Abstract
Background
Post-traumatic stress disorder (PTSD) is associated with enhanced noradrenergic activity. Animal and human studies demonstrate that noradrenergic stimulation augments consolidation of fear learning. Retrieval of well-established memories by presenting a learned fear cue triggers reconsolidation processes during which memories may be updated, weakened, or strengthened. We previously reported that noradrenergic blockade in the rat amygdala impairs reconsolidation of fear memories. Here we investigated the effects of noradrenergic enhancement on reconsolidation of learned fear.
Methods
Using auditory fear conditioning in rats, we tested the effects of post-retrieval intra-amygdala infusion of the beta-adrenergic receptor agonist isoproterenol or the antagonist propranolol on conditioned fear in the amygdala.
Results
A single intra-amygdala infusion of isoproterenol following retrieval of a well-consolidated memory enhanced fear memory elicited by the learned fear stimulus and impaired extinction of this memory forty-eight hours later. Intra-amygdala infusion of the beta-adrenergic receptor antagonist propranolol following a consecutive retrieval trial blocked the enhancing effects of isoproterenol on fear memory.
Conclusions
Postretrieval beta-adrenergic stimulation in the amygdala enhances reconsolidation of fear memories, making them resistant to extinction. Noradrenergic augmentation during retrieval of fear memories may thus contribute to persistence and severity of traumatic memories. Reconsolidation may be a useful tool in understanding the pathology of PTSD and may thus help in developing new and in modifying existing treatments of traumatic memories.
Keywords: fear, anxiety, fear conditioning, amygdala, reconsolidation, extinction, isoproterenol, propranolol, posttraumatic stress disorder, PTSD, late-onset PTSD, beta-adrenergic receptor, norepinephrine
INTRODUCTION
Posttraumatic stress disorder (PTSD) develops following an emotional trauma and is characterized by the recurrence of intrusive memories, avoidance and hyperarousal (American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). One of the most common behavioral treatments for PTSD is exposure therapy (Foa, 2006). Exposure therapy is based on extinction processes in which repeated exposure to trauma-related cues (thoughts, feelings and situations) reduces symptoms of posttraumatic stress (Cukor et al., 2010; Keane et al., 2006). Pharmacological treatments are also used either alone (Cukor et al., 2010; Hetrick et al., 2010; Berