putative neuropeptide derived from the nociceptin/orphanin FQ precursor polypeptide (280), specifically inhibits GABA and glycine release in the dorsal horn (418). This synaptic action can be prevented by pre-treating spinal cord slices with pertussis toxin demonstrating the involvement of Gi/o coupled GPCRs (418). However, other groups have shown that NST depolarizes neurons in the central amygdala via activation of Gq/11 and the phospholipase C dependent opening of TRPC channels (66). It is conceivable that activation of this pathway triggers the production or release of another signaling molecule responsible for the pertussis toxin-sensitive effects of NST on synaptic transmission. In rats, intrathecal injection of nanomolar doses of NST increases nociceptive responses in the formalin test (418) and in the chronic constriction injury model of neuropathic pain (270). At lower doses NST has an antinociceptive action, which is blocked by pretreatment with D-serine, an endogenous agonist at the glycine binding site of NMDA receptor. The latter effect is likely to occur as a result of reduced availability of glycine at pronociceptive NMDA receptors (8, 270). These reports suggest that pathological pain states lead to an increase in the release of synaptic glycine and the activation of neighbouring NMDA receptors via spill-over. Such a process is consistent with the increase in activity of glycinergic neurons that is observed during prolonged pain states (161).
Ghrelin is a peptide hormone originally described in the gastrointestinal tract as a regulator of appetite and feeding. Ghrelin and its receptor, the growth hormone secretagogue receptor (GHSR) type 1a, are also expressed in the mouse spinal dorsal horn. After peripheral, or intracerebroventricular, injection ghrelin exhibits significant antihyperalgesic activity (344). When tested in spinal cord slices ghrelin dramatically increases the frequency of mIPSCs in the deep dorsal horn (384).
E. Acetylcholine
Acetylcholine facilitates inhibitory neurotransmission in the dorsal horn by activating muscarinic and nicotinic receptors. The muscarinic agonist carbachol enhances the excitability of GABAergic dorsal horn neurons and facilitates synaptic release of GABA through non-M1, non-M2 muscarinic receptors (22). Nicotinic receptors are found on the presynaptic terminals of inhibitory neurons (121, 358) and on the spinal terminals of serotonergic neurons descending from the raphe magnus nucleus (79). In both cases nicotinic receptor activation facilitates transmitter release and inhibits spinal nociception. This action persists in the presence of dihydro-β-erythroidine and methyllycaconitine indicating that it is not mediated by α4β2 or α7 containing nicotine receptors (ref. 358, see also ref. 194). Single cell RT-PCR experiments revealed that α4α6β2 nicotine receptors predominate on inhibitory neurons in the dorsal horn (81) making this receptor isoform an attractive target for possible nicotinergic analgesics.
F. Endocannabinoids
N-arachidonoylethanolamide (AEA, also called anandamide) and 2-arachidonyl-glycerol (2-AG) are endogenous lipid signaling molecules (endocannabinoids), which activate G-protein coupled cannabinoid receptors. 2-AG is active almost exclusively at CB1 receptors, while AEA activates both CB1 and CB2 receptors. In many CNS areas, endocannabinoids and CB1 receptors function as retrograde messengers in response to prolonged depolarization or intense glutamatergic stimulation and subsequent activation of group I metabotropic glutamate receptors. Upon binding to presynaptic CB1 receptors, endocannabinoids can reduce the release of glutamate or of GABA and glycine and thereby act as mediators of homosynaptic or heterosynaptic plasticity (73, 383). In the trigeminal dorsal horn, activation of CB1 receptors reduces synaptic transmission between primary afferent nociceptors and second order neurons (216). Similar actions also occur in the spinal dorsal horn (A. Kato, A.J. Pernia-Andrade, P. Punnakkal, H.U. Zeilhofer, unpublished). By contrast, activation of CB1 receptors does not interfere with excitatory synaptic transmission in the case of intrinsic dorsal horn neurons. However, GABAergic and glycinergic synaptic transmission is reduced in the trigeminal and spinal dorsal horn (177, 289). A possible function for this heterosynaptic plasticity in spinal pain processing is discussed in Chapter XIV E. Finally, endocannabinoids and some cannabinoid derivatives may also exert direct allosteric effects on glycine receptors (151, 224, 400, 405).
G. Zinc
Zinc can be considered an endogenous allosteric modulator of glycine receptors. It is stored in various presynaptic vesicles and released into the synaptic cleft upon stimulation (87, 115). It exerts a bi-phasic modulation consisting of potentiation at low (< 10 μM) concentrations and inhibition at higher (>10 μM) concentrations (46, 90, 209). This bi-directional modulation occurs via distinct sites. Potentiation is dependent on an allosteric site, which increases the affinity of the receptor to glycine, whereas inhibition occurs through reduced efficacy. Amino acid residues involved in the potentiating action include D80, E192, E194 (209, 230), while inhibition involves H107, H109, T112, and T133 (all positions refer to GlyRα1) (142, 209, 249). The various glycine receptor isoforms differ in their susceptibility to modulation by glycine with GlyRα2 and GlyRα3 being inhibited by zinc to a lesser extent than GlyRα1. This discrepancy is due to the substitution of the H107 residue in GlyRα1 for an asparagine residue at the corresponding position in GlyRα2 and GlyRα3.
Point mutated “knock-in” mice, carrying a D80A substitution, are largely resistant to the potentiating effects of zinc while the glycine sensitivity, expression level, and receptor trafficking to the synapse remain normal (154). Homozygous D80A point mutated mice exhibit a hyperekplexia-like phenotype, at approximately day P12, when α1 glycine receptors replace embryonic α2 glycine receptors. These mutants thus clearly reveal a physiological function for this modulatory site (and for zinc itself) in spinal cord neuronal circuits.
H. Other modulators of GABAergic and glycinergic synaptic transmission
The modulatory role of GABA acting as a feedback signal on inhibitory transmitter release, or as a postsynaptic modulator, is discussed in Chapter VI. Effects of PGE2 on glycine receptor function are discussed in Chapter XIV D.
XIV. Changes in inhibitory synaptic transmission in chronic pain syndromes
Melzack and Wall’s gate control theory of pain (248) suggested that inhibitory interneurons in the substantia gelatinosa of the spinal dorsal horn act as “gate control” units for nociceptive signals entering the CNS from the periphery. At the time, this was merely speculation and some of the synaptic arrangements proposed in their original publication turned out to be incorrect (128). However, the advent of pharmacological tools capable of manipulating synaptic inhibition in the dorsal horn has led to the discovery that blockade of GABAA or inhibitory glycine receptors strongly enhances nociceptive sensitivity in rodents.
A. Pain behavior elicited by blockade of spinal GABAA and glycine receptors
In rats, intrathecal injection of strychnine at sub-convulsive doses causes recurring stereotypic behaviors such as coordinated grooming, scratching and biting at the skin (43). In addition, these animals also develop heat hyperalgesia (65) and vocalize upon light mechanical stimulation of the skin (43). At least some of the nociceptive responses produced by light mechanical stimulation in strychnine-treated rodents are resistant to treatment with morphine suggesting that they are triggered by non-nociceptive primary afferent fibers (222, 343). Intrathecal application of either bicuculline or picrotoxin also provokes vocalizations in response to innocuous mechanical stimulation with von Frey filaments. Furthermore, thresholds of vocalization in response to electrical stimulation of the tail are also significantly reduced (321).
These behavioral observations correlate well with morphological studies aimed at quantifying changes in c-fos expression following blockade of spinal inhibitory neurotransmission. The number of c-fos positive neurons in the deep dorsal horn increased significantly following treatment with both strychnine and picrotoxin. In contrast, significant increases in the number of c-fos-positive neurons in the superficial dorsal horn were only obtained using picrotoxin (84). An isobolographic study, aimed at investigating the effects of intrathecal administration of strychnine and bicuculline, indicated that the effects of both antagonists amount to more than an additive effect and thus suggest non-identical mechanisms of action of both transmitter systems in pain (222). Electrophysiological recordings from α motoneurons, which produce the motor output after noxious skin stimulation, show that blockade of segmental GABAergic and glycinergic inhibition not only facilitates their activation in response to noxious stimuli but also renders these neurons responsive to innocuous stimulation (347). Miraucourt et al. (250) recently investigated the effect of reduced glycinergic inhibition in the trigeminal system. The authors found that blockade of glycinergic inhibition by strychnine increased action potential firing in both wide dynamic range neurons and in nociception-specific neurons. The latter newly acquired responsiveness to innocuous stimuli and were therefore converted into wide dynamic range neurons. Interestingly, increases in blood pressure, normally a reliable indicator of pain in anesthetized animals, only occurs after increased activation of nociception-specific neurons, not after activation of wide dynamic range neurons.
B. Changes in functional dorsal horn circuits after blockade of GABAA and glycine receptors
The role of diminished inhibitory neurotransmission has also been examined in electrophysiological studies aimed at addressing the effect of reduced synaptic inhibition on signal processing in the dorsal horn neuronal circuit. Baba et al. (21) recorded primary afferent evoked EPSCs from lamina II neurons in spinal cord slices. Bicuculline had a negligible effect on the initial fast EPSC but led to the appearance of long-lasting polysynaptic responses after stimulation of primary afferent fibers at Aβ, Aδ and C fiber strength. These long-lasting polysynaptic events disappeared in the presence of NMDA receptor blockers APV or ketamine. Interestingly the effect of bicuculline was less pronounced in spinal cord slices obtained from mice with spared nerve injury possibly indicating that GABAergic neurotransmission is already impaired in these mice. The same group also observed an increased incidence of polysynaptic Aβ fiber input onto substantia gelatinosa neurons in slices prepared from mice with inflamed paws (19). Whether or not, this polysynaptic Aβ input arose from reduced inhibition was not addressed but other studies suggest that this might well be the case (140, 266). In similar experiments, Torsney and MacDermott (375) studied two types of postsynaptic neurons located in either lamina I or III. NK1 receptor-positive (presumed projection) neurons and NK1 receptor-negative neurons. NK1 receptor-positive neurons in lamina I received monosynaptic input from high threshold primary afferent fibers (Aδ and C fibers), while NK1 receptor-positive neurons in lamina III received monosynaptic input from low threshold (Aβ) fibers. Co-application of bicuculline and strychnine induced polysynaptic input onto NK1 receptor-positive lamina I neurons which was mainly Aβ fiber mediated. As shown by Baba et al. (21) this polysynaptic input depends on NMDA receptor activation.
Since these initial electrophysiological studies, several other groups have provided direct evidence to support the theory that inhibitory neurotransmission becomes impaired during abnormal pain states. A number of studies have also proposed mechanisms to explain this observation. Deficits in synaptic inhibition have been shown to occur in at least three forms of pathological pain: (i) neuropathic pain elicited through peripheral nerve damage, (ii) pain in response to peripheral inflammation, and (iii) activity dependent pain sensitization which refers to central sensitization triggered by intense nociceptor input to the dorsal horn in the absence of immunological inflammation or primary nerve damage.
C. Changes during neuropathic pain
De Koninck’s group has proposed a potential mechanism for neuropathic pain following nerve damage in which inhibitory dorsal horn interneurons play a central role (Figure 15A). The authors found that injury to peripheral nerves induces a depolarizing shift in the chloride equilibrium potential of dorsal horn neurons (83). This depolarizing shift originates from a reduction in the expression of the potassium chloride exporter KCC2 whose function is required to maintain a low intracellular chloride concentration. As a consequence, GABAergic and glycinergic input is rendered less inhibitory and may even acquire a net excitatory effect, i.e. induce depolarizations sufficiently large to trigger action potentials. Since the initial injury involves peripheral nerve fibers and the changes in chloride concentration occur in central neurons, this hypothesis is dependent upon the action of one or more diffusible messengers released in the dorsal horn. Subsequent work by the same group ultimately identified activation of dorsal horn microglia and the release of brain derived neurotrophic factor (BDNF) as critical steps in this process. The initial trans-synaptic trigger is likely to be the release of cytokine CCL2 (also called macrophage chemoattractant protein-1, MCP-1) from damaged nerve fibers into the dorsal horn, which then activates resident microglia (360, 421). ATP acting primarily on P2X4 receptors (312, 377) is thought to trigger the release of BDNF from activated microglia cells (82). BDNF then binds to trkB receptors on dorsal horn neurons leading to the down-regulation of KCC2 (82). It should be noted that P2X7 receptors are also expressed at high levels by spinal microglia (312) and that some doubt remains over whether P2X4 or P2X7 receptors are more relevant (135). Both receptors have hence received considerable attention as potential targets for novel analgesics directed against both inflammatory and neuropathic pain (72, 156).
Figure 15.
Possible changes in synaptic inhibition during pathological pain states. (A) Three pathways leading to reduced synaptic inhibition in the dorsal horn. Peripheral nerve damage and microglia-induced changes in the inhibitory control by GABA and glycine (pathway shown in green). Inflammation-induced reduction in glycinergic neurotransmission (blue). Reduced glycine and GABA release triggered by intense C fiber input and subsequent release of endocannabinoids and activation of presynaptic CB1 receptors (magenta). (B) Dorsal root reflexes as a possible source of secondary hyperalgesia and allodynia (modified from ref. 64). Input from the lower axon excites a GABAergic interneuron, which depolarizes the top axon terminal. If the intracellular chloride concentration of the top axon is sufficiently high and input from the GABAergic interneuron strong enough to reach the action potential threshold, the top axon would give rise to the activation of additional central neurons and to release of proinflammatory peptides through action potentials retrogradely invading the periphery.
In vivo electrophysiology experiments have since shown that peripheral nerve damage, the transplant of ATP-activated microglia, and pharmacological disruption of the transmembrane chloride gradient all lead to similar phenotypical switches in lamina I projection neurons (190). Under physiological conditions these neurons are not spontaneously active and only responsive to noxious stimuli. All three of the aforementioned manipulations induce spontaneous activity, increase firing responses to noxious stimulation and transform nociceptive-specific neurons into wide dynamic range neurons. Studies carried out in spinal cord slices have demonstrated that changes in the local spinal cord circuitry are sufficient to trigger this type of phenotypical switch (336). In these experiments, the spread of excitation through the dorsal horn, “across modality borders”, was assessed using FURA-2-based Ca2+ measurements. In control animals, neuronal excitation resulting from electrical stimulation of Aβ fibers or local glutamate injection into the deep dorsal horn remained confined to the termination area of these fibers or to the site of glutamate injection. However, in neuropathic animals neuronal excitation spread from the deep dorsal horn to the superficial layers, which normally only respond to noxious stimuli. This effect could be mimicked by blockade of inhibitory transmission with bicuculline and strychnine.
Deficits in GABAergic inhibition could also arise as a consequence of reduced GABA content caused e.g. by a selective loss of inhibitory dorsal horn neurons (259, 337). However, the issue of apoptotic cell death within subpopulations of dorsal horn neurons and whether this factor contributes to neuropathic pain remains controversial (295-297).
Pathological pain states are not only triggered by damage to the peripheral nervous system. They also occur following spinal cord injury and diminished inhibition has also been suggested to occur under these conditions. For example, a reduction in the effect of bicuculline on the activity and receptive field sizes of inhibitory interneurons has been reported in allodynic rats after spinal cord contusion injury (95).
Whether or not a microglia-induced disturbance of chloride homeostasis contributes to non-neuropathic pain remains unclear. The phenotypes of different mouse mutations may provide some hints. Mice lacking the CCL2 receptor CCR2 show a dramatic decrease in nociceptive responses after peripheral nerve damage and in the formalin test but only a relatively minor phenotype and no microglia activation after intraplantar injection of complete Freund’s adjuvant (CFA) (1). Intrathecal injection of the microglial activation inhibitor fluocitrate partially reverses zymosan A-induced mechanical hypersensitivity but has no effect in CFA treated rats (75). Furthermore, while expression of the chloride exporter KCC2 is down-regulated in the dorsal horn after peripheral nerve damage, it is reported to be up-regulated, together with the chloride importer NKCC1, in the superficial dorsal horn during arthritis (261). The net effect of up-regulation of both chloride transporters, which transport chloride in opposite directions, is not known and difficult to predict. However, the switch from analgesic to hyperalgesic action of intrathecally administered gabazine may indicate its functional relevance to pain (16).
At a first glance, the idea of GABA acting as an excitatory transmitter in neuropathic pain states seems to be at odds with the observation that facilitation of GABAA receptors by benzodiazepines, under these conditions, produces anti-hyperalgesic effects (196, 200). There are several possible explanations for this apparent paradox. (i) the GABAA receptors relevant for antihyperalgesia may reside at the terminals of primary nociceptors which do not express KCC2, (ii) the main mechanism behind the anti-hyperalgesic action may involve an increase in shunting conductance which would be retained despite changes in the chloride gradient, (iii) in the majority of dorsal horn neurons the reversal potential of chloride may become less negative but still remain hyperpolarizing after induction of neuropathy, (iv) spinal output neurons may retain low intracellular chloride concentrations even in the presence of neuropathy.
D. Changes during inflammatory pain
Diminished synaptic inhibition in the spinal dorsal horn also occurs in response to peripheral inflammation (140, 266, 314) (Figure 15 A). Peripheral inflammation results in increased expression of the inducible isoform of prostaglandin H synthase-2, known colloquially as cyclooxygenase-2 (COX-2) (35, 330), and of inducible prostaglandin E synthase-1 (mPGES-1) (76, 100, 134, 239). This leads to production of the pro-nociceptive and pro-inflammatory PGE2 in the spinal cord (314, 330). PGE2 specifically reduces strychnine-sensitive glycinergic inhibition in the superficial dorsal horn through a postsynaptic mechanism involving EP2 receptors and PKA-dependent phosphorylation of α3 subunit containing glycine receptors (GlyRα3) (7, 203, 276). This subunit is specifically expressed in the superficial layers of the dorsal horn where the majority of nociceptive fibers terminate (140). Mice deficient in GlyRα3 exhibit normal responses to acute nociceptive stimuli, which may indicate an up-regulation of other glycine receptor subunits in these mice, which are not inhibited by PKA-dependent phosphorylation, such as GlyRα1 (311). Despite unchanged responses to acute nociceptive stimulation, these mice (and EP2-deficient mice) recovered from inflammatory pain sensitization much more quickly than corresponding wild-type mice (314, 382) (reviewed in ref. (416). PGE2-, EP2 receptor- and PKA-mediated reduction of glycinergic inhibition may also explain the decreased spinal PGE2 evoked hyperalgesia in mice lacking in neuronal PKA (236). More recent studies have shown that PGE2-mediated inhibition of GlyRα3 appears to be restricted to immunological inflammation as it does not contribute to pain elicited by peripheral nerve damage or acute chemical irritation of C fibers following capsaicin, formalin or acetic acid injection (143, 159, 310).
E. Changes during activity dependent central sensitization
In the early 1980s Woolf and coworkers reported that intense nociceptor activation can alter the central (spinal) processing of both painful and non-painful signals resulting in increased nociceptive responses after injury (399). Subsequent work has then shown that intense C fiber input increases receptive field sizes of dorsal horn neurons and rendered then responsive to input from non-nociceptive fibers (78). Sivilotti and Woolf (347) later showed that this sensitized state could be mimicked by applying GABAA or glycine receptor antagonists onto the spinal cord. This phenomenon of C fiber activity-evoked sensitization is now commonly referred to as central sensitization and is generally studied experimentally by employing local injection of capsaicin or by electrical stimulation of the peripheral nociceptor terminals. It is insensitive to COX inhibition and thus apparently independent of prostaglandin synthesis (99). However, capsaicin-induced secondary hyperalgesia is reduced by antagonists of group I metabotropic glutamate receptors and NK1 receptors (91).
Interestingly, both NK1 (94) and group I mGluRs (289) trigger the release of spinal endocannabinoids. This is particularly noteworthy since CB1 receptor activation in the dorsal horn reduces IPSC amplitudes by inhibiting the synaptic release of glycine and GABA (177, 289). Deletion of the CB1 receptor either globally or specifically in inhibitory dorsal horn neurons prevents the development of mechanical sensitization after subcutaneous capsaicin injection (289). Spinal endocannabinoids might therefore serve as mediators of heterosynaptic spinal plasticity by linking intense C fiber input to decreased synaptic inhibition by GABA and/or glycine (Figure 15A). Since the spinal application of CB1 receptor activators exerts a net anti-hyperalgesic action in most animal models of inflammatory and neuropathic pain (for a review see ref. 387), central sensitization, as a result of exaggerated input from C fibers, may not play a major role in these pain models. On the other hand, the idea that endocannabinoids and CB1 receptors support spinal disinhibition is consistent with reports which showed pro-nociceptive rather than analgesic actions of cannabinoids on acute pain in humans (201, 273) or in acute postoperative pain in patients (34, 57).
