INTRODUCTION
Children and adolescents are increasingly being diagnosed with psychopathology, with approximately 21% of youth in the United States ages 9 through 17 having a diagnosable mental illness with some degree of impairment.1 An early onset of many psychiatric disorders in youth has been linked with a more severe course of illness, morbidities such as suicide attempts and substance abuse, as well as the presence of comorbidities and complications such as poor academic and job performance, interpersonal conflicts, or legal problems.2–5 Despite vigorous efforts to find effective treatments for psychiatric conditions in youth, treatment challenges are frequent and illness carries high rates of complications, including mortality.6
Comprehensive treatment plans are often required for youth with psychopathology to address a complex array of symptoms and associated morbidities. In general, a multimodal treatment approach combining pharmacologic agents and psychosocial interventions is suggested, with the goal of improving symptoms, providing psychoeducation about the mental illness, and promoting treatment adherence for relapse prevention and attenuation of long-term complications from the illness.7,8 Clinicians are encouraged to advocate for prevention, early intervention, and bio-psychosocial treatments that promote the healthy growth and development of all children affected by psychopathology, in any cultural context.9 At this point, however, we know relatively little about the mechanisms that underlie treatment and presume that the effects of pharmacologic and nonpharmacologic interventions on the brain are multifactorial.
An efficient way to elucidate neural mechanisms that underlie the effects of treatment is to use magnetic resonance imaging (MRI) technology to assess in vivo brain differences in youth before and after an intervention has been made. For example, specific differences in brains exposed rather than unexposed to medications may suggest intrinsic biological pathways that may clarify the mechanisms by which such treatments are functioning to reduce symptom burden. In some instances, medications have been found to have either no effect or a normalizing effect on brain MRI findings compared with healthy controls.10 Some neuroimaging studies have attempted to examine the effects of medications on structural and functional outcomes in the brain post hoc, and have found no direct influence of medication exposure on primary findings.11,12 In other cases, researchers have tried to avoid the potential confounding effects of medications on brain MRI results by studying only unmedicated or medication-naïve youth.13–16 Although there are some clear advantages to examining unmedicated youth with psychopathology, such individuals are difficult to find and may represent a subset of the population with relatively low symptom severity, thereby limiting generalizability of the results to the overall population.
This article evaluates studies in which medications were not treated as a confounder, but rather as a variable of interest on neural outcome. Structural, functional, neurochemical, and other neuroimaging modalities used to study the neurophysiologic alterations associated with psychotropic medication exposure in youth are reviewed. In addition, the authors review neuroimaging studies examining the effects of psychotherapeutic interventions, in an effort to explore the potential effects of nonpharmacologic treatment in selected disorders. After reviewing each modality as it applies to youth with various psychiatric diagnoses, the article concludes by illustrating how taken together, these studies suggest that therapeutic interventions during childhood do indeed affect brain structure and function in a detectable manner. Finally, areas of future study that will further explain the biological correlates of treating psychopathology are proposed.
METHODS
A literature search using PubMed was conducted to identify peer-reviewed neuroimaging studies of children and adolescents for the period 1966 to June 2012. The following terms were included in the search: “medication” or “psychotropic” or “psychotherapy” or “treatment” with “psychiatric” or diagnostic categories, and “adolescents,” “children,” “youth,” “juvenile,” or “pediatric,” followed by “neuroimaging,” “magnetic resonance imaging (MRI),” “diffusion tensor imaging (DTI),” “functional MRI (fMRI),” or “spectroscopy (MRS).” References from identified articles were also reviewed to ensure that all relevant articles were included.
RESULTS
Data were reviewed from more than 50 studies published from 1966 to 2012 that reported on neuroimaging applications in selected child psychiatric diagnoses. Available data were examined from structural MRI, diffusion tensor imaging (DTI), functional MRI (fMRI), and magnetic resonance spectroscopy (MRS) studies in youth with selected psychiatric disorders including anorexia nervosa (AN), attention deficit with hyperactivity disorder (ADHD), autism, bipolar disorder (BD), depressive disorders, obsessive-compulsive disorder (OCD), and schizophrenia.
Anorexia Nervosa
In youth with eating disorders such as AN, some studies have demonstrated that the cognitive effects of this disorder may be ameliorated by intervention in general. Although weight recovery is the main treatment end point for youth with this disorder, an understanding of the interaction between specific mechanisms by which weight recovery is achieved and neurocognitive outcomes is still under development. For example, 9 patients with AN were assessed by neuroimaging before and after 7 months of inpatient multidisciplinary treatment, involving a combination of biological management (eg, with selective serotonin reuptake inhibitors [SSRIs]), nutritional rehabilitation, a behavioral program standardized to improve eating patterns and weight, individual and group cognitive treatment, and parent counseling, all aimed at weight recovery.17 Before treatment, the AN group showed significantly higher activation than controls in temporal and parietal areas, and especially in the temporal superior gyrus, during performance of a working memory task. A negative correlation was found between brain activation and body mass index and a positive correlation was found between activation and depressive symptomatology. At follow-up after weight recovery, AN patients showed a decrease in brain activation in these areas and did not present differences relative to controls, suggesting that differences with respect to controls disappeared after weight recovery. These investigators also showed that posterior gray matter structural deficits assessed by serial voxel-based morphometry (VBM)18 and prefrontal N-acetyl aspartate (NAA), and other neurochemical deficits assessed by MRS,19 are also reversible by nutritional recovery. The multimodal approach used by these researchers provides them with the opportunity to develop important theoretical models of how treatment of AN can influence all levels of brain function. Future studies would benefit from teasing apart the specific neural effects of the various interventions (biological and psychosocial) presented to this population.
Attention Deficit with Hyperactivity
In youth with ADHD, a few studies have examined the neural effects of psychostimulant medications. One study showed that total frontal, prefrontal, and caudate volumes were larger for children and adolescents with ADHD compared with controls, and that youth with ADHD without a treatment history had smaller right anterior cingulate cortex (ACC) volumes than those of ADHD youth with a treatment history.20 Although the treatment group and the nontreatment group were comparable in terms of ADHD symptom severity assessed by parents, this study did not explicitly evaluate or control for symptom severity, which may have biased their results such that those who received treatment had more severe illness warranting pharmacologic intervention in comparison with those who did not receive treatment.
