Abstract
The aquaporins (AQPs) are small, integralmembrane proteins that selectively transport water across cell plasma membranes. A subset of AQPs, the aquaglyceroporins, also transport glycerol. AQPs are strongly expressed in tumor cells of different origins, particularly aggressive tumors. Recent discoveries of AQP involvement in cell migration and proliferation suggest that AQPs play key roles in tumor biology. AQP1 is ubiquitously expressed in tumor vascular endothelium, and AQP1-null mice show defective tumor angiogenesis resulting from impaired endothelial cell migration. AQP-expressing cancer cells show enhanced migration in vitro and greater local tumor invasion, tumor cell extravasation, and metastases in vivo. AQP-dependent cell migration may involve AQP-facilitated water influx into lamellipodia at the front edge of migrating cells. The aquaglyceroporin AQP3, which is found in normal epidermis and becomes upregulated in basal cell carcinoma, facilitates cell proliferation in different cell types. Remarkably, AQP3-null mice are resistant to skin tumorigenesis by a mechanism that may involve reduced tumor cell glycerol metabolism and ATP generation. Together, the data suggest that AQP expression in tumor cells and tumor vessels facilitates tumor growth and spread, suggesting AQP inhibition as a novel antitumor therapy.
Keywords: Angiogenesis, Aquaglyceroporin, Cell migration, Cell motility, Cell proliferation, Water channel
Introduction
The aquaporins (AQPs) are a family of small (~30 kDa/monomer) membrane transport proteins that assemble in membranes as tetramers and act primarily as water-selective pores, facilitating osmotically driven water transport across cell plasma membranes [1, 2]. A subset of the AQP family, the aquaglyceroporins (AQP3, AQP7, AQP9), transport both water and glycerol. Studies largely from knockout mice implicate AQPs in many expected physiological functions, including urine concentration and exocrine gland secretion, as well as in several unanticipated functions, including brain swelling, neural signal transduction, skin moisturization, and fat metabolism [3, 4]. Compelling new evidence for involvement of AQPs in cell migration and proliferation, as reviewed here, add AQPs to an expanding list of effectors in tumor biology.
AQP expression in tumors
As summarized in Table 1, at least 12 different tumor cell types have been reported to express AQPs in vivo in humans and rodents. For some tumors, positive correlations have been established between histological tumor grade and the amount of AQP expression, as for AQP4 expression in diffuse astrocytomas [5, 6]. The four studies reporting reduced AQP expression in tumors should be interpreted with caution because only single AQPs were studied without exploring the possibility that other AQPs may be upregulated. For example, in human colorectal cancer, one study reported reduced AQP8 expression [7] but another study showed increased expression of AQPs 1, 3, and 5 [8], suggesting that, in some cases, AQPs may become expressed in tumors even when these AQPs are not found in the normal cells of origin. Overall, it appears that AQP expression becomes upregulated in tumors, but it is not known from expression studies whether this is an aberrant phenomenon or whether it plays a role in tumor biology.
Table 1.
AQP expression in tumors
| Tumor type | AQP | Species | Expression | References |
|---|---|---|---|---|
| Cholangiocarcinoma | AQP1 | Human | ↓ | [27] |
| AQP1 | Human | ↑ | [28] | |
| Glioma | AQP9 | Human | ↑ | [29] |
| AQP4 | Human | ↑ | [5, 9, 30] | |
| AQP1 | Human | ↑ | [30, 31] | |
| AQP1 | Mouse | ↑ | [32] | |
| AQP1, 3, 5 | Human | ↑ | [33, 34] | |
| Laryngeal cancer | AQP1 | Human | ↑ | [35] |
| Hepatocellular | AQP8, 9 | Human | ↓ | [36] |
| Hemangioblastoma | AQP1 | Human | ↑ | [37] |
| Lung adenocarcinoma | AQP3 | Human | ↑ | [38] |
| AQP1 | Human | ↑ | [39] | |
| Choroid plexus tumor | AQP1 | Human | ↑ | [40] |
| Epithelial Ovarian | AQP5 | Human | ↑ | [41] |
| Follicular Thyroid | AQP7 | Human | ↑ | [42] |
| Renal cell | AQP3 | Human | ↑ | [43] |
| AQP1 | Human | ↓ | [44] | |
| Colorectal | AQP8 | Human | ↓ | [7] |
| AQP1, 3, 5 | Human | ↑ | [8] | |
| Mammary | AQP1 | Mouse | ↑ | [32] |
Because AQPs transport water, most expression studies speculate that AQPs in tumor cells allow water to rapidly penetrate into the growing tumor mass. Tumor AQP expression may thus cause tumor expansion by exacerbating tumor-associated edema, a particularly troublesome complication in the case of brain tumors. In astrocytomas, for example, tumor AQP4 expression correlates with the amount of edema but not with patient survival [6, 9]. Because of the noncompliant skull, brain tumor edema contributes to increased intracranial pressure, which reduces cerebral blood flow and produces brain herniation. Recent evidence for the involvement of AQPs in angiogenesis, and in tumor cell migration and proliferation [10-14], suggest new roles of AQPs in tumor biology, in addition to their potential role in tumor-associated edema.