F. Changes in presynaptic inhibition and primary afferent depolarization during inflammation and neuropathy
As discussed in Chapter XI C, activation of GABAA receptors at the central terminals of primary afferent fibers cannot only evoke presynaptic inhibition but also elicit dorsal root reflexes. Dorsal root reflexes have been recorded from individual Aδ nociceptors after tactile and noxious mechanical cutaneous stimulation (reviewed in ref. 395) and in C fibers after conditioning cutaneous capsaicin injection (219). C fiber dorsal root reflexes have been reported to contribute to the spread of capsaicin-induced flare responses beyond the site of injection (218). Peripheral capsaicin injection facilitates the appearance of dorsal root reflexes in C fiber nociceptors (219). Enhanced GABA release at axo-axonic synapses onto the C fiber terminals, sensitization of their GABAA receptors, or increases in intracellular chloride concentration could mediate this facilitation. Indeed, intense C fiber input as a result of intracolonic application of capsaicin promotes membrane trafficking and phosphorylation of NKCC1 in lumbosacral spinal cord tissue (117). If these results reflect changes in primary nociceptor NKCC1, they may promote NKCC1-mediated chloride accumulation in these cells, as well as increase PAD and dorsal root reflexes (for a recent review see ref. 305). In line with this concept, blockade of spinal NKCC1 activity with intrathecal bumetanide prevented increases in the incidence of dorsal root reflexes by capsaicin and capsaicin-induced hyperalgesia and allodynia (379). Because PAD and dorsal root reflexes can be generated in C fiber nociceptors by input from touch-sensitive Aβ fibers (112, 219), it has been speculated that dorsal root reflexes may support touch-evoked pain or allodynia (64, 205, 206) (Figure 15B). However, Wasner et al. (389) failed to evoke vasodilatory responses by Aβ fiber stimulation in humans after capsaicin injection. The discrepancy between these results and those of Cervero and Laird (63) sparked an interesting scientific dispute (62, 388).
G. Inhibitory dorsal horn neurons in the control of itch
Like pain, itch serves a protective function in that it stimulates a scratching behavior aimed at the removal of a potentially harmful object or agent from the skin. Recent evidence suggests that pruritogenic (itch provoking) stimuli are detected by specialized primary afferent sensory neurons which release gastrin-releasing peptide at their spinal synapses. Here gastrin-releasing peptide activates its cognate receptor, the gastrin-releasing peptide receptors (GRPR), on lamina I neurons. Recordings from single histamine-sensitive C fibers in humans also indicate that a specific class of C fibers, distinct from typical nociceptors, are excited by pruritogenic stimuli (333). Mice deficient in GRPR show reduced scratching behavior upon exposure to puritogenic stimuli (354). Similarly, ablation of GRPR expressing neurons strongly reduces the responses of mice to such stimuli suggesting a “labeled line” for itch sensation, at least at the peripheral and spinal cord level (355). It should be noted, however, that a close interaction is likely to exist between itch and pain processing cells at the level of the CNS (170). A recent study has identified a small population of inhibitory interneurons in the superficial dorsal horn which require the transcription factor Bhlhb5 for survival (323). Deletion of Bhlhb5 causes mice to develop spontaneous scratching behavior although responses to acute noxious stimuli remain the same. These mice do however exhibit signs of increased central sensitization in response to chemical irritants and inflammatory stimuli.
XV. Restoring dorsal horn synaptic inhibition as a potential new therapeutic approach to pathological pain
As discussed in the previous chapter, work from several laboratories indicates that diminished synaptic inhibition in the dorsal horn is a major contributor to pathological pain states. Peripheral nerve damage (82, 83) and inflammation (140, 266, 314) as well as intense C fiber input to the spinal dorsal horn (289, 347) lead to a reduction in inhibitory pain control. Drugs that facilitate dorsal horn GABAA receptors, and thus restore this inhibition, should represent a new approach to the treatment of pathological pain syndromes. Injection of benzodiazepines such as diazepam (196) or midazolam (200) into the subarachnoid space of the spinal canal normalizes abnormal pain sensitivity in a wide range of rodent pain models and also show efficacy in humans (378). Similar anti-hyperalgesic actions are also obtained after systemic treatment with direct GABAA receptor agonists (195, 202), and inhibitors of the GABA degrading enzyme GABA transaminase (55, 56, 278). Furthermore, anecdotal evidence exists that supports an analgesic or anti-hyperalgesic role for systemic benzodiazepines in chronic pain patients (108, 138, 165). However, this role remained difficult to prove due to the confounding sedative effects of these drugs.
The generation of GABAA receptor point-mutated mice, in which the four types of benzodiazepine-sensitive GABAA receptor subunits have been rendered diazepam-insensitive individually (327), has helped attribute the different in vivo actions of diazepam to specific GABAA receptor subtypes. Most importantly, the sedative effects of diazepam have been assigned to α1-GABAA receptors (326), while the anxiolytic actions occur through α2-GABAA receptors (223). The evaluation of these mice in different pain models has revealed that diazepam-induced antihyperalgesia does not require activation of α1-GABAA receptors and is therefore independent of the sedative effects of benzodiazepines. Subsequent and more detailed analyses revealed that α2-GABAA receptors make the biggest contribution to the spinal antihyperalgesic actions of classical benzodiazepines. α3- and α5-GABAA receptors also contribute in some models depending on the pain model and the pain stimulus (heat, cold or mechanical) used (196). Most recently, mice were investigated which express diazepam-resistant α1-GABAA receptors and which are hence not sedated by systemic diazepam. These mice exhibited a clear analgesic or anti-hyperalgesic response to the drug in the absence of sedation. This antihyperalgesia was again mediated by α2-and α3-GABAA receptors (197).
Figure 16.
Antihyperalgesic actions of spinally injected diazepam and the contribution of the different GABAA receptor subtypes. (A) Antihyperalgesic action of intrathecal injection of diazepam (0.09 mg/kg, red symbols; vehicle, black) in mice with inflammatory hyperalgesia induced by subcutaneous injection of zymosan A. Open symbols contralateral non-inflamed paw. Note the lack of effect on the contralateral non-inflamed paw. (B) Contribution of the different diazepam-sensitive GABAA receptor subtypes to antihyperalgesia against inflammatory pain. (C) Antihyperalgesic activity of systemically administered L-838,417, a subtype selective (α1-sparing) GABAA receptor modulator visualized in a rat functional magnetic resonance imaging (fMRI) experiment. Inflamed and non-inflamed (contralateral) hindpaws were stimulated with a defined heat stimulus and brain activation was measured. Note again the pronounced reduction in brain activation after stimulation of the inflamed paw, and the considerably smaller effect on activation following stimulation of the non-inflamed paw. Modified from (196).
Several studies have meanwhile provided proof-of-principle evidence that profound antihyperalgesic activity can be obtained using α1-sparing subtype-specific benzodiazepine-site ligands. The majority of these compounds were developed in the quest for non-sedative anxiolytic drugs, but several of them have also been tested in rodent pain models. One such compound, L-838,417, which is a partial agonist at α2-, α3- and α5-GABAA receptors and lacks agonistic activity at α1 (246), has not only been tested in behavioral pain models but also in rat functional magnetic resonance (fMRI) experiments. There, it elicited a significant reduction in noxious heat stimulus-induced activation of pain-related brain areas, the so called pain matrix (196), but had only minor effects on brain activation elicited by acute nociceptive stimuli which was consistent with its antihyperalgesic action in behavioral tests. Several other compounds with various degrees of subtype selectivity and agonistic activity have also been scrutinized (197, 269). Compounds with the desired subunit selectivity and sufficiently high intrinsic activity at α2 and α3 showed considerable antihyperalgesic activity at non-sedative doses (for reviews see ref. 251, 268, 417). It is not yet known whether these results translate to humans, but the on-going development of such compounds may soon answer this question.
XVI. Conclusions
It is firmly established that inhibitory interneurons in the spinal dorsal horn serve important functions in the central processing of sensory information. A deficit in synaptic inhibition at this site immediately leads to changes in the sizes of receptive fields of dorsal horn neurons. In pathological and chronic pain states it is a major factor contributing to central pain sensitization. To date, substantial gains have been made in our understanding of the specific function of GABA and glycine and of their receptor isoforms in these processes. Ultimately it is hoped that this will result in the development of novel pharmacological approaches to pain treatment. The function of defined subtypes of dorsal horn interneurons and their integration in dorsal horn neuronal circuits is much less clear. Current models such as the one shown in Figure 17 are to date only partially based on direct experimental evidence. Recently developed techniques such as optogenetics, which allow precise spatial and temporal control of the activity of defined interneuron populations, as well as the targeted ablation of specific interneuron populations in vivo promise exciting new insights over the next few years.
Figure 17.
Possible integration of inhibitory interneurons in the spinal dorsal horn based on the findings discussed in this review. Excitatory and inhibitory axon terminals with red and yellow vesicles, respectively.
Acknowledgements
The authors would like to thanks Dres. Dietmar Benke, Edmund Foster, Hanns Möhler, and Jean-Marc Fritschy for critical reading of the manuscript and for helpful comments.
Grants The authors’ work related to spinal synaptic inhibition has been supported by the Swiss National Science Foundation, the NCCR Neural Plasticity and Repair, the Deutsche Forschungsgemeinschaft, and by the European Research Council through an Advanced Investigator Grant.