Structural MRI for ADHD
Because ADHD persists into adulthood in 50% to 70% of cases, a recent meta-analysis aiming to identify MRI-based structural differences between adults and children with ADHD discovered significant positive effects of treatment.21 Specifically, volumetric reductions in basal ganglia regions (eg, right globus pallidus, right putamen, and caudate), as well as alterations in limbic regions (ACC and amygdala), were more pronounced in nontreated populations and appeared to diminish over time from childhood to adulthood. Treatment also appeared to have a normalizing effect on brain structure such that a higher percentage of treated participants showed fewer structural anomalies than those who were untreated. Consistent with this study, another meta-analysis of structural imaging data also found abnormalities in the basal ganglia associated with ADHD.22 This analysis evaluated the effects of age and treatment in these studies, and found that patients with ADHD may catch up on disorder-related developmental delay with the use of stimulant medication, which was associated with normalization of structural abnormalities in the lentiform nucleus extending into the caudate nucleus. Both of these meta-analyses demonstrate the need for within-subject prospective studies to confirm the impact of psychostimulants on brain structure in youth with ADHD.
Functional MRI for ADHD
fMRI studies in this area used longitudinal designs by performing prestimulant and poststimulant exposure fMRI scans. In an fMRI study of 18 youths with ADHD, researchers used a Sternberg working memory task in a randomized, double-blind, placebo-controlled design. Results demonstrated that a clinically effective dose of a psychostimulant led to the recruitment of additional brain regions that were not engaged in the networks when participants were on placebo.23 In addition, psychostimulant therapy strengthened connectivity between frontoparietal networks that were engaged during working memory, with many connectivity changes being directly related to improved working memory reaction time. This study showed strong evidence for regional functional connectivity changes following medication in structures previously implicated as abnormal in ADHD, such as anterior cingulate, ventrolateral prefrontal cortex, and precuneus, suggesting a mechanism underlying the beneficial effects of medication on working memory performance.
In another fMRI study designed to assess the effects of single-dose methylphenidate on error-processing regions of the brain during a stop task, 12 medication-naïve boys with ADHD were scanned twice, under either a single clinical dose of methylphenidate or placebo, in a randomized, double-blind design.24 Brain activation was compared within patients under either drug condition, and to test for the potential normalization effects of methylphenidate, brain activation in ADHD patients under either drug condition was compared with that of 13 healthy age-matched boys. This study found that during failed inhibition, boys with ADHD taking placebo showed reduced brain activation relative to control subjects in performance-monitoring areas of dorsomedial and left ventrolateral prefrontal cortices, thalamus, cingulate, and parietal regions. Methylphenidate, in comparison with placebo, upregulated activation in these brain regions within patients and normalized all activation differences between patients and control subjects. During successful inhibition, methylphenidate normalized reduced activation observed in patients taking placebo compared with control subjects in parietotemporal and cerebellar regions. This study demonstrated that a single dose of a psychostimulant normalizes levels of brain activity in attentional brain networks in youth with ADHD.
Functional near-infrared spectroscopy for ADHD
Similar to ADHD, olfactory sensitivity, discrimination, and identification are mediated by dopamine metabolism. Twenty-seven youths with ADHD and a history of chronic methylphenidate exposure were washed out from their medication and assessed along with healthy controls for olfactory function by functional near-infrared spectroscopy (fNIRS) during presentation of 2-phenylethanol.25 Results from this study showed that cessation of methylphenidate led to significant increases in olfactory discrimination, but decreased inferior frontal and temporal brain activation in youth with ADHD. Of interest, this activation pattern associated with olfaction normalized with the reintroduction of medication to the ADHD group, providing in vivo evidence that psychostimulants modulate related dopaminergic systems.
Magnetic resonance spectroscopy for ADHD
Data demonstrating macroscopic structural and functional brain changes in youth with ADHD suggest underlying cellular and molecular changes that may be due to treatment. MRS is a noninvasive neuroimaging method that yields molecular-level biochemical data to quantitatively examine neuronal function. Several studies in youth with ADHD have demonstrated in vivo neurochemical changes in response to different medication treatments. In a case series of 2 children showing symptom improvement with methylphenidate and 2 children treated with atomoxetine, a decrease in the glutamate/creatine ratio (mean change 56.1%) was observed in the striatum between 14 and 18 weeks of therapy in all 4 children with ADHD.26 In the prefrontal cortex, however, changes in the glutamate/creatine ratio were noted only in subjects receiving atomoxetine.26 In another study by the same group, MRS data were collected from the prefrontal cortex and striatum in 14 children with ADHD, before and after treatment with psychostimulants or atomoxetine.27 The glutamate/glutamine/γ-aminobutyric acid-to-creatine/phosphocreatine ratio decreased significantly in the striatum, suggesting that striatal glutamate may be involved in treatment response in ADHD.
Treatment of the prefrontal region for ADHD
Hammerness and colleagues28 recently found that glutamatergic abnormalities may also be ameliorated with treatment in a prefrontal region of youth with ADHD. In an open-label design, adolescents with ADHD were scanned by MRS in the ACC before and after administration of extended-release methylphenidate. Untreated youth with ADHD showed higher metabolite ratios (glutamate/myoinositol, glutamine/myoinositol, glutamate + glutamine/myoinositol) in the ACC compared with controls and treated ADHD youth, but these group differences did not reach statistical significance. Although these preliminary findings suggest possible glutamatergic abnormalities in adolescents with ADHD, which may normalize with methylphenidate treatment, a larger sample and controlled study design are needed to confirm these results.
Another study using a pretest and posttest design examined the effect on prefrontal neurometabolites of 12 weeks of treatment with long-acting methylphenidate, 20 mg per day, in 21 medication-naïve children with ADHD.29 These youths had increases in NAA/creatine ratios and decreases in glutamate/creatine, choline/creatine, and myoinositol/creatine ratios in the right and left prefrontal cortices after stimulant treatment. The investigators speculated that the significant neurochemical changes may reflect medication-related functional improvement and improved neuroplasticity in the prefrontal cortices of children with ADHD.
Behavioral treatment for ADHD
Behavioral treatment of ADHD symptoms appears to also have an effect on brain functioning. In a recent study of youth attending summer camp training for treatment of ADHD, fMRI with a Go/No-Go paradigm was performed twice in 12 children with ADHD before and after a response cost and token (RCT) program, and in 12 healthy control children, to investigate the influence of RCT training on attention and impulsivity.30 The No-Go condition revealed weak activation in the dorsal part of the ACC, parietal cortex, and dorsolateral prefrontal cortex (DLPFC) before the training in children with ADHD compared with healthy children, which was significantly more pronounced after the training. This increase in hemodynamic response was not attributed merely to repetition of the measurement, because the effect was not observed in healthy children. The increase in hemodynamic response in the ACC and right DLPFC was significantly associated with a reduction in response-time variability and clinical symptoms in ADHD patients. This study showed that after the RCT training, youth with ADHD showed more pronounced activation of cortical structures that function in response monitoring and self-control.
Combination treatments for ADHD
Collectively these studies suggest significant effects of pharmacologic and behavioral interventions on brain structure and function in youth with ADHD. Additional controlled studies and longer-term follow-up would aid in determining the long-term benefits of these interventions in ameliorating brain dysfunction associated with ADHD.
Autism
Despite a wealth of preclinical investigation, there are surprisingly few human studies in youth with autism that directly examine the effects of intervention on brain functioning. One study examined functional connectivity during a phonological decision-making task in adolescents with autism-spectrum disorders.31 This study found that in youth taking propranolol, there was increased functional connectivity in regions including the left inferior frontal cortex, left fusiform gyrus, left parietal cortex, and left middle temporal gyrus.
A few other studies in youth with autism have evaluated the effects of medication directly on receptor and transporter functioning in the brain. For example, a positron emission tomography (PET) study showed that a high value of dopamine D2 receptor binding in the caudate and putamen decreased by about 10% toward the normal level after treating 6 children between 3 and 5 years of age with infantile autism for 3 months with enzymatic cofactor 6R-L-erythro-5,6,7,8-tetrahydrobiopterin.32 Another study aimed to correlate striatal dopamine transporter (DAT) binding and cerebrospinal fluid insulin-like growth factor 1 (CSF IGF-1) with clinical response in autistic children (n = 13, age 5–16 years) after 6 months of fluoxetine treatment.33 Good clinical responders (n = 6) had a decrease (P = .031) in DAT binding as assessed using single-photon emission computed tomography with [123I]nor-β-CIT (2β-carbomethoxy-3β-(4-iodo-phenyl)nortropane), whereas poor responders had a trend toward an increase. This study showed that fluoxetine decreases DAT binding, which may also have a neuroprotective effect against dopamine-induced neurotoxicity in autistic children.
Medications do not appear to have any direct effects on white matter microstructure, as described in youth and young adults with high-functioning autism.34 However, long-term cognitive and behavioral therapies beginning in adolescence have been shown to improve structural integrity in the uncinate fasciculus in low-functioning young adults with autism.35 These few studies in youth with autism point to specific mechanisms by which interventions may reduce symptom severity, but additional studies are needed to specifically evaluate how these interventions work to provide neural protection.
Bipolar Disorder
There are several emerging pharmacologic neuroimaging studies in youth with BD. Bipolar symptoms are severe in youth and create a significant level of impairment, such that it is challenging to find youth unmedicated for this disorder when they enroll in a neuroimaging study. Moreover, sometimes youth with BD do not show significant departures from normal development in brain structure and function, possibly because of the normalizing effects of medication. Researchers have attempted to evaluate the contribution of medication to neural effects insofar as examining subsamples of youth with and without medication exposure, or to compare neuroimaging outcome measures among youth with specific medication exposures. For example, a recent study showed that youth with BD who were exposed to lithium had larger hippocampal volumes than those who were not exposed to lithium.36 Other studies have found that youth with BD who had past exposure to mood stabilizers (either lithium or divalproex) had significantly greater posterior subgenual ACC and amygdalar volumes compared with BD youth without mood-stabilizer exposure and controls.37,38 The effects on white matter microstructure have been less studied, with one post hoc analysis finding no effects of medication exposure on DTI findings in youth with BD.39
Functional MRI for bipolar disorder
Recently, a few researchers have begun to directly examine the effects of pharmacologic intervention in fMRI activation in youth with BD. In an open-label study, Chang and colleagues40 examined the neural effects of lamotrigine in adolescents with bipolar depression and found that BD youth treated with lamotrigine for 8 weeks demonstrated less amygdala activation when viewing negative stimuli as depressive symptoms improved; whether the changes in fMRI activation were due to lamotrigine exposure or improvements in depressive symptoms (as a consequence of lamotrigine treatment) could not be determined. Another study examined 17 youths with BD after 14 weeks of treatment with a second-generation antipsychotic (SGA) followed by adjunctive lamotrigine monotherapy, and compared fMRI activation with that of healthy subjects while performing an affective color-matching task.41 The investigators observed treatment-related decreases in the ventromedial prefrontal cortex (VMPFC) and the DLPFC in the BD subjects, showing that pharmacotherapy resulted in differential brain activation patterns within the BD group, with persistently increased activity in the affective regions and decreased activity in the cognitive regions relative to a healthy comparison group. This same group showed that treatment with an SGA followed by lamotrigine monotherapy enhanced ventrolateral prefrontal cortical (VLPFC) and temporal lobe activity during a response-inhibition task, demonstrating reversal of disorder-relevant neural circuitry dysfunction in patients with adolescent BD.42 Behavioral performance was not slowed down in patients on this treatment regimen. Finally, an affective working memory task was presented before and after sequential treatment for 8 weeks with an SGA followed by 6 weeks of lamotrigine to previously unmedicated youth with BD, showing that pharmacotherapy resulted in normalization of symptoms and higher prefrontal cortical and cognitive regional activation in youth with BD versus healthy subjects, but did not normalize amygdala overactivation.43 Improvement on Young Mania Rating Scale (YMRS) score significantly correlated with decreased activity in the VMPFC within the patient group, suggesting a normalizing effect of treatment on fMRI activation, which may be due to either direct medication effects or improvement in symptoms.
Other studies have looked more broadly at the effects of pharmacotherapy on neurocognitive systems in pediatric BD. Wegbreit and colleagues44 aimed to determine functional connectivity differences in youth with BD who were responders (n = 22) versus nonresponders (n = 12) to 1 of 3 mood-stabilizing medications (divalproex, risperidone, or lamotrigine) and compared with healthy controls (n = 14). Participants performed a color-matching task during fMRI whereby they had to match the color of positive, negative, or neutral words with colored dots. A frontolimbic network was identified that showed impaired functional integration in youth with BD relative to healthy controls when participants viewed negatively valenced words. Medication responders in the BD group showed greater connectivity of the amygdala into the network before and after treatment compared with nonresponders, with responders showing a pattern more similar to healthy controls than to nonresponders. The degree of amygdala functional connectivity predicted medication response as well as the improvement in YMRS scores across responders and nonresponders regardless of medication type. From these results the investigators inferred that increased functional integration of the amygdala within the frontolimbic network might be a biomarker of broad responsivity to mood stabilizers in BD.