AQP1 facilitates tumor angiogenesis and endothelial cell migration
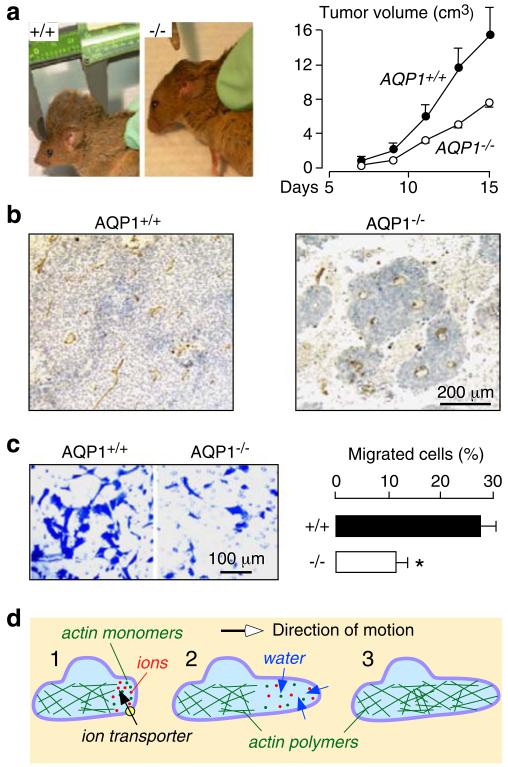
AQP1 is expressed in vascular endothelial cells throughout the body, except in the normal central nervous system [15]. The function of AQP1 in vascular endothelial cells was established using an in vivo tumor angiogenesis assay in which wild-type and AQP1-null mice were subcutaneously implanted with B16F10 melanoma cells [14]. Tumor growth was reduced in AQP1-null mice (Fig. 1a) due to impaired tumor angiogenesis causing extensive tumor necrosis (Fig. 1b). The underlying defect in the AQP1-null endothelial cells was determined using cell culture models. Cultured aortic endothelial cells from wild-type and AQP1 null mice had comparable morphology, growth, and adherence to different surfaces but showed remarkable impairment in their migration as determined using transwell (Fig. 1c) and in vitro scratch assays. These findings suggested that the strong AQP1 expression observed in tumor microvascular endothelial cells facilitates their migration, an essential component of tumor angiogenesis.
Fig. 1.
Impaired angiogenesis and endothelial cell migration in AQP1 knockout mice. a Tumor in wild-type vs AQP1 null mouse, 2 weeks after subcutaneous injection of 106 B16F10 melanoma cells (left). Tumor growth data (10 mice per group, P<0.001) (right). b Tumor tissue stained with the endothelial marker isolectin-B4 (brown). c Migration of aortic endothelial cells for 6 h towards 10% serum shown in a transwell assay (left). Picture shows migrated wild-type and AQP1 null endothelial cells (stained with Coomassie blue) after scraping. Data summary (S.E., n=16–20, asterisk P<0.001) (right). d Proposed mechanism of AQP-facilitated endothelial cell migration: 1 Actin depolymerization and ion movements increase osmolality at the anterior end of the cell. 2 Water entry increases local hydrostatic pressure, producing cell membrane expansion to form a protrusion. AQP polarizes to the leading edge of the cell membrane facilitating water entry into the cell. 3 Actin repolymerizes stabilizing the protrusion. Adapted from [14, 17]
Follow-up experiments showed that AQPs facilitate cell migration independent of AQP and cell types. AQP1 and AQP4 accelerate the migration of cultured Chinese Hamster Ovary and Fisher Rat Thyroid cells [14]; AQP4 facilitates astrocyte cell migration [12, 13]; and AQP1 facilitates the migration of cultured renal proximal tubule cells [10], B16F10 melanoma, and 4T1 breast cancer cells [16]. Two mechanisms have been proposed to explain how AQPs facilitate cell migration [17]: According to the first theory, AQPs facilitate rapid water flow across the plasma membrane into the front end of migrating cells (Fig. 1d). The rapid transmembrane water influx is driven by changes in osmolality produced by transmembrane ion flux and actin depolymerization. This theory would explain why AQPs tend to polarize to the front end of migrating cells. According to the second theory, cells migrating through the irregularly shaped extracellular space undergo rapid changes in their volume, accompanied by rapid changes in trasmembrane water fluxes.