REFERENCES
- 1.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 2009;159:316–324. doi: 10.1016/j.neuroscience.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 4.Adams RH, Sato K, Shimada S, Tohyama M, Püschel AW, Betz H. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi S, Kotalla C, Guhring H, Takeshima H, Pahl A, Zeilhofer HU. Modulation of synaptic transmission by nociceptin/orphanin FQ and nocistatin in the spinal cord dorsal horn of mutant mice lacking the nociceptin/orphanin FQ receptor. Mol Pharmacol. 2001;59:612–618. doi: 10.1124/mol.59.3.612. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. doi: 10.1126/science.1083970. [DOI] [PubMed] [Google Scholar]
- 9.Airan RD, Hu ES, Vijaykumar R, Roy M, Meltzer LA, Deisseroth K. Integration of light-controlled neuronal firing and fast circuit imaging. Curr Opin Neurobiol. 2007;17:587–592. doi: 10.1016/j.conb.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Ghamdi KS, Polgar E, Todd AJ. Soma size distinguishes projection neurons from neurokinin 1 receptor-expressing interneurons in lamina I of the rat lumbar spinal dorsal horn. Neuroscience. 2009;164:1794–1804. doi: 10.1016/j.neuroscience.2009.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Khater KM, Kerr R, Todd AJ. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol. 2008;511:1–18. doi: 10.1002/cne.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez FJ, Kavookjian AM, Light AR. Synaptic interactions between GABA-immunoreactive profiles and the terminals of functionally defined myelinated nociceptors in the monkey and cat spinal cord. J Neurosci. 1992;12:2901–2917. doi: 10.1523/JNEUROSCI.12-08-02901.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez FJ, Kavookjian AM, Light AR. Ultrastructural morphology, synaptic relationships, and CGRP immunoreactivity of physiologically identified C-fiber terminals in the monkey spinal cord. J Comp Neurol. 1993;329:472–490. doi: 10.1002/cne.903290405. [DOI] [PubMed] [Google Scholar]
- 14.Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125:113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 15.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 18.Aubrey KR, Rossi FM, Ruivo R, Alboni S, Bellenchi GC, Le Goff A, Gasnier B, Supplisson S. The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotype. J Neurosci. 2007;27:6273–6281. doi: 10.1523/JNEUROSCI.1024-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba H, Goldstein PA, Okamoto M, Kohno T, Ataka T, Yoshimura M, Shimoji K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology. 2000;92:485–492. doi: 10.1097/00000542-200002000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 22.Baba H, Kohno T, Okamoto M, Goldstein PA, Shimoji K, Yoshimura M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol. 1998;508(Pt 1):83–93. doi: 10.1111/j.1469-7793.1998.083br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–484. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baccei ML, Fitzgerald M. Intrinsic firing properties of developing rat superficial dorsal horn neurons. Neuroreport. 2005;16:1325–1328. doi: 10.1097/01.wnr.0000175612.08560.10. [DOI] [PubMed] [Google Scholar]
- 26.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 27.Barber RP, Vaughn JE, Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Res. 1982;238:305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- 28.Barber RP, Vaughn JE, Saito K, McLaughlin BJ, Roberts E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978;141:35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- 29.Barnard EA. The Molecular Architecture of GABA-A Receptors. In: Möhler H, editor. Pharmacology of GABA and Glycine Neurotransmission. Springer; Berlin: 2001. pp. 79–99. [Google Scholar]
- 30.Barnard EA, Endo M. Pharmacology of ionic channel fucntion. Springer; 1998. The range of structures of the transmitter-gated channels; pp. 363–390. [Google Scholar]
- 31.Bateson AN, Lasham A, Darlison MG. γ-Aminobutyric acidA receptor heterogeneity is increased by alternative splicing of a novel β-subunit gene transcript. J Neurochem. 1991;56:1437–1440. doi: 10.1111/j.1471-4159.1991.tb11443.x. [DOI] [PubMed] [Google Scholar]
- 32.Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors: Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 33.Baumann SW, Baur R, Sigel E. Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth. 2006;53:769–775. doi: 10.1007/BF03022793. [DOI] [PubMed] [Google Scholar]
- 35.Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- 36.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 38.Benke D, Honer M, Michel C, Möhler H. GABAA receptor subtypes differentiated by their γ-subunit variants: prevalence, pharmacology and subunit architecture. Neuropharmacology. 1996;35:1413–1423. doi: 10.1016/s0028-3908(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 39.Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- 40.Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- 41.Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betz H, Graham D, Rehm H. Identification of polypeptides associated with a putative neuronal nicotinic acetylcholine receptor. J Biol Chem. 1982;257:11390–11394. [PubMed] [Google Scholar]
- 43.Beyer C, Roberts LA, Komisaruk BR. Hyperalgesia induced by altered glycinergic activity at the spinal cord. Life Sci. 1985;37:875–882. doi: 10.1016/0024-3205(85)90523-5. [DOI] [PubMed] [Google Scholar]
- 44.Bicknell HR, Jr., Beal JA. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol. 1984;226:508–522. doi: 10.1002/cne.902260406. [DOI] [PubMed] [Google Scholar]
- 45.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- 47.Bohlhalter S, Möhler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res. 1994;642:59–69. doi: 10.1016/0006-8993(94)90905-9. [DOI] [PubMed] [Google Scholar]
- 48.Bohlhalter S, Weinmann O, Möhler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradaia A, Schlichter R, Trouslard J. Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord. J Physiol. 2004;559:169–186. doi: 10.1113/jphysiol.2004.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABAA receptor-mediated inhibition in the cerebral cortex. J Neurochem. 2008;105:1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- 51.Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, Rorsman P. γ-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bremner L, Fitzgerald M, Baccei M. Functional GABAA-receptor-mediated inhibition in the neonatal dorsal horn. J Neurophysiol. 2006;95:3893–3897. doi: 10.1152/jn.00123.2006. [DOI] [PubMed] [Google Scholar]
- 53.Bremner LR, Fitzgerald M. Postnatal tuning of cutaneous inhibitory receptive fields in the rat. J Physiol. 2008;586:1529–1537. doi: 10.1113/jphysiol.2007.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brohl D, Strehle M, Wende H, Hori K, Bormuth I, Nave KA, Muller T, Birchmeier C. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev Biol. 2008;322:381–393. doi: 10.1016/j.ydbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Buckett WR. Induction of analgesia and morphine potentiation by irreversible inhibitors of GABA-transaminase [proceedings] Br J Pharmacol. 1980;68:129P–130P. [PMC free article] [PubMed] [Google Scholar]
- 56.Buckett WR. Irreversible inhibitors of GABA transaminase induce antinociceptive effects and potentiate morphine. Neuropharmacology. 1980;19:715–722. doi: 10.1016/0028-3908(80)90062-3. [DOI] [PubMed] [Google Scholar]
- 57.Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral Δ-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172. doi: 10.1016/s0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 58.Cabot JB, Bushnell A, Alessi V, Mendell NR. Postsynaptic gephyrin immunoreactivity exhibits a nearly one-to-one correspondence with γ-aminobutyric acid-like immunogold-labeled synaptic inputs to sympathetic preganglionic neurons. J Comp Neurol. 1995;356:418–432. doi: 10.1002/cne.903560309. [DOI] [PubMed] [Google Scholar]
- 59.Carlton SM, Hayes ES. Light microscopic and ultrastructural analysis of GABA-immunoreactive profiles in the monkey spinal cord. J Comp Neurol. 1990;300:162–182. doi: 10.1002/cne.903000203. [DOI] [PubMed] [Google Scholar]
- 60.Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat Rev Neurosci. 2003;4:289–297. doi: 10.1038/nrn1073. [DOI] [PubMed] [Google Scholar]
- 61.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cervero F, Laird JM. Absence of evidence is not evidence of absence (again) Pain. 2000;84:114–115. doi: 10.1016/S0304-3959(99)00180-3. [DOI] [PubMed] [Google Scholar]
- 63.Cervero F, Laird JM. Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. Neuroreport. 1996;7:526–528. [PubMed] [Google Scholar]
- 64.Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–351. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 65.Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008;139:603–609. doi: 10.1016/j.pain.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Chen YL, Li AH, Yeh TH, Chou AH, Wang HL. Nocistatin and nociceptin exert opposite effects on the excitability of central amygdala nucleus-periaqueductal gray projection neurons. Mol Cell Neurosci. 2009;40:76–88. doi: 10.1016/j.mcn.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, Jin N, Narasaraju T, Chen J, McFarland LR, Scott M, Liu L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem Biophys Res Commun. 2004;319:774–780. doi: 10.1016/j.bbrc.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 68.Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 69.Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- 70.Chery N, De Koninck Y. GABAB receptors are the first target of released GABA at lamina I inhibitory synapses in the adult rat spinal cord. J Neurophysiol. 2000;84:1006–1011. doi: 10.1152/jn.2000.84.2.1006. [DOI] [PubMed] [Google Scholar]
- 71.Chery N, de Koninck Y. Junctional versus extrajunctional glycine and GABAA receptor-mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. J Neurosci. 1999;19:7342–7355. doi: 10.1523/JNEUROSCI.19-17-07342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 73.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 74.Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Claveau D, Sirinyan M, Guay J, Gordon R, Chan CC, Bureau Y, Riendeau D, Mancini JA. Microsomal prostaglandin E synthase-1 is a major terminal synthase that is selectively up-regulated during cyclooxygenase-2-dependent prostaglandin E2 production in the rat adjuvant-induced arthritis model. J Immunol. 2003;170:4738–4744. doi: 10.4049/jimmunol.170.9.4738. [DOI] [PubMed] [Google Scholar]
- 77.Colin I, Rostaing P, Augustin A, Triller A. Localization of components of glycinergic synapses during rat spinal cord development. J Comp Neurol. 1998;398:359–372. [PubMed] [Google Scholar]
- 78.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 79.Cordero-Erausquin M, Changeux JP. Tonic nicotinic modulation of serotoninergic transmission in the spinal cord. Proc Natl Acad Sci U S A. 2001;98:2803–2807. doi: 10.1073/pnas.041600698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: impact of chloride extrusion capacity. J Neurosci. 2005;25:9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cordero-Erausquin M, Pons S, Faure P, Changeux JP. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain. 2004;109:308–318. doi: 10.1016/j.pain.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 82.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 83.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 84.Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA- and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain. 2004;112:156–163. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 86.Dahlhaus A, Ruscheweyh R, Sandkuhler J. Synaptic input of rat spinal lamina I projection and unidentified neurones in vitro. J Physiol. 2005;566:355–368. doi: 10.1113/jphysiol.2005.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danscher G, Stoltenberg M. Zinc-specific autometallographic in vivo selenium methods: tracing of zinc-enriched (ZEN) terminals, ZEN pathways, and pools of zinc ions in a multitude of other ZEN cells. J Histochem Cytochem. 2005;53:141–153. doi: 10.1369/jhc.4R6460.2005. [DOI] [PubMed] [Google Scholar]
- 88.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 89.De Koninck Y, Henry JL. Prolonged GABAA-mediated inhibition following single hair afferent input to single spinal dorsal horn neurones in cats. J Physiol. 1994;476:89–100. [PMC free article] [PubMed] [Google Scholar]
- 90.Doi A, Kishimoto K, Ishibashi H. Modulation of glycine-induced currents by zinc and other metal cations in neurons acutely dissociated from the dorsal motor nucleus of the vagus of the rat. Brain Res. 1999;816:424–430. doi: 10.1016/s0006-8993(98)01172-x. [DOI] [PubMed] [Google Scholar]
- 91.Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- 92.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drew CA, Johnston GA, Weatherby RP. Bicuculline-insensitive GABA receptors: studies on the binding of (-)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984;52:317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- 94.Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J Neurosci. 2009;29:7220–7229. doi: 10.1523/JNEUROSCI.4362-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Druzin M, Haage D, Johansson S. Bicuculline free base blocks voltage-activated K+ currents in rat medial preoptic neurons. Neuropharmacology. 2004;46:285–295. doi: 10.1016/j.neuropharm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Möhler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eccles JC, Schmidt R, Willis WD. Pharmacological Studies on Presynaptic Inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eisenach JC, Curry R, Tong C, Houle TT, Yaksh TL. Effects of intrathecal ketorolac on human experimental pain. Anesthesiology. 2010;112:1216–1224. doi: 10.1097/ALN.0b013e3181d94d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 101.Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor ρ1 and ρ2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 102.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 103.Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev. 2010;63:103–112. doi: 10.1016/j.brainresrev.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia. 2010;58:1066–1073. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- 105.Falcon M, Guendellman D, Stolberg A, Frenk H, Urca G. Development of thermal nociception in rats. Pain. 1996;67:203–208. doi: 10.1016/0304-3959(96)03070-9. [DOI] [PubMed] [Google Scholar]
- 106.Feng YP, Li YQ, Wang W, Wu SX, Chen T, Shigemoto R, Mizuno N. Morphological evidence for GABA/glycine-cocontaining terminals in synaptic contact with neurokinin-1 receptor-expressing neurons in the sacral dorsal commissural nucleus of the rat. Neurosci Lett. 2005;388:144–148. doi: 10.1016/j.neulet.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 107.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Elsevier; 2006. pp. 125–142. [Google Scholar]
- 108.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Clonazepam open clinical treatment trial for myofascial syndrome associated chronic pain. Pain Med. 2000;1:332–339. doi: 10.1046/j.1526-4637.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 109.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 110.Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fitzgerald M, Woolf CJ. Effects of cutaneous nerve and intraspinal conditioning of C-fibre afferent terminal excitability in decerebrate spinal rats. J Physiol. 1981;318:25–39. doi: 10.1113/jphysiol.1981.sp013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleming AA, Todd AJ. Thyrotropin-releasing hormone- and GABA-like immunoreactivity coexist in neurons in the dorsal horn of the rat spinal cord. Brain Res. 1994;638:347–351. doi: 10.1016/0006-8993(94)90670-x. [DOI] [PubMed] [Google Scholar]
- 114.Fodor L, Boros A, Dezso P, Maksay G. Expression of heteromeric glycine receptor-channels in rat spinal cultures and inhibition by neuroactive steroids. Neurochem Int. 2006;49:577–583. doi: 10.1016/j.neuint.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 115.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 116.Fritschy JM, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 117.Galan A, Cervero F. Painful stimuli induce in vivo phosphorylation and membrane mobilization of mouse spinal cord NKCC1 co-transporter. Neuroscience. 2005;133:245–252. doi: 10.1016/j.neuroscience.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 118.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gassner M, Ruscheweyh R, Sandkühler J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain. 2009;145:204–210. doi: 10.1016/j.pain.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 120.Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2005;1031:229–237. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 122.Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- 123.Gobel S. Golgi studies in the substantia gelatinosa neurons in the spinal trigeminal nucleus. J Comp Neurol. 1975;162:397–415. doi: 10.1002/cne.901620308. [DOI] [PubMed] [Google Scholar]
- 124.Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- 125.Gomeza J, Ohno K, Hülsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003;40:797–806. doi: 10.1016/s0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- 126.Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol. 2002;12:508–515. doi: 10.1016/s0959-4388(02)00371-9. [DOI] [PubMed] [Google Scholar]
- 127.Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 128.Gregor M, Zimmermann M. Dorsal root potentials produced by afferent volleys in cutaneous group 3 fibers. J Physiol. 1973;232:413–425. doi: 10.1113/jphysiol.1973.sp010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 130.Grudt TJ, Henderson G. Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by mu-opioid and GABAB agonists. J Physiol. 1998;507(Pt 2):473–483. doi: 10.1111/j.1469-7793.1998.473bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 133.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 134.Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- 135.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 136.Hantman AW, Perl ER. Molecular and genetic features of a labeled class of spinal substantia gelatinosa neurons in a transgenic mouse. J Comp Neurol. 2005;492:90–100. doi: 10.1002/cne.20709. [DOI] [PubMed] [Google Scholar]
- 137.Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harkins S, Linford J, Cohen J, Kramer T, Cueva L. Administration of clonazepam in the treatment of TMD and associated myofascial pain: a double-blind pilot study. J Craniomandib Disord. 1991;5:179–186. [PubMed] [Google Scholar]
- 139.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- 140.Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Müller U. GlyRα3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 141.Harvey RJ, Kim HC, Darlison MG. Molecular cloning reveals the existence of a fourth γ subunit of the vertebrate brain GABAA receptor. FEBS Lett. 1993;331:211–216. doi: 10.1016/0014-5793(93)80339-v. [DOI] [PubMed] [Google Scholar]
- 142.Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor α1 subunit. J Physiol. 1999;520(Pt 1):53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harvey VL, Caley A, Muller UC, Harvey RJ, Dickenson AH. A Selective Role for α3 Subunit Glycine Receptors in Inflammatory Pain. Front Mol Neurosci. 2009;2:14. doi: 10.3389/neuro.02.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hathway G, Harrop E, Baccei M, Walker S, Moss A, Fitzgerald M. A postnatal switch in GABAergic control of spinal cutaneous reflexes. Eur J Neurosci. 2006;23:112–118. doi: 10.1111/j.1460-9568.2005.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haverkamp S, Müller U, Zeilhofer HU, Harvey RJ, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the α2 subunit. J Comp Neurol. 2004;477:399–411. doi: 10.1002/cne.20267. [DOI] [PubMed] [Google Scholar]
- 147.Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 148.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heinemann U, Schaible HG, Schmidt RF. Changes in extracellular potassium concentration in cat spinal cord in response to innocuous and noxious stimulation of legs with healthy and inflamed knee joints. Exp Brain Res. 1990;79:283–292. doi: 10.1007/BF00608237. [DOI] [PubMed] [Google Scholar]
- 150.Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkühler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Δ9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- 152.Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 154.Hirzel K, Müller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, Betz H, Laube B. Hyperekplexia phenotype of glycine receptor α1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 155.Hodgson AJ, Penke B, Erdei A, Chubb IW, Somogyi P. Antisera to γ-aminobutyric acid. I. Production and characterization using a new model system. J Histochem Cytochem. 1985;33:229–239. doi: 10.1177/33.3.3973378. [DOI] [PubMed] [Google Scholar]
- 156.Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- 157.Hori K, Cholewa-Waclaw J, Nakada Y, Glasgow SM, Masui T, Henke RM, Wildner H, Martarelli B, Beres TM, Epstein JA, Magnuson MA, Macdonald RJ, Birchmeier C, Johnson JE. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes Dev. 2008;22:166–178. doi: 10.1101/gad.1628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 159.Hösl K, Reinold H, Harvey RJ, Müller U, Narumiya S, Zeilhofer HU. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor α3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 160.Hosli E, Otten U, Hosli L. Expression of GABAA receptors by reactive astrocytes in explant and primary cultures of rat CNS. Int J Dev Neurosci. 1997;15:949–960. doi: 10.1016/s0736-5748(97)00041-5. [DOI] [PubMed] [Google Scholar]
- 161.Hossaini M, Duraku LS, Sarac C, Jongen JL, Holstege JC. Differential distribution of activated spinal neurons containing glycine and/or GABA and expressing c-fos in acute and chronic pain models. Pain. 2010;151:356–365. doi: 10.1016/j.pain.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 162.Hossaini M, French PJ, Holstege JC. Distribution of glycinergic neuronal somata in the rat spinal cord. Brain Res. 2007;1142:61–69. doi: 10.1016/j.brainres.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 163.Howard RF, Walker SM, Mota PM, Fitzgerald M. The ontogeny of neuropathic pain: postnatal onset of mechanical allodynia in rat spared nerve injury (SNI) and chronic constriction injury (CCI) models. Pain. 2005;115:382–389. doi: 10.1016/j.pain.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 164.Huang M, Huang T, Xiang Y, Xie Z, Chen Y, Yan R, Xu J, Cheng L. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev Biol. 2008;322:394–405. doi: 10.1016/j.ydbio.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 165.Hugel H, Ellershaw JE, Dickman A. Clonazepam as an adjuvant analgesic in patients with cancer-related neuropathic pain. J Pain Symptom Manage. 2003;26:1073–1074. doi: 10.1016/j.jpainsymman.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 166.Hugel S, Schlichter R. Convergent control of synaptic GABA release from rat dorsal horn neurones by adenosine and GABA autoreceptors. J Physiol. 2003;551:479–489. doi: 10.1113/jphysiol.2003.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hunt SP, Kelly JS, Emson PC, Kimmel JR, Miller RJ, Wu JY. An immunohistochemical study of neuronal populations containing neuropeptides or γ-aminobutyrate within the superficial layers of the rat dorsal horn. Neuroscience. 1981;6:1883–1898. doi: 10.1016/0306-4522(81)90029-4. [DOI] [PubMed] [Google Scholar]