Differential neural effects of medications for bipolar disorder
Differential neural effects between two medications have also been demonstrated. Pavuluri and colleagues45–47 recently investigated the relative effects of risperidone and divalproex on 3 different cognitive functions in unmedicated manic patients randomized to either treatment or healthy control. In the first task, participants matched the color of a positive, negative, or neutral word with one of two colored circles. After treatment and relative to healthy controls, the risperidone-treated group showed increased activation in the right pregenual and subgenual ACC, and decreased activation in the bilateral middle frontal gyrus, left inferior, medial, and right middle frontal gyri, left inferior parietal lobe, and right striatum. In the divalproex-treated group, relative to healthy controls, increased activations were found in the right superior temporal gyrus, left medial frontal gyrus, and right precuneus. The differential effects of medication were also evaluated in this sample while subjects performed a response inhibition task whereby a motor response, already “on the way” to execution, had to be voluntarily inhibited in trials where a stop signal was presented.46 Youth taking risperidone and divalproex differentially engaged an evaluative affective circuit (EAC: bilateral inferior frontal gyrus, middle frontal gyrus, ACC, middle temporal gyrus, insulae, caudate, and putamen) during task performance. Within the EAC, posttreatment and relative to healthy controls, greater engagement was seen in left insula in the risperidone group and left subgenual ACC in the divalproex group. Finally, during a working memory task under emotional duress, divalproex enhanced activation in a frontotemporal circuit whereas risperidone increased activation in the dopamine (D2) receptor-rich ventral striatum.47 Thus, risperidone and divalproex yield differential patterns of neural activity during emotion processing, response inhibition, and working memory tasks in youth with BD. These studies illustrate that psychotropic medication effects on the brain may be task dependent as well as specific for different types of medications.
Multimodal neuroimaging for bipolar disorder
In a multimodal neuroimaging study, Chang and colleagues48 examined the effect of divalproex on brain structure, chemistry, and function in symptomatic youth at high risk for BD. Although there were no detectable effects on brain structure or neurochemistry after 12 weeks of treatment with divalproex, decreases in prefrontal brain activation correlated with decreases in depressive symptom severity.48 Thus, this change in brain activation may have been due to symptom improvement rather than direct effects of the medication. A placebo arm may help to differentiate medication effects from changes resulting from symptomatic change.
Magnetic resonance spectroscopy for bipolar disorder
Other studies have used MRS in youth with BD, primarily using proton (1H-MRS) acquisitions focused on key prefrontal cortical regions. For example, studies of youth with BD have shown altered medial and dorsolateral prefrontal concentrations of NAA and phosphocreatine/creatine, healthy nerve cell markers putatively involved in maintaining energy production and myelin formation in the brain.49 In addition, higher prefrontal myoinositol levels, a marker for cellular metabolism and second-messenger signaling pathways, have also been found in youth with50 or at familial risk51 for BD. These levels appear to be sensitive to lithium treatment in that children with BD exposed to lithium have relative decreases in prefrontal myoinositol.52 Some, but not all, prior studies have demonstrated that alterations in neurometabolite concentrations may explain the pathophysiology of BD53 and may be sensitive to the effects of psychotropic medications in this population.
Lithium treatment for bipolar disorder
Both NAA and myoinositol concentrations have changed in response to lithium treatment in pediatric populations.52,54 Specifically, Davanzo and colleagues52 found that after 1 week of acute lithium treatment, baseline elevated levels of myoinositol/creatine ratios in the ACC in 11 youths with BD decreased, and this decrement was higher for lithium responders than for nonresponders. However, Patel and colleagues55 observed that in 12- to 18-year-old youth with bipolar depression, lithium did not have any acute (1 week) or chronic (42 days) effects on myoinositol levels in the medial and lateral prefrontal cortices. In a different study, Patel and colleagues54 did find that after 42 days of lithium administration, a sample of 12- to 18-year-old youth with BD demonstrated reductions in NAA concentration in the ventral but not the lateral prefrontal cortex. In this study, there was a time-by-remission-status interaction of NAA concentrations in the right ventrolateral prefrontal cortex, such that youth who remitted developed decreased mean NAA concentration from day 7 to day 42 whereas nonremitters showed an increase in mean NAA concentration during that same time period. The investigators speculated that higher lithium levels earlier in the treatment course might have resulted in lithium-induced increases in prefrontal metabolism.54 However, in adults, chronic lithium exposure has been shown to non-selectively increase NAA concentrations in prefrontal, temporal, parietal, and occipital regions,56 thereby perhaps increasing neuronal viability and function. Some of these findings suggest that by modulating neurometabolites involved in neuronal cell fluid balance and the second-messenger–related neurometabolite myoinositol, lithium exerts its action either by fluid shifts or through intracellular calcium signaling pathways. These studies provide clues about the mechanisms by which lithium and other mood stabilizers exert their therapeutic effect,57 and are consistent with the aforementioned effects of lithium on brain regional volume.
Prefrontal neurometabolite concentrations in bipolar disorder
Prefrontal neurometabolite levels in youth have also been examined after treatment with divalproex48 and the atypical antipsychotic olanzapine.58 In a cohort of youth at high risk for developing BD, there were no statistically changes in pre-divalproex to post-divalproex NAA/creatine ratios, but there was a large effect size (d = 0.94) for a decrease in right dorsolateral prefrontal NAA/creatine after treatment with divalproex. Hospitalized adolescents with bipolar I disorder, experiencing a manic or mixed episode, who achieved remission with olanzapine demonstrated increases in ventral prefrontal NAA compared with nonremitting patients, who showed decreases in prefrontal NAA concentrations. Thus it is unclear as to exactly what these increases or decreases in NAA/creatine ratios mean, but given the potential neurogenic effects of these medications in rat brains59 and neural stem cells,60 additional studies examining in vivo effects of these medications in individuals with BD would help clarify their role in reversing the pathophysiologic effects of this disorder.
Another prefrontal neurometabolite measurable by 1H-MRS that may be associated with abnormal mood regulation is the excitatory neurotransmitter glutamate, its precursor and storage form glutamine, or a combined contribution of glutamate and glutamine (Glx). Moore and colleagues61 used 1H-MRS and found decreased levels of glutamine in the ACC in unmedicated youth with BD in comparison with healthy controls and medicated youth on a variety of different agents for BD. This group also found that unmedicated children with BD exhibiting manic symptoms severe enough to warrant treatment had lower Glx to creatine ratios in the ACC than children with BD who were stably treated with risperidone.62 Mania severity correlated negatively with ACC Glx/creatine levels.
Medications outcomes in bipolar disorder
Taken together, these studies suggest that medications appear to consistently have a normalizing effect on brain function and on some brain volumes in youth with BD. This notion is also consistent with findings from studies in adults with BD.63 Larger controlled studies in individuals who were previously medication naïve would aid in understanding the specific effects of medication exposure on neural activation in BD.
Depressive Disorders
Youth with depressive disorders demonstrate abnormalities in brain structure and function.64 However, although effective treatments are available, the impact of treatment of depression on the brain in youth is understudied. In the only fMRI study examining changes in brain activity with treatment in pediatric depression, Tao and colleagues65 showed that after 8 weeks of open-label fluoxetine treatment, 19 depressed youths with baseline overactivation in prefrontal and temporal regions showed normalization of brain activation in these areas. Further region-of-interest analyses of the areas involved in emotion processing indicated that before treatment, depressed youth had significantly greater activations of fearful relative to neutral facial expressions than did healthy comparison subjects in the amygdala, orbitofrontal cortex, and subgenual ACC bilaterally. Fluoxetine treatment appeared to decrease activations in all 3 regions. This study is limited by the lack of depressed youth exposed to a placebo to substantiate that fluoxetine was truly normalizing activations in these regions.
A smaller open-label study evaluated the potential neurochemical benefit of supplementing fluoxetine with creatine for 8 weeks in 5 adolescent females who had been stabilized on fluoxetine but continued to have persistent depressive symptoms.66 This study used phosphorus MRS and found that compared with healthy controls, creatine-treated adolescents demonstrated a significant increase in brain phosphocreatine concentration (P = .02) on follow-up MRS brain scans. This study warrants replication because of its small sample size and lack of a placebo control group. Moreover, additional neuroimaging studies are needed to investigate the neural effects of widely accepted pharmacologic and psychotherapeutic treatments for pediatric depression.
Obsessive-Compulsive Disorder
A single case report describes the pharmacologic effect of paroxetine on neurochemistry in an 8-year-old with OCD.67 In this report, OCD symptoms improved markedly in an 8-year-old girl treated for 14 months with the SSRI paroxetine (titrated from 10 to 40 mg/d). Paroxetine dose was then decreased in 10-mg decrements and discontinued without symptom recurrence. Serial 1H-MRS examinations were acquired before and after 12 weeks of paroxetine treatment (40 mg/d) and 3 months after discontinuation of medication. A striking decrease in caudate Glx was observed after 12 weeks of treatment, which persisted after discontinuation of medication. These data provide support for a reversible glutamatergically mediated dysfunction of the caudate nucleus in OCD that may serve as a marker for pathophysiology and treatment response. It is clear that more investigation in this area would advance our understanding of brain-based disease and treatment correlates.
Schizophrenia
Children and adolescents with schizophrenia share a similar pattern of phenomenologic, genetic, and cognitive abnormalities with adults with this disorder. However, early-onset schizophrenia (before age 18 years) is associated with a higher frequency of developmental delays and schizophrenia-spectrum disorders in family members along with a worse long-term outcome. Neurobiologically, schizophrenia in childhood is associated with a high frequency of cytogenetic abnormalities such as de novo chromosomal aberrations, and brain-imaging research in adolescents with schizophrenia has revealed a progressive loss of cortical gray matter after onset of psychosis and subtle abnormalities in white matter microstructure.68 Although more likely to be treatment-refractory than adults with schizophrenia, there are some data supporting that youth with schizophrenia are particularly responsive to clozapine.
Caudate volume in schizophrenia treated with clozapine
The neural effects of this medication were demonstrated in a study of 8 adolescents with early-onset psychosis who were scanned along with matched controls at baseline before starting clozapine, and a rescanned after 2 years of treatment with clozapine.69 Caudate volume was found to be higher than normal in the patients at the initial scanning, showing a slope of declining volume between scans, and did not differ significantly between the patients and the healthy control subjects at the second scanning. This study suggested a potential beneficial role of atypical antipsychotics in normalizing striatal volumes after a period of maintenance treatment.
Cortical thickness in schizophrenia treated with clozapine and olanzapine
Another study compared the effects of clozapine and its nearest related drug, olanzapine, on brain cortical thickness in youth with schizophrenia, contrasted with gray matter trajectories of matched healthy controls.70 There were no significant differences in the trajectories of cortical thickness between clozapine-treated and olanzapine-treated groups, except in a small area in the right prefrontal cortex where olanzapine-treated youth had a thicker cortex. Both treatment groups showed relative cortical thinning over time compared with healthy controls. The fact that olanzapine showed a generally comparable neural trajectory to clozapine provides clinicians with additional information during their discussion with patients about choice of medication for long-term use in this population, particularly in treatment-refractory states.71
Cortical thickness and remission status in schizophrenia
Remission status also appears to affect cortical thickness. One study aimed to examine cortical thickness in 56 youths with childhood-onset schizophrenia between the time of hospital admission and discharge, on average 3 months later.72 This study found that compared with patients with continued symptoms, the patients who were remitted at discharge (n = 16 [29%]) had thicker regional cortex in left orbitofrontal, left superior, and middle temporal gyri, and bilateral postcentral and angular gyri (P≤.008). Although a specific medication was not evaluated, this study still provides some neuroanatomic correlates of clinical remission in schizophrenia as well as preliminary evidence that response to treatment may be mediated by these cortical brain regions.
Structural brain changes in schizophrenia
Because schizophrenia is characterized by a chronic course, structural brain changes seen over time in some patients may relate to poor outcome, whereas other changes may be predictive of recovery.73 There are data about the safety and efficacy of multimodal interventions for childhood-onset schizophrenia starting even in the prodromal phases of illness. However, just as for depression, there are few studies that have examined the neural processes that might underlie their benefit. It is certainly possible that intervention may not directly influence the neural processes studied at all. For example, in one study examining prefrontal neurochemical levels in children with symptoms of schizophrenia-spectrum disorders (n = 16; mean age 11 years) and a healthy comparison group (n = 12; mean age 10.8 years), mean ratios of NAA/creatine were significantly lower in schizophrenia-spectrum subjects than in the comparison group (1.67 vs 1.92; P<.05), but medication status did not affect results in schizophrenia-spectrum subjects.74 However, post hoc analyses searching for medication effects are often underpowered to detect significant or specific differences in medicated and unmedicated subsamples. Taken together, the preliminary findings in youth with schizophrenia suggest that atypical antipsychotics in this population tend to normalize brain structure, although perhaps not completely. Antipsychotic effects on brain function, connectivity, and white matter of youth with schizophrenia-spectrum disorders are not known.
SUMMARY
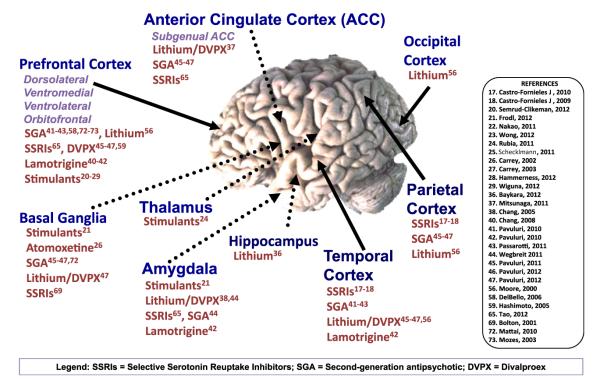
Neuroimaging studies have shown great promise in advancing our understanding of potential mechanisms of action of effective treatments for a range of psychiatric disorders. The good news is that, taken together, intervention appears to have a normalizing effect on brain structure and function in youth suffering from psychopathology. Across disorders, the neurotropic effects of lithium on amygdala and hippocampal volumes appear to be normalizing and are correlated with symptom improvement, and there appears to be normalization of functional activations while performing a wide array of neurocognitive tasks after treatment with psychostimulants, antipsychotics, antidepressants, and mood stabilizers (Fig. 1). Additional information is needed to better understand the critical periods of benefit from these interventions, and how they compare relative to one another. These kinds of investigations will enrich the field of child psychiatry and substantiate the importance of early identification and intervention in youth. Longitudinal studies tracking youth well into adulthood will also provide important supporting evidence for the long-term beneficial effects or deleterious consequences of treatment.
Fig. 1.
Selected brain regions affected by medication exposure in youth versus healthy volunteers. Superscript numbers indicate appropriate references listed on the right. DVPX, divalproex; SGA, second-generation antipsychotic; SSRIs, selective serotonin reuptake inhibitors.
It is also clear from this review that using neuroimaging tools to probe intervention effects in psychiatry may be associated with unique methodological considerations. Such factors include the psychometric effects of repeated scans, how to assess potential relations between the effects of an intervention on symptoms and on specific structural, neurochemical, or brain activation patterns, and how to best make causal inferences about intervention effects on brain function.77 For example, it is often unclear from the studies described whether neural differences observed after treatment are due to the intervention or due to the symptom improvement that resulted from the intervention (ie, we do not know if we are observing a medication effect or a brain-improvement effect with changing symptoms). Future studies should be designed with placebo arms to distinguish these related but separate effects on brain structure and function.
In addition, the study of treatment effects in disorders that manifest in childhood presents additional unique challenges related to brain maturation, analysis methods, and the potential for motion artifacts. Methodological advancements to minimize confounders associated with artifact and optimize analytical techniques to enable predictive inferences will be important steps in advancing this field. It is also true that the interventions examined in the literature reviewed were studied in youth who need them rather than experimentally exposing these interventions to typically developing healthy youth, which proves to be ethically challenging. For example, whereas it may be the case that lithium restores brain structure and function in youth with BD, it is less clear whether it has the same effect in healthy youth. This aspect limits our interpretation of the results, as there may be an interaction between certain neural characteristics and medications that produce a unique effect, one that may not be generalizable to all humans.
There are many justifiable concerns about the adverse effects of psychotropic medication on the developing body and brain. All of the studies in this review reported either null effect or benefit of treatment, but our knowledge of the long-term risks of the various interventions is very limited. However, we do know from prospective observations about the short-term risks of untreated depression, BD, schizophrenia, and other psychiatric disorders in which the levels of morbidity and mortality are very high if left untreated. Therefore, while there are adverse effects that may arise in both the body and the brain, the potential beneficial effects on the brain require further understanding. This kind of investigation is certain to substantiate why behavioral and functional improvements are observed at the clinical level. Nevertheless, adverse brain effects should also be studied with neuroimaging. It would be important to determine if there are predictors of response as well as predictors of adverse effects that are detectable. For example, is it possible that neuroimaging could help us determine which depressed adolescents will respond well to SSRIs and which would have a high likelihood of developing antidepressant-induced mania?78
When we learn more about the effects of treatment on brain structure and function, and if there is a particular window during development when they are optimal (or most problematic), we can begin to develop more targeted and thoughtful approaches to treatment. The possibility remains that acute intervention with proper medications at a critical point in time will allow for shorter duration of treatment needed, and perhaps neuroprotection or neuroplastic change that will then eliminate the need for a lifetime of medications. This dream is one shared by all practitioners caring for youth with psychopathology, who wish that these youth achieve full and permanent remission of all symptoms and are able to eventually be taking as few medications as possible, and ideally be medication free, before reaching adulthood.
CLINICAL VIGNETTE.
Although neuroimaging cannot currently be used to diagnose youth with psychopathology,75 one can imagine that this tool will become more accessible and, it is hoped, lead to more targeted treatments for youth with psychiatric illnesses. In some young individuals, treatments directed to more specific brain structures such as repetitive transcranial magnetic stimulation have shown some promise for tolerability and efficacy.76 For the great majority of youth, however, pharmacologic and psychosocial interventions will likely remain the mainstay of treatment. A hypothetical example is given here to illustrate how information from neuroimaging studies might advance our treatments for youth with severe psychiatric illness.
A 16-year-old girl presents to the clinic after a week of euphoria, decreased need for sleep, increased goal-directed activities, racing thoughts, grandiosity, distractibility, increased motor activity, and hypersexuality, preceded by a 2-week period of depressed mood, suicidal ideation, irritability, lethargy, and hypersomnia. These symptoms are above and beyond what would be expected of a typical adolescent, and she meets criteria for bipolar I disorder. Currently unmedicated, she and her family elect to first undergo multimodal neuroimaging. Her structural MRI scan shows that she has reduced volumes in the subgenual anterior cingulate cortex, amygdala, and hippocampus. Her fMRI scan shows amygdalar overactivation and impaired functional integration of limbic structures with prefrontal areas during emotional tasks. Her MRS scan shows increased prefrontal myoinositol, decreased NAA, and decreased glutamine levels. With hope that lithium might restore her structural and neurochemical aberrancies, the clinician begins a trial of lithium for 12 weeks. The patient achieves remission of her manic symptoms and receives a follow-up MRI scan, which reveals normalization of subgenual ACC, amygdalar, and hippocampal volumes, restoration of typical neurometabolite levels, and normalization of activation patterns and improved functional integration between limbic structures and the prefrontal cortex. Three months later, she experiences another depressive episode and is rescanned. Amygdalar and VLPFC activation is high during an fMRI emotion task, and prefrontal glutamate levels are increased. After 8 weeks of adjunctive lamotrigine she is rescanned, with normalization of activation in these areas. With additional family and individual therapy, the patient remains in remission for the next 2 years.
KEY POINTS.
Little is known about the neurobiological effects of psychotropic medications on the developing brain.
An efficient way to elucidate neural mechanisms that underlie the effects of treatment is to use magnetic resonance imaging technology to assess in vivo brain differences in youth before and after an intervention has been made.
Across disorders, the neurotropic effects of lithium on amygdala and hippocampal volumes appear to be normalizing and are correlated with symptom improvement, and there appears to be normalization of functional activations while performing a wide array of neurocognitive tasks after treatment with psychostimulants, antipsychotics, antidepressants, and mood stabilizers.
Additional information is needed to better understand the critical periods of benefit from these interventions, and how they compare relative to one another.