AQPs facilitate tumor cell extravasation and metastasis
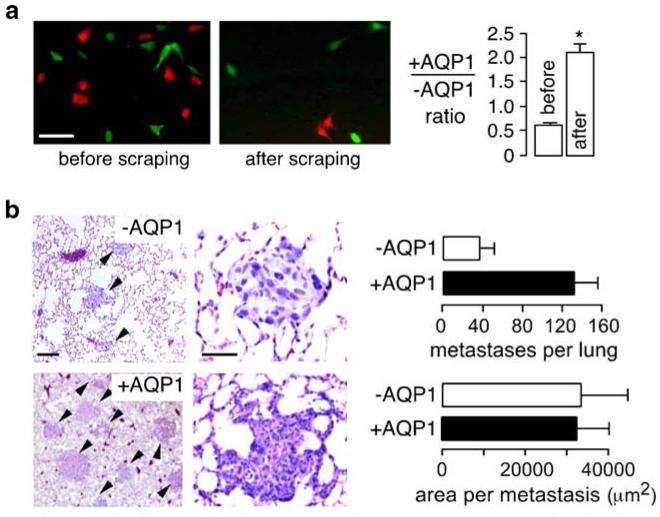
The generality of AQP-facilitated cell migration to different cell types and AQPs suggests that AQP expression in tumor cells may increase local tumor invasiveness and the ability of tumor cells to metastasize by crossing microvascular endothelial barriers. To test this possibility, tumor cell migration, invasiveness, and metastatic potential were evaluated using two established mouse cell lines (B16F10 melanoma and 4T1 breast tumor) without vs with AQP1 expression [16]. AQP1 expression did not affect the appearance, size, growth, or substrate adherence of the tumor cells in culture, but it increased their plasma membrane osmotic water permeability by 5- to 10-fold. In vitro analysis of cell migration using transwell migration and scratch assays showed greatly increased migration of the AQP1-expressing tumor cells, as anticipated. Also, as found for migrating endothelial and other types cells, AQP1 expression increased the number and shape of lamellipodia. To study tumor cell extravasation across microvascular endothelia, tumor cells were labeled with fixable fluorescent dyes so that they could be identified after intravenous injection in mice. Cells were stained with cell-permeable red or green fluorescent dyes, which become entrapped in cells by covalent reaction with cytoplasmic proteins. The two-color cell labeling strategy for simultaneous detection of control and AQP1-expressing cells was validated using the in vitro transwell assay in which a mixture of red-labeled control 4T1 cells and green-labeled AQP1-expressing 4T1 cells was added to the upper chamber of a transwell filter. Figure 2a (left panel) shows the red and green adherent cells at 6 h after cell addition. More green than red cells remained after scraping the upper surface of the filter (middle panel). From counting many cells on multiple filters, the ratio of AQP1-expressing vs control cells was significantly increased after scraping cells (Fig. 2a, right), indicating increased migration of the AQP1-expressing cells. The same approach was used in mice to study tumor cell extravasation. Following tail vein injection of a mixture of fluorescently labeled cells, there was a >1.5-fold increase in the number of AQP1-expressing vs control cells that had migrated across lung microvascular endothelia at 6 h.
Fig. 2.