- 169.Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 170.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 171.Ingram RA, Fitzgerald M, Baccei ML. Developmental changes in the fidelity and short-term plasticity of GABAergic synapses in the neonatal rat dorsal horn. J Neurophysiol. 2008;99:3144–3150. doi: 10.1152/jn.01342.2007. [DOI] [PubMed] [Google Scholar]
- 172.Inquimbert P, Rodeau JL, Schlichter R. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III-IV of the young rat spinal cord. Eur J Neurosci. 2007;26:2940–2949. doi: 10.1111/j.1460-9568.2007.05919.x. [DOI] [PubMed] [Google Scholar]
- 173.Inquimbert P, Rodeau JL, Schlichter R. Regional differences in the decay kinetics of GABAA receptor-mediated miniature IPSCs in the dorsal horn of the rat spinal cord are determined by mitochondrial transport of cholesterol. J Neurosci. 2008;28:3427–3437. doi: 10.1523/JNEUROSCI.5076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iyadomi M, Iyadomi I, Kumamoto E, Tomokuni K, Yoshimura M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain. 2000;85:385–393. doi: 10.1016/S0304-3959(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 175.Jang IS, Rhee JS, Kubota H, Akaike N, Akaike N. Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J Physiol. 2001;536:505–519. doi: 10.1111/j.1469-7793.2001.0505c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jennings E, Fitzgerald M. C-fos can be induced in the neonatal rat spinal cord by both noxious and innocuous peripheral stimulation. Pain. 1996;68:301–306. doi: 10.1016/s0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- 177.Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534:805–812. doi: 10.1111/j.1469-7793.2001.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- 179.Jiang MC, Gebhart GF. Development of mustard oil-induced hyperalgesia in rats. Pain. 1998;77:305–313. doi: 10.1016/S0304-3959(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 180.Jiang P, Yang CX, Wang YT, Xu TL. Mechanisms of modulation of pregnanolone on glycinergic response in cultured spinal dorsal horn neurons of rat. Neuroscience. 2006;141:2041–2050. doi: 10.1016/j.neuroscience.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 181.Jimenez I, Rudomin P, Solodkin M. Mechanisms involved in the depolarization of cutaneous afferents produced by segmental and descending inputs in the cat spinal cord. Exp Brain Res. 1987;69:195–207. doi: 10.1007/BF00247042. [DOI] [PubMed] [Google Scholar]
- 182.Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- 183.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 184.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 185.Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 186.Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 187.Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. J Neurosci. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 189.Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. α2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 190.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5α-reduced neurosteroids is developmentally regulated and shapes GABAA miniature IPSCs in lamina II of the spinal cord. J Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- 194.Kiyosawa A, Katsurabayashi S, Akaike N, Pang ZP. Nicotine facilitates glycine release in the rat spinal dorsal horn. J Physiol. 2001;536:101–110. doi: 10.1111/j.1469-7793.2001.t01-1-00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kjaer M, Nielsen H. The analgesic effect of the GABA-agonist THIP in patients with chronic pain of malignant origin. A phase-1-2 study. Br J Clin Pharmacol. 1983;16:477–485. doi: 10.1111/j.1365-2125.1983.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy J-M, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 197.Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 198.Kneussel M, Brandstatter JH, Laube B, Stahl S, Müller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999;518(Pt 3):803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kontinen VK, Dickenson AH. Effects of midazolam in the spinal nerve ligation model of neuropathic pain in rats. Pain. 2000;85:425–431. doi: 10.1016/S0304-3959(99)00298-5. [DOI] [PubMed] [Google Scholar]
- 201.Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101–110. doi: 10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- 202.Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. GABAA agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol. 2004;68:1573–1580. doi: 10.1016/j.bcp.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 203.Kuhse J, Schmieden V, Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990;265:22317–22320. [PubMed] [Google Scholar]
- 204.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci Lett. 2004;361:200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 206.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 207.Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lao LJ, Kawasaki Y, Yang K, Fujita T, Kumamoto E. Modulation by adenosine of Aδ and C primary-afferent glutamatergic transmission in adult rat substantia gelatinosa neurons. Neuroscience. 2004;125:221–231. doi: 10.1016/j.neuroscience.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 209.Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522(Pt 2):215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- 213.Li H, Lang B, Kang JF, Li YQ. Serotonin potentiates the response of neurons of the superficial laminae of the rat spinal dorsal horn to γ-aminobutyric acid. Brain Res Bull. 2000;52:559–565. doi: 10.1016/s0361-9230(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 214.Li P, Calejesan AA, Zhuo M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- 215.Li Y, Wu LJ, Legendre P, Xu TL. Asymmetric cross-inhibition between GABAA and glycine receptors in rat spinal dorsal horn neurons. J Biol Chem. 2003;278:38637–38645. doi: 10.1074/jbc.M303735200. [DOI] [PubMed] [Google Scholar]
- 216.Liang YC, Huang CC, Hsu KS, Takahashi T. Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices. J Physiol. 2004;555:85–96. doi: 10.1113/jphysiol.2003.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- 218.Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- 219.Lin Q, Zou X, Willis WD. Aδ and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol. 2000;84:2695–2698. doi: 10.1152/jn.2000.84.5.2695. [DOI] [PubMed] [Google Scholar]
- 220.Liu B, Liu Z, Chen T, Li H, Qiang B, Yuan J, Peng X, Qiu M. Selective expression of Bhlhb5 in subsets of early-born interneurons and late-born association neurons in the spinal cord. Dev Dyn. 2007;236:829–835. doi: 10.1002/dvdy.21061. [DOI] [PubMed] [Google Scholar]
- 221.Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 222.Loomis CW, Khandwala H, Osmond G, Hefferan MP. Coadministration of intrathecal strychnine and bicuculline effects synergistic allodynia in the rat: an isobolographic analysis. J Pharmacol Exp Ther. 2001;296:756–761. [PubMed] [Google Scholar]
- 223.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 224.Lozovaya N, Yatsenko N, Beketov A, Tsintsadze T, Burnashev N. Glycine receptors in CNS neurons as a target for nonretrograde action of cannabinoids. J Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lu T, Rubio ME, Trussell LO. Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron. 2008;57:524–535. doi: 10.1016/j.neuron.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 226.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol. 2007;582:127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luque JM, Nelson N, Richards JG. Cellular expression of glycine transporter 2 messenger RNA exclusively in rat hindbrain and spinal cord. Neuroscience. 1995;64:525–535. doi: 10.1016/0306-4522(94)00404-s. [DOI] [PubMed] [Google Scholar]
- 230.Lynch JW, Jacques P, Pierce KD, Schofield PR. Zinc potentiation of the glycine receptor chloride channel is mediated by allosteric pathways. J Neurochem. 1998;71:2159–2168. doi: 10.1046/j.1471-4159.1998.71052159.x. [DOI] [PubMed] [Google Scholar]
- 231.Ma W, Behar T, Chang L, Barker JL. Transient increase in expression of GAD65 and GAD67 mRNAs during postnatal development of rat spinal cord. J Comp Neurol. 1994;346:151–160. doi: 10.1002/cne.903460111. [DOI] [PubMed] [Google Scholar]
- 232.Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- 233.Mackie M, Hughes DI, Maxwell DJ, Tillakaratne NJ, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience. 2003;119:461–472. doi: 10.1016/s0306-4522(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 234.Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 235.Maeda A, Katafuchi T, Oba Y, Shiokawa H, Yoshimura M. Enhancement of GABAergic tonic currents by midazolam and noradrenaline in rat substantia gelatinosa neurons in vitro. Anesthesiology. 2010;113:429–437. doi: 10.1097/ALN.0b013e3181e19bd4. [DOI] [PubMed] [Google Scholar]
- 236.Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 238.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. Embo J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mancini JA, Blood K, Guay J, Gordon R, Claveau D, Chan CC, Riendeau D. Cloning, expression, and up-regulation of inducible rat prostaglandin E synthase during lipopolysaccharide-induced pyresis and adjuvant-induced arthritis. J Biol Chem. 2001;276:4469–4475. doi: 10.1074/jbc.M006865200. [DOI] [PubMed] [Google Scholar]
- 240.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 241.Marsh D, Dickenson A, Hatch D, Fitzgerald M. Epidural opioid analgesia in infant rats I: mechanical and heat responses. Pain. 1999;82:23–32. doi: 10.1016/S0304-3959(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 242.Marshall GE, Shehab SA, Spike RC, Todd AJ. Neurokinin-1 receptors on lumbar spinothalamic neurons in the rat. Neuroscience. 1996;72:255–263. doi: 10.1016/0306-4522(95)00558-7. [DOI] [PubMed] [Google Scholar]
- 243.Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Maxwell DJ, Réthelyi M. Ultrastructure and synaptic connections of cutaneous afferent fibres in the spinal cord. Trends in Neurosciences. 1987;10:117–123. [Google Scholar]
- 245.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 246.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 247.McLaughlin BJ, Barber R, Saito K, Roberts E, Wu JY. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J Comp Neurol. 1975;164:305–321. doi: 10.1002/cne.901640304. [DOI] [PubMed] [Google Scholar]
- 248.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 249.Miller PS, Beato M, Harvey RJ, Smart TG. Molecular determinants of glycine receptor αβ subunit sensitivities to Zn2+-mediated inhibition. J Physiol. 2005;566:657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCγ interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mirza NR, Munro G. The role of GABAA receptor subtypes as analgesic targets. Drug News Perspect. 2010;23:351–360. doi: 10.1358/dnp.2010.23.6.1489909. [DOI] [PubMed] [Google Scholar]
- 252.Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 254.Mitchell K, Spike RC, Todd AJ. An immunocytochemical study of glycine receptor and GABA in laminae I-III of rat spinal dorsal horn. J Neurosci. 1993;13:2371–2381. doi: 10.1523/JNEUROSCI.13-06-02371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- 256.Möhler H. Functions of GABAA Receptors: Pharmacology and Pathophysiology. In: Möhler H, editor. Pharmacology of GABA and Glycine Neurotransmission. Springer; Berlin: 2001. pp. 101–116. [Google Scholar]
- 257.Möhler H, Crestani F, Rudolph U. GABAA-receptor subtypes: a new pharmacology. Curr Opin Pharmacol. 2001;1:22–25. doi: 10.1016/s1471-4892(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 258.Mokha SS, McMillan JA, Iggo A. Dorsal root potentials in the cat: effects of bicuculline. Brain Res. 1983;259:313–318. doi: 10.1016/0006-8993(83)91265-9. [DOI] [PubMed] [Google Scholar]