REFERENCES
- 1.Opler M, Sodhi D, Zaveri D, et al. Primary psychiatric prevention in children and adolescents. Ann Clin Psychiatry. 2010;22(4):220–34. [PubMed] [Google Scholar]
- 2.Carlson GA, Kotov R, Chang SW, et al. Early determinants of four-year clinical outcomes in bipolar disorder with psychosis. Bipolar Disord. 2012;14(1):19–30. doi: 10.1111/j.1399-5618.2012.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olino TM, Seeley JR, Lewinsohn PM. Conduct disorder and psychosocial outcomes at age 30: early adult psychopathology as a potential mediator. J Abnorm Child Psychol. 2010;38(8):1139–49. doi: 10.1007/s10802-010-9427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023–35. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- 5.Klein DN, Shankman SA, Rose S. Dysthymic disorder and double depression: prediction of 10-year course trajectories and outcomes. J Psychiatr Res. 2008;42(5):408–15. doi: 10.1016/j.jpsychires.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezo J, Paris J, Barker ED, et al. Natural history of suicidal behaviors in a population-based sample of young adults. Psychol Med. 2007;37(11):1563–74. doi: 10.1017/S003329170700058X. [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503–26. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 8.Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 9.McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107–25. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 10.Phillips ML, Travis MJ, Fagiolini A, et al. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–20. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh MK, Chang KD, Mazaika P, et al. Neural correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20(1):15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Spielman D, Adleman N, et al. Brain glutamatergic characteristics of pediatric offspring of parents with bipolar disorder. Psychiatry Res. 2010;182(2):165–71. doi: 10.1016/j.pscychresns.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169(2):205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chantiluke K, Halari R, Simic M, et al. Fronto-striato-cerebellar dysregulation in adolescents with depression during motivated attention. Biol Psychiatry. 2012;71(1):59–67. doi: 10.1016/j.biopsych.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Freitag CM, Luders E, Hulst HE, et al. Total brain volume and corpus callosum size in medication-naïve adolescents and young adults with autism spectrum disorder. Biol Psychiatry. 2009;66(4):316–9. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickstein DP, van der Veen JW, Knopf L, et al. Proton magnetic resonance spectroscopy in youth with severe mood dysregulation. Psychiatry Res. 2008;163(1):30–9. doi: 10.1016/j.pscychresns.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Fornieles J, Caldú X, Andrés-Perpiñá S, et al. A cross-sectional and follow-up functional MRI study with a working memory task in adolescent anorexia nervosa. Neuropsychologia. 2010;48(14):4111–6. doi: 10.1016/j.neuropsychologia.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Castro-Fornieles J, Bargalló N, Lázaro L, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. 2009;43(3):331–40. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Fornieles J, Bargalló N, Lázaro L, et al. Adolescent anorexia nervosa: cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J Psychiatr Res. 2007;41(11):952–8. doi: 10.1016/j.jpsychires.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Semrud-Clikeman M, Pliszka SR, Bledsoe J, et al. [Accessed June 30, 2012];Volumetric MRI differences in treatment naive and chronically treated adolescents with ADHD-combined type. J Atten Disord. 2012 doi: 10.1177/1087054712443158. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22653807. [DOI] [PubMed]
- 21.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125(2):114–26. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakao T, Radua J, Rubia K, et al. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168(11):1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 23.Wong CG, Stevens MC. The effects of stimulant medication on working memory functional connectivity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71(5):458–66. doi: 10.1016/j.biopsych.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubia K, Halari R, Mohammad AM, et al. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70(3):255–62. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schecklmann M, Schaldecker M, Aucktor S, et al. Effects of methylphenidate on olfaction and frontal and temporal brain oxygenation in children with ADHD. J Psychiatr Res. 2011;45(11):1463–70. doi: 10.1016/j.jpsychires.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Carrey N, MacMaster FP, Sparkes SJ, et al. Glutamatergic changes with treatment in attention deficit hyperactivity disorder: a preliminary case series. J Child Adolesc Psychopharmacol. 2002;12(4):331–6. doi: 10.1089/104454602762599871. [DOI] [PubMed] [Google Scholar]
- 27.Carrey N, MacMaster FP, Fogel J, et al. Metabolite changes resulting from treatment in children with ADHD: a 1H-MRS study. Clin Neuropharmacol. 2003;26(4):218–21. doi: 10.1097/00002826-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Hammerness P, Biederman J, Petty C, et al. Brain biochemical effects of methylphenidate treatment using proton magnetic spectroscopy in youth with attention-deficit hyperactivity disorder: a controlled pilot study. CNS Neurosci Ther. 2012;18(1):34–40. doi: 10.1111/j.1755-5949.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiguna T, Guerrero AP, Wibisono S, et al. Effect of 12-week administration of 20-mg long-acting methylphenidate on Glu/Cr, NAA/Cr, Cho/Cr, and mI/Cr ratios in the prefrontal cortices of school-age children in Indonesia: a study using 1H magnetic resonance spectroscopy (MRS) Clin Neuropharmacol. 2012;35(2):81–5. doi: 10.1097/WNF.0b013e3182452572. [DOI] [PubMed] [Google Scholar]
- 30.Siniatchkin M, Glatthaar N, von Müller GG, et al. Behavioural treatment increases activity in the cognitive neuronal networks in children with attention deficit/hyperactivity disorder. Brain Topogr. 2012;25(3):332–44. doi: 10.1007/s10548-012-0221-6. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan A, White CA, Saklayen S, et al. Effect of propranolol on functional connectivity in autism spectrum disorder—a pilot study. Brain Imaging Behav. 2010;4(2):189–97. doi: 10.1007/s11682-010-9098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernell E, Watanabe Y, Adolfsson I, et al. Possible effects of tetrahydrobiopterin treatment in six children with autism—clinical and positron emission tomography data: a pilot study. Dev Med Child Neurol. 1997;39(5):313–8. doi: 10.1111/j.1469-8749.1997.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 33.Makkonen I, Kokki H, Kuikka J, et al. Effects of fluoxetine treatment on striatal dopamine transporter binding and cerebrospinal fluid insulin-like growth factor-1 in children with autism. Neuropediatrics. 2011;42(5):207–9. doi: 10.1055/s-0031-1291242. [DOI] [PubMed] [Google Scholar]
- 34.Lange N, Dubray MB, Lee JE, et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3(6):350–8. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardini M, Elia M, Garaci FG, et al. Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. J Autism Dev Disord. 2012;42(4):585–92. doi: 10.1007/s10803-011-1281-2. [DOI] [PubMed] [Google Scholar]