Increased migration and metastasis of AQP-expressing tumor cells. a Control (red fluorescent) and AQP1-expressing (green fluorescent) tumor cells were mixed 1:1 and applied to transwell filters. The upper chamber contained 1% serum and the lower chamber contained 10% serum. Fluorescence micrographs showing red and green cells before (left) and after (middle) scraping nonmigrated cells from the upper surface of the porous membrane (scale bar 50 μm). Summary of ratio of AQP1-expressing vs control (+AQP1/−AQP1) cells at 6 h before and after scraping of nonmigrating cells (asterisk P<0.05) (right). b Hematoxylin and eosinstained paraffin sections of mouse lung tissue at 14 days after tail vein injection of 106 control or AQP1-expressing tumor cells (left). Tumor metastases indicated by arrows. Number of metastases per lung, and area per metastasis (S.E.) (right). Adapted from [16]
Tumor cell growth and spread were also studied. In one set of studies, lung metastases were counted and their size was determined at 15 days after tail vein injection of control or AQP1-expressing tumor cells. Figure 2b (left) shows more metastases in lungs of mice injected with AQP1-expressing tumor cells. Also, in most mice receiving the AQP1-expressing cells, there was evidence for tumor infiltration of the alveolar wall (middle panel). The data summary in the right panel shows a significantly greater number of lung metastases produced by the AQP1-expressing tumor cells but no difference in the average size of individual metastases. In another set of experiments, mice were implanted subcutaneously with control or AQP1-expressing 4T1 or B16F10 cells to study tumor growth and local invasion. Tumor growth, as assessed by tumor volume at different times after implantation, was not affected by AQP1 expression. However, finger-like projections into subcutaneous adipose tissue were seen with AQP1-expressing but not with control cells. Together, these studies support the idea that AQP expression in tumor cells can increase their migration, leading to greater local invasiveness and metastatic potential.
AQP3 deletion in mice prevents skin tumor formation
We recently discovered a remarkable phenotype in AQP3 null mice—resistance to the development of skin tumors [18]. AQP3 is an aquaglyceroporin (transporting water and glycerol) expressed at the basolateral membrane of epithelial cells in kidney collecting duct, airways, and intestine, as well as in urinary bladder, conjunctiva, and cornea [19]. In mammalian skin, AQP3 is expressed strongly in plasma membranes of the basal epidermal cell layer. Phenotype analysis of AQP3 null mice showed dry skin and delayed barrier recovery after removal of the stratum corneum [20-22]. These defects were attributed to the absence of AQP3-facilitated glycerol transport, resulting in reduced stratum corneum and epidermal cell glycerol content [20, 21].
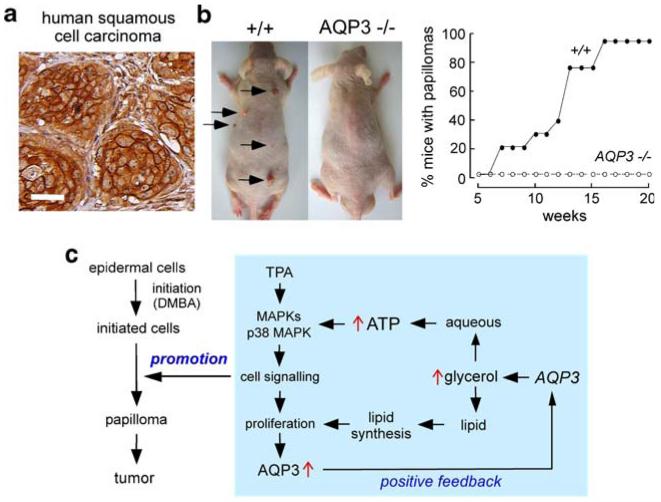
Our motivation for studying AQP3 and skin tumors was the strong expression of AQP3 in basal cells in human skin squamous cell carcinomas. Figure 3a shows one of many such examples. Also, we recently discovered proliferation defects in AQP3 knockout mice in normally AQP3-expressing cells in cornea [23], resulting in delayed healing of corneal wounds; in colon [24], producing severe colitis in experimental models; and in skin [11], slowing cutaneous wound healing.
Fig. 3.
AQP3 expression in human squamous cell carcinoma and protection against cutaneous papillomas in AQP3-null mice. a AQP3 immunostaining in human skin squamous cell carcinoma. Bar, 50 μm. b Dorsal skin of mice was treated with a single application of DMBA, followed by twice-weekly applications of TPA for 20 weeks (left). Representative photographs showing multiple papillomas in wild-type mouse but no papillomas in AQP3 null mouse. Percentage of mice with papillomas (right). c Proposed cellular mechanism of AQP3-facilitated tumorigenesis. Adapted from [18]
An established multistage carcinogenesis model was used to study skin tumor formation, which involved skin exposure to a tumor initiator and phorbol ester promoter [25]. The phenotype finding, as shown in Fig. 3b, was the absence of cutaneous papillomas in AQP3 knockout mice under identical treatment conditions in which multiple tumors were produced in wild-type mice [18].