- 259.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Moragues N, Ciofi P, Lafon P, Odessa MF, Tramu G, Garret M. cDNA cloning and expression of a γ-aminobutyric acid A receptor ε-subunit in rat brain. Eur J Neurosci. 2000;12:4318–4330. [PubMed] [Google Scholar]
- 261.Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis. 2004;17:62–69. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 262.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 263.Mori M, Gähwiler BH, Gerber U. β-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol. 2002;539:191–200. doi: 10.1113/jphysiol.2001.013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mori M, Kose A, Tsujino T, Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- 265.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr., Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Müller F, Heinke B, Sandkühler J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience. 2003;122:799–805. doi: 10.1016/j.neuroscience.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 267.Müller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 268.Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 269.Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EO, Larsen JS, Ahring PK, Mirza NR. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitr ile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- 270.Muth-Selbach U, Dybek E, Kollosche K, Stegmann JU, Holthusen H, Lipfert P, Zeilhofer HU. The spinal antinociceptive effect of nocistatin in neuropathic rats is blocked by D-serine. Anesthesiology. 2004;101:753–758. doi: 10.1097/00000542-200409000-00025. [DOI] [PubMed] [Google Scholar]
- 271.Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- 272.Nabekura J, Xu TL, Rhee JS, Li JS, Akaike N. α2-adrenoceptor-mediated enhancement of glycine response in rat sacral dorsal commissural neurons. Neuroscience. 1999;89:29–41. doi: 10.1016/s0306-4522(98)00303-0. [DOI] [PubMed] [Google Scholar]
- 273.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral Δ-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 274.Narikawa K, Furue H, Kumamoto E, Yoshimura M. In vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation. J Neurophysiol. 2000;84:2171–2174. doi: 10.1152/jn.2000.84.4.2171. [DOI] [PubMed] [Google Scholar]
- 275.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 276.Nikolic Z, Laube B, Weber RG, Lichter P, Kioschis P, Poustka A, Mülhardt C, Becker CM. The human glycine receptor subunit α3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J Biol Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- 277.Nishi S, Minota S, Karczmar AG. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974;13:215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- 278.Novak V, Kanard R, Kissel JT, Mendell JR. Treatment of painful sensory neuropathy with tiagabine: a pilot study. Clin Auton Res. 2001;11:357–361. doi: 10.1007/BF02292767. [DOI] [PubMed] [Google Scholar]
- 279.Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 280.Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- 281.Oliva AA, Jr., Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ottersen OP. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 1989;180:1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- 285.Pan YZ, Li DP, Pan HL. Inhibition of glutamatergic synaptic input to spinal lamina IIo neurons by presynaptic α2-adrenergic receptors. J Neurophysiol. 2002;87:1938–1947. doi: 10.1152/jn.00575.2001. [DOI] [PubMed] [Google Scholar]
- 286.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 287.Pape JR, Bertrand SS, Lafon P, Odessa MF, Chaigniau M, Stiles JK, Garret M. Expression of GABAA receptor α3-, θ-, and ε-subunit mRNAs during rat CNS development and immunolocalization of the epsilon subunit in developing postnatal spinal cord. Neuroscience. 2009;160:85–96. doi: 10.1016/j.neuroscience.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pastor A, Chvatal A, Sykova E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci. 1995;7:1188–1198. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 289.Pernía-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schüttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–764. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- 291.Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- 292.Poisbeau P, Patte-Mensah C, Keller AF, Barrot M, Breton JD, Luis-Delgado OE, Freund-Mercier MJ, Mensah-Nyagan AG, Schlichter R. Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J Neurosci. 2005;25:11768–11776. doi: 10.1523/JNEUROSCI.3841-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Polgar E, Al-Khater KM, Shehab S, Watanabe M, Todd AJ. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. J Neurosci. 2008;28:13150–13160. doi: 10.1523/JNEUROSCI.4053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C γin rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- 295.Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 296.Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 298.Polgar E, Puskar Z, Watt C, Matesz C, Todd AJ. Selective innervation of lamina I projection neurones that possess the neurokinin 1 receptor by serotonin-containing axons in the rat spinal cord. Neuroscience. 2002;109:799–809. doi: 10.1016/s0306-4522(01)00304-9. [DOI] [PubMed] [Google Scholar]
- 299.Polgar E, Shehab SA, Watt C, Todd AJ. GABAergic neurons that contain neuropeptide Y selectively target cells with the neurokinin 1 receptor in laminae III and IV of the rat spinal cord. J Neurosci. 1999;19:2637–2646. doi: 10.1523/JNEUROSCI.19-07-02637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pow DV, Wright LL, Vaney DI. The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods. 1995;56:115–123. doi: 10.1016/0165-0270(94)00113-u. [DOI] [PubMed] [Google Scholar]
- 301.Powell JJ, Todd AJ. Light and electron microscope study of GABA-immunoreactive neurones in lamina III of rat spinal cord. J Comp Neurol. 1992;315:125–136. doi: 10.1002/cne.903150202. [DOI] [PubMed] [Google Scholar]
- 302.Poyatos I, Ponce J, Aragon C, Gimenez C, Zafra F. The glycine transporter GLYT2 is a reliable marker for glycine-immunoreactive neurons. Brain Res Mol Brain Res. 1997;49:63–70. doi: 10.1016/s0169-328x(97)00124-1. [DOI] [PubMed] [Google Scholar]
- 303.Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol. 2002;539:817–836. doi: 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Price TJ, Hargreaves KM, Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res. 2006;1112:146–158. doi: 10.1016/j.brainres.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Proudlock F, Spike RC, Todd AJ. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neurol. 1993;327:289–297. doi: 10.1002/cne.903270210. [DOI] [PubMed] [Google Scholar]
- 308.Puskar Z, Polgar E, Todd AJ. A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord. Neuroscience. 2001;102:167–176. doi: 10.1016/s0306-4522(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 309.Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q. Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev. 2002;16:1220–1233. doi: 10.1101/gad.982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Racz I, Schütz B, Abo-Salem OM, Zimmer A. Visceral, inflammatory and neuropathic pain in glycine receptor α3-deficient mice. Neuroreport. 2005;16:2025–2028. doi: 10.1097/00001756-200512190-00011. [DOI] [PubMed] [Google Scholar]
- 311.Rajalu M, Müller UC, Caley A, Harvey RJ, Poisbeau P. Plasticity of synaptic inhibition in mouse spinal cord lamina II neurons during early postnatal development and after inactivation of the glycine receptor α3 subunit gene. Eur J Neurosci. 2009;30:2284–2292. doi: 10.1111/j.1460-9568.2009.07018.x. [DOI] [PubMed] [Google Scholar]
- 312.Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 313.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol. 1995;484(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, Pahl A, Brune K, Narumiya S, Müller U, Zeilhofer HU. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rethelyi M, Light AR, Perl ER. Synaptic complexes formed by functionally defined primary afferent units with fine myelinated fibers. J Comp Neurol. 1982;207:381–393. doi: 10.1002/cne.902070409. [DOI] [PubMed] [Google Scholar]
- 316.Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- 317.Rhee JS, Wang ZM, Nabekura J, Inoue K, Akaike N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J Physiol. 2000;524(Pt 2):471–483. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ribeiro-da-Silva A. Substantia gelatinosa of the spinal cord. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. [Google Scholar]
- 319.Ribeiro-Da-Silva A, Castro-Lopes JM, Coimbra A. Distribution of glomeruli with fluoride-resistant acid phosphatase (FRAP)-containing terminals in the substantia gelatinosa of the rat. Brain Res. 1986;377:323–329. doi: 10.1016/0006-8993(86)90875-9. [DOI] [PubMed] [Google Scholar]
- 320.Ribeiro-da-Silva A, Tagari P, Cuello AC. Morphological characterization of substance P-like immunoreactive glomeruli in the superficial dorsal horn of the rat spinal cord and trigeminal subnucleus caudalis: a quantitative study. J Comp Neurol. 1989;281:497–415. doi: 10.1002/cne.902810402. [DOI] [PubMed] [Google Scholar]
- 321.Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- 322.Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J Neurophysiol. 2008;100:169–184. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 325.Rowan S, Todd AJ, Spike RC. Evidence that neuropeptide Y is present in GABAergic neurons in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1993;53:537–545. doi: 10.1016/0306-4522(93)90218-5. [DOI] [PubMed] [Google Scholar]
- 326.Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 327.Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 328.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- 329.Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- 330.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 331.Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- 332.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schmitt B, Knaus P, Becker CM, Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987;26:805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- 335.Schoenen J. The dendritic organization of the human spinal cord: the dorsal horn. Neuroscience. 1982;7:2057–2087. doi: 10.1016/0306-4522(82)90120-8. [DOI] [PubMed] [Google Scholar]
- 336.Schoffnegger D, Ruscheweyh R, Sandkühler J. Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain. 2008;135:300–310. doi: 10.1016/j.pain.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 337.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003;121:907–916. doi: 10.1016/s0306-4522(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 339.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Seddik R, Schlichter R, Trouslard J. Corelease of GABA/glycine in lamina-X of the spinal cord of neonatal rats. Neuroreport. 2007;18:1025–1029. doi: 10.1097/WNR.0b013e3281667c0c. [DOI] [PubMed] [Google Scholar]
- 341.Seybold VS, Hylden JL, Wilcox GL. Intrathecal substance P and somatostatin in rats: behaviors indicative of sensation. Peptides. 1982;3:49–54. doi: 10.1016/0196-9781(82)90141-3. [DOI] [PubMed] [Google Scholar]
- 342.Shay BL, Hochman S. Serotonin alters multi-segmental convergence patterns in spinal cord deep dorsal horn and intermediate laminae neurons in an in vitro young rat preparation. Pain. 2002;95:7–14. doi: 10.1016/s0304-3959(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 343.Sherman SE, Loomis CW. Morphine insensitive allodynia is produced by intrathecal strychnine in the lightly anesthetized rat. Pain. 1994;56:17–29. doi: 10.1016/0304-3959(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 344.Sibilia V, Lattuada N, Rapetti D, Pagani F, Vincenza D, Bulgarelli I, Locatelli V, Guidobono F, Netti C. Ghrelin inhibits inflammatory pain in rats: involvement of the opioid system. Neuropharmacology. 2006;51:497–505. doi: 10.1016/j.neuropharm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 345.Simmons DR, Spike RC, Todd AJ. Galanin is contained in GABAergic neurons in the rat spinal dorsal horn. Neurosci Lett. 1995;187:119–122. doi: 10.1016/0304-3940(95)11358-4. [DOI] [PubMed] [Google Scholar]