- 36.Baykara B, Inal-Emiroglu N, Karabay N, et al. Increased hippocampal volumes in lithium treated adolescents with bipolar disorders: a structural MRI study. J Affect Disord. 2012;138(3):433–9. doi: 10.1016/j.jad.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Mitsunaga MM, Garrett A, Howe M, et al. Increased subgenual cingulate cortex volume in pediatric bipolar disorder associated with mood stabilizer exposure. J Child Adolesc Psychopharmacol. 2011;21(2):149–55. doi: 10.1089/cap.2010.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang K, Karchemskiy A, Barnea-Goraly N, et al. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(6):565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 39.Barnea-Goraly N, Chang KD, Karchemskiy A, et al. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66(3):238–44. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Chang KD, Wagner C, Garrett A, et al. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10(3):426–31. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 41.Pavuluri MN, Passarotti AM, Parnes SA, et al. A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20(5):395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavuluri MN, Passarotti AM, Harral EM, et al. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010;71(11):1526–34. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pretreatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011;216(4):485–99. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wegbreit E, Ellis JA, Nandam A, et al. Amygdala functional connectivity predicts pharmacotherapy outcome in pediatric bipolar disorder. Brain Connect. 2011;1(5):411–22. doi: 10.1089/brain.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavuluri MN, Passarotti AM, Lu LH, et al. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Res. 2011;193(1):28–37. doi: 10.1016/j.pscychresns.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavuluri MN, Ellis JA, Wegbreit E, et al. Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behav Brain Res. 2012;226(2):493–503. doi: 10.1016/j.bbr.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavuluri MN, Passarotti AM, Fitzgerald JM, et al. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2012;51(2):157–170. doi: 10.1016/j.jaac.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang K, Karchemskiy A, Kelley R, et al. Effect of divalproex on brain morphometry, chemistry, and function in youth at high-risk for bipolar disorder: a pilot study. J Child Adolesc Psychopharmacol. 2009;19(1):51–9. doi: 10.1089/cap.2008.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang K, Adleman N, Dienes K, et al. Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53(11):1059–65. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- 50.Patel NC, Cecil KM, Strakowski SM, et al. Neurochemical alterations in adolescent bipolar depression: a proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. J Child Adolesc Psychopharmacol. 2008;18(6):623–7. doi: 10.1089/cap.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cecil KM, DelBello MP, Sellars MC, et al. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13(4):545–55. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 52.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24(4):359–69. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 53.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):969–95. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Patel NC, DelBello MP, Cecil KM, et al. Temporal change in N-acetyl-aspartate concentrations in adolescents with bipolar depression treated with lithium. J Child Adolesc Psychopharmacol. 2008;18(2):132–9. doi: 10.1089/cap.2007.0088. [DOI] [PubMed] [Google Scholar]
- 55.Patel NC, DelBello MP, Cecil KM, et al. Lithium treatment effects on Myo-inositol in adolescents with bipolar depression. Biol Psychiatry. 2006;60(9):998–1004. doi: 10.1016/j.biopsych.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol Psychiatry. 2000;48(1):1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 57.Glitz DA, Manji HK, Moore GJ. Mood disorders: treatment-induced changes in brain neurochemistry and structure. Semin Clin Neuropsychiatry. 2002;7(4):269–80. doi: 10.1053/scnp.2002.35226. [DOI] [PubMed] [Google Scholar]
- 58.DelBello MP, Cecil KM, Adler CM, et al. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31(6):1264–73. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto R, Fujimaki K, Jeong MR, et al. Neuroprotective actions of lithium. Seishin Shinkeigaku Zasshi. 2003;105(1):81–6. [in Japanese] [PubMed] [Google Scholar]
- 60.Laeng P, Pitts RL, Lemire AL, et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem. 2004;91(1):238–51. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- 61.Moore CM, Frazier JA, Glod CA, et al. Glutamine and glutamate levels in children and adolescents with bipolar disorder: a 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry. 2007;46(4):524–34. doi: 10.1097/chi.0b013e31802f5f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore CM, Biederman J, Wozniak J, et al. Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: a proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Affect Disord. 2007;99(1–3):19–25. doi: 10.1016/j.jad.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hafeman DM, Chang KD, Garrett AS, et al. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 64.Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5(4):307–28. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao R, Calley CS, Hart J, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169(4):381–8. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondo DG, Sung YH, Hellem TL, et al. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord. 2011;135(1–3):354–61. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolton J, Moore GJ, MacMillan S, et al. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):903–6. doi: 10.1097/00004583-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Kumra S, Asarnow R, Grace A, et al. From bench to bedside: translating new research from genetics and neuroimaging into treatment development for early-onset schizophrenia. Early Interv Psychiatry. 2009;3(4):243–58. doi: 10.1111/j.1751-7893.2009.00142.x. [DOI] [PubMed] [Google Scholar]
- 69.Frazier JA, Giedd JN, Kaysen D, et al. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry. 1996;153(4):564–6. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- 70.Mattai A, Chavez A, Greenstein D, et al. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 2010;116(1):44–8. doi: 10.1016/j.schres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mozes T, Greenberg Y, Spivak B, et al. Olanzapine treatment in chronic drug-resistant childhood-onset schizophrenia: an open-label study. J Child Adolesc Psychopharmacol. 2003;13(3):311–7. doi: 10.1089/104454603322572642. [DOI] [PubMed] [Google Scholar]
- 72.Greenstein DK, Wolfe S, Gochman P, et al. Remission status and cortical thickness in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47(10):1133–40. doi: 10.1097/CHI.0b013e3181825b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeLisi LE, Sakuma M, Ge S, et al. Association of brain structural change with the heterogeneous course of schizophrenia from early childhood through five years subsequent to a first hospitalization. Psychiatry Res. 1998;84(2–3):75–88. doi: 10.1016/s0925-4927(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 74.Brooks WM, Hodde-Vargas J, Vargas LA, et al. Frontal lobe of children with schizophrenia spectrum disorders: a proton magnetic resonance spectroscopic study. Biol Psychiatry. 1998;43(4):263–9. doi: 10.1016/S0006-3223(97)00462-9. [DOI] [PubMed] [Google Scholar]
- 75.Chang K, Adleman N, Wagner C, et al. Will neuroimaging ever be used to diagnose pediatric bipolar disorder? Dev Psychopathol. 2006;18(4):1133–46. doi: 10.1017/S0954579406060548. [DOI] [PubMed] [Google Scholar]
- 76.Mattai A, Miller R, Weisinger B, et al. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain Stimul. 2011;4(4):275–80. doi: 10.1016/j.brs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dichter GS, Sikich L, Song A, et al. [Accessed July 5, 2012];Functional neuroimaging of treatment effects in psychiatry: methodological challenges and recommendations. Int J Neurosci. 2012 doi: 10.3109/00207454.2012.678446. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22471393. [DOI] [PMC free article] [PubMed]
- 78.Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4):225–43. doi: 10.2165/11591660-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]