Studies to establish the cellular mechanisms responsible for the skin tumor phenotype, utilizing living mice and keratinocyte cell cultures, showed impaired promoter-induced cell proliferation in AQP3-null or knock-down keratinocytes. AQP3-deficient keratinocytes had reduced content of glycerol, its metabolite glycerol-3-phosphate, and ATP, without impairment of mitochondrial function. Glycerol supplementation or AQP3 adenoviral infection (but not AQP1 adenoviral infection) corrected the defects in keratinocyte proliferation and reduced ATP generation. Further studies revealed correlations between cell proliferation and ATP and glycerol content. As diagrammed in Fig. 3c, we propose that AQP3-facilitated glycerol transport is an important determinant of epidermal cell proliferation and tumorigenesis by a novel mechanism in which cellular glycerol is a key regulator of cellular ATP energy. The mechanism also shows glycerol biosynthetic incorporation into lipids, ATP-facilitated MAP kinase signaling, and positive feedback in which cell proliferation increases AQP3 expression. It is not known whether this mechanism applies to nonepidermal, AQP3-expressing cells.
Tumor-committed cells generally have an aggressive energy metabolic profile, allowing them to compete with surrounding cells, proliferate, and form characteristic structure [26]. The resistance to skin tumorigenesis in AQP3 deficiency provides a rational basis for AQP3 inhibition/suppression in the therapy of skin and possibly other cancers associated with overexpression of aquaglyceroporins. Prevention of uptake of cell substrates during early tumorigenesis represents a novel paradigm in tumor therapy.
Clinical implications
The involvement of AQPs in angiogenesis and tumor cell migration and proliferation has potentially important clinical implications. First, it provides a functional explanation for AQP expression in tumor cells and tumor microvessels and for the correlations between tumor aggressiveness and AQP expression. Second, it provides a rational basis to evaluate AQP inhibitors, when available, for tumor therapy, both for reduction of tumor angiogenesis and tumor spread. Last, the remarkable resistance to skin tumor formation in AQP3 deficiency suggests the possible use of topical AQP3 inhibitors for tumor prevention and therapy.
Acknowledgements
Supported by grants EB00415, DK35124, EY13574, HL59198, DK72517, and HL73856 from the National Institutes of Health, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation (to ASV) and by a Wellcome Trust Clinician–Scientist Fellowship and grants from the Neurosciences Research Foundation (to MCP).
Biography

Alan S. Verkman completed S.B. degrees in Physics and Biology at M.I.T., Ph.D. in physics at Harvard, and M.D. from Harvard Medical School. He completed clinical training in Medicine at Harvard and in Nephrology at University of California at San Francisco (UCSF). Dr. Verkman is Professor of Medicine and Physiology at UCSF. His research interests include water and chloride transport mechanisms, drug discovery, and new optical methods to study living cells.

Marios C. Papadopoulos received BA and MD degrees from the Universities of Cambridge and BM BCh degrees from Oxford. He completed Neurosurgery training in London and is a Fellow of the Royal College of Surgeons. He is currently a Consultant Neurosurgeon at St. George’s Hospital, University of London. His research interests include aquaporins, brain edema, and proteomics, and his clinical interests include spinal and vascular neurosurgery.