- 346.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABAA receptor genes in the human genome. J Biol Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 347.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 348.Somogyi R, Wen X, Ma W, Barker JL. Developmental kinetics of GAD family mRNAs parallel neurogenesis in the rat spinal cord. J Neurosci. 1995;15:2575–2591. doi: 10.1523/JNEUROSCI.15-04-02575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- 350.Spike RC, Todd AJ, Johnston HM. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993;335:320–333. doi: 10.1002/cne.903350303. [DOI] [PubMed] [Google Scholar]
- 351.Spike RC, Watt C, Zafra F, Todd AJ. An ultrastructural study of the glycine transporter GLYT2 and its association with glycine in the superficial laminae of the rat spinal dorsal horn. Neuroscience. 1997;77:543–551. doi: 10.1016/s0306-4522(96)00501-5. [DOI] [PubMed] [Google Scholar]
- 352.Stil A, Liabeuf S, Jean-Xavier C, Brocard C, Viemari JC, Vinay L. Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience. 2009;164:809–821. doi: 10.1016/j.neuroscience.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 353.Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- 354.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 355.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- 358.Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-α4β2, non-α7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 359.Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 360.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 361.Todd AJ. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience. 1990;39:387–394. doi: 10.1016/0306-4522(90)90275-9. [DOI] [PubMed] [Google Scholar]
- 362.Todd AJ. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur J Neurosci. 1996;8:2492–2498. doi: 10.1111/j.1460-9568.1996.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 363.Todd AJ. Immunohistochemical evidence that acetylcholine and glycine exist in different populations of GABAergic neurons in lamina III of rat spinal dorsal horn. Neuroscience. 1991;44:741–746. doi: 10.1016/0306-4522(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 364.Todd AJ, Koerber HR. Wall and Mezack’s textbook of pain. Elsevier; 2006. Neuroanatomical substrates of spinal nociception; pp. 73–90. [Google Scholar]
- 365.Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 366.Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989;31:799–806. doi: 10.1016/0306-4522(89)90442-9. [DOI] [PubMed] [Google Scholar]
- 367.Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance P-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Todd AJ, Russell G, Spike RC. Immunocytochemical evidence that GABA and neurotensin exist in different neurons in laminae II and III of rat spinal dorsal horn. Neuroscience. 1992;47:685–691. doi: 10.1016/0306-4522(92)90176-3. [DOI] [PubMed] [Google Scholar]
- 369.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- 370.Todd AJ, Spike RC, Chong D, Neilson M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur J Neurosci. 1995;7:1–11. doi: 10.1111/j.1460-9568.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 371.Todd AJ, Spike RC, Polgar E. A quantitative study of neurons which express neurokinin-1 or somatostatin sst2a receptor in rat spinal dorsal horn. Neuroscience. 1998;85:459–473. doi: 10.1016/s0306-4522(97)00669-6. [DOI] [PubMed] [Google Scholar]
- 372.Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- 373.Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Torsney C, Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. J Neurophysiol. 2002;87:1311–1317. doi: 10.1152/jn.00462.2001. [DOI] [PubMed] [Google Scholar]
- 375.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci U S A. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 378.Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98:1521–1527. doi: 10.1213/01.ANE.0000112434.68702.E4. [DOI] [PubMed] [Google Scholar]
- 379.Valencia-de Ita S, Lawand NB, Lin Q, Castaneda-Hernandez G, Willis WD. Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95:3553–3561. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- 380.Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- 381.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 383.Vaughan CW, Christie MJ. Retrograde signalling by endocannabinoids. Handb Exp Pharmacol. 2005:367–383. doi: 10.1007/3-540-26573-2_12. [DOI] [PubMed] [Google Scholar]
- 384.Vergnano AM, Ferrini F, Salio C, Lossi L, Baratta M, Merighi A. The gastrointestinal hormone ghrelin modulates inhibitory neurotransmission in deep laminae of mouse spinal cord dorsal horn. Endocrinology. 2008;149:2306–2312. doi: 10.1210/en.2007-1164. [DOI] [PubMed] [Google Scholar]
- 385.Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Waldvogel HJ, Baer K, Eady E, Allen KL, Gilbert RT, Möhler H, Rees MI, Nicholson LF, Faull RL. Differential localization of γ-aminobutyric acid type A and glycine receptor subunits and gephyrin in the human pons, medulla oblongata and uppermost cervical segment of the spinal cord: an immunohistochemical study. J Comp Neurol. 2010;518:305–328. doi: 10.1002/cne.22212. [DOI] [PubMed] [Google Scholar]
- 387.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 388.Wasner G, Baron R, Jänig W. Absence of evidence is not evidence of absence (again) response to Dr. Fernando Cervero and Dr. Jennifer M.A Laird. Pain. 2000;84:439–440. doi: 10.1016/s0304-3959(99)00191-8. [DOI] [PubMed] [Google Scholar]
- 389.Wasner G, Baron R, Jänig W. Dynamic mechanical allodynia in humans is not mediated by a central presynaptic interaction of Aβ-mechanoreceptive and nociceptive C-afferents. Pain. 1999;79:113–119. doi: 10.1016/s0304-3959(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 390.Watson AH. Synaptic interactions between the terminals of slow-adapting type II mechanoreceptor afferents and neurones expressing γ-aminobutyric acid- and glycine-like immunoreactivity in the rat spinal cord. J Comp Neurol. 2004;471:168–179. doi: 10.1002/cne.20043. [DOI] [PubMed] [Google Scholar]
- 391.Watson AH, Hughes DI, Bazzaz AA. Synaptic relationships between hair follicle afferents and neurones expressing GABA and glycine-like immunoreactivity in the spinal cord of the rat. J Comp Neurol. 2002;452:367–380. doi: 10.1002/cne.10410. [DOI] [PubMed] [Google Scholar]
- 392.Wegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saarma M, Reeben M. Distribution of GABA receptor rho subunit transcripts in the rat brain. Eur J Neurosci. 1998;10:350–357. doi: 10.1046/j.1460-9568.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 393.White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. J Neurophysiol. 1990;64:57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]
- 394.Wildner H, Müller T, Cho SH, Brohl D, Cepko CL, Guillemot F, Birchmeier C. dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development. 2006;133:2105–2113. doi: 10.1242/dev.02345. [DOI] [PubMed] [Google Scholar]
- 395.Willis WD., Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 396.Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res Mol Brain Res. 1991;10:179–183. doi: 10.1016/0169-328x(91)90109-b. [DOI] [PubMed] [Google Scholar]
- 397.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Witschi R, Punnakkal P, Paul J, Walczak J-S, Cervero F, Fritschy J-M, Kuner R, Keist R, Rudolph U, Zeilhofer H. Presynaptic α2-GABA-A receptors in primary afferent depolarization in spinal pain control. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.6328-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 400.Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, Zhang L. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol. 2011 doi: 10.1038/nchembio.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Xu TL, Gong N. Glycine and glycine receptor signaling in hippocampal neurons: diversity, function and regulation. Prog Neurobiol. 2010;91:349–361. doi: 10.1016/j.pneurobio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 402.Xu TL, Nabekura J, Akaike N. Protein kinase C-mediated enhancement of glycine response in rat sacral dorsal commissural neurones by serotonin. J Physiol. 1996;496(Pt 2):491–501. doi: 10.1113/jphysiol.1996.sp021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Xu TL, Pang ZP, Li JS, Akaike N. 5-HT potentiation of the GABAA response in the rat sacral dorsal commissural neurones. Br J Pharmacol. 1998;124:779–787. doi: 10.1038/sj.bjp.0701896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Yang K, Fujita T, Kumamoto E. Adenosine inhibits GABAergic and glycinergic transmission in adult rat substantia gelatinosa neurons. J Neurophysiol. 2004;92:2867–2877. doi: 10.1152/jn.00291.2004. [DOI] [PubMed] [Google Scholar]
- 405.Yang Z, Aubrey KR, Alroy I, Harvey RJ, Vandenberg RJ, Lynch JW. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem Pharmacol. 2008;76:1014–1023. doi: 10.1016/j.bcp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 406.Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Möhler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yeo EJ, Cho DK, Paik SK, A. Y, Park MJ, Ahn DK, Moon C, Kim YS, Bae YC. Ultrastructural analysis of the synaptic connectivity of TRPV1-expressing primary afferent terminals in the rat trigeminal caudal nucleus. The Journal of Comparative Neurology. 2010;518:4134–4146. doi: 10.1002/cne.22369. [DOI] [PubMed] [Google Scholar]
- 411.Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. Modulation of glycine-activated ion channel function by G-protein βγ subunits. Nat Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- 412.Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. J Physiol. 1995;482(Pt 1):29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yu XH, Ribeiro-da-Silva A, De Koninck Y. Morphology and neurokinin 1 receptor expression of spinothalamic lamina I neurons in the rat spinal cord. J Comp Neurol. 2005;491:56–68. doi: 10.1002/cne.20675. [DOI] [PubMed] [Google Scholar]
- 414.Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 416.Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Zeilhofer HU, Möhler H, Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009;30:397–402. doi: 10.1016/j.tips.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 418.Zeilhofer HU, Muth-Selbach U, Gühring H, Erb K, Ahmadi S. Selective suppression of inhibitory synaptic transmission by nocistatin in the rat spinal cord dorsal horn. J Neurosci. 2000;20:4922–4929. doi: 10.1523/JNEUROSCI.20-13-04922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 420.Zeilhofer HU, Witschi R, Johansson T. Fast inhibitory transmission of pain in the spinal cord. In: Malcangio M, editor. Synaptic plasticity in pain. Springer; Heidelberg: 2009. [Google Scholar]
- 421.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 422.Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zheng W, Xie W, Zhang J, Strong JA, Wang L, Yu L, Xu M, Lu L. Function of γ-aminobutyric acid receptor/channel ρ1 subunits in spinal cord. J Biol Chem. 2003;278:48321–48329. doi: 10.1074/jbc.M307930200. [DOI] [PubMed] [Google Scholar]
- 424.Zieglgänsberger W, Herz A. Changes of cutaneous receptive fields of spino-cervical-tract neurones and other dorsal horn neurones by microelectrophoretically administered amino acids. Exp Brain Res. 1971;13:111–126. doi: 10.1007/BF00234080. [DOI] [PubMed] [Google Scholar]