Contributor Information
A. S. Verkman, Departments of Medicine and Physiology, University of California, 1246 Health Sciences East Tower, San Francisco, CA 94143-0521, USA
Mariko Hara-Chikuma, Departments of Medicine and Physiology, University of California, 1246 Health Sciences East Tower, San Francisco, CA 94143-0521, USA; Department of Dermatology, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
Marios C. Papadopoulos, Departments of Medicine and Physiology, University of California, 1246 Health Sciences East Tower, San Francisco, CA 94143-0521, USA; Academic Neurosurgery Unit, St. George’s, University of London, London, UK
References
- 1.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 3.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 4.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 5.Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 2004;107:311–318. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 6.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer H, Stenling R, Rubio C, Lindblom A. Differential expression of aquaporin 8 in human colonic epithelial cells and colorectal tumors. BMC Physiol. 2001;1:1. doi: 10.1186/1472-6793-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, Trink B, Chang YS, Sidransky D, Mao L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- 9.Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- 10.Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- 11.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2007 doi: 10.1007/s00109-007-0272-4. doi:10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 12.Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 13.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 14.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 15.Bondy C, Chin E, Smith BL, Preston GM, Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993;90:4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos MC, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0357-5. doi:10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 20.Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002;277:46616–46621. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- 21.Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci U S A. 2003;100:7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002;277:17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- 23.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 24.Thiagarajah JR, Zhao D, Verkman AS. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut. 2007;56:1529–1535. doi: 10.1136/gut.2006.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS Lett. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 27.Aishima S, Kuroda Y, Nishihara Y, Taguchi K, Iguchi T, Taketomi A, Maehara Y, Tsuneyoshi M. Down-regulation of aquaporin-1 in intrahepatic cholangiocarcinoma is related to tumor progression and mucin expression. Hum Pathol. 2007;38:1819–1825. doi: 10.1016/j.humpath.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Mazal PR, Susani M, Wrba F, Haitel A. Diagnostic significance of aquaporin-1 in liver tumors. Hum Pathol. 2005;36:1226–1231. doi: 10.1016/j.humpath.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Warth A, Mittelbronn M, Hulper P, Erdlenbruch B, Wolburg H. Expression of the water channel protein aquaporin-9 in malignant brain tumors. Appl Immunohistochem Mol Morphol. 2007;15:193–198. doi: 10.1097/01.pai.0000213110.05108.e9. [DOI] [PubMed] [Google Scholar]
- 30.Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, Manley GT. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56:375–381. doi: 10.1227/01.neu.0000148904.57841.6b. discussion 375-381. [DOI] [PubMed] [Google Scholar]
- 32.Endo M, Jain RK, Witwer B, Brown D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc Res. 1999;58:89–98. doi: 10.1006/mvre.1999.2158. [DOI] [PubMed] [Google Scholar]
- 33.Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Boon K, Edwards JB, Eberhart CG, Riggins GJ. Identification of astrocytoma associated genes including cell surface markers. BMC Cancer. 2004;4:39. doi: 10.1186/1471-2407-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan B, Zhu D, Dong Z, Yang Z. Expression and distribution of aquaporin 1 in laryngeal carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21:269–272. [PubMed] [Google Scholar]
- 36.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM, Jr, Fausto N, Pierce RH, McKillop IH. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Tachibana O, Oda M, Xu R, Hamada J, Yamashita J, Hashimoto N, Takahashi JA. Increased expression of aquaporin 1 in human hemangioblastomas and its correlation with cyst formation. J Neurooncol. 2006;80:219–225. doi: 10.1007/s11060-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 38.Liu YL, Matsuzaki T, Nakazawa T, Murata S, Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata K, Katoh R. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum Pathol. 2007;38:171–178. doi: 10.1016/j.humpath.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, Mao L, Sidransky D, Moon C. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–1353. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A. Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg. 2006;42:228–233. doi: 10.1159/000092359. [DOI] [PubMed] [Google Scholar]
- 41.Yang JH, Shi YF, Cheng Q, Deng L. Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecol Oncol. 2006;100:294–299. doi: 10.1016/j.ygyno.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 42.Lacroix L, Lazar V, Michiels S, Ripoche H, Dessen P, Talbot M, Caillou B, Levillain JP, Schlumberger M, Bidart JM. Follicular thyroid tumors with the PAX8-PPARgamma1 rearrangement display characteristic genetic alterations. Am J Pathol. 2005;167:223–231. doi: 10.1016/s0002-9440(10)62967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kafe H, Verbavatz JM, Cochand-Priollet B, Castagnet P, Vieillefond A. Collecting duct carcinoma: an entity to be redefined? Virchows Arch. 2004;445:637–640. doi: 10.1007/s00428-004-1124-z. [DOI] [PubMed] [Google Scholar]
- 44.Takenawa J, Kaneko Y, Kishishita M, Higashitsuji H, Nishiyama H, Terachi T, Arai Y, Yoshida O, Fukumoto M, Fujita J. Transcript levels of aquaporin 1 and carbonic anhydrase IV as predictive indicators for prognosis of renal cell carcinoma patients after nephrectomy. Int J Cancer. 1998;79:1–7. doi: 10.1002/(sici)1097-0215(19980220)79:1<1::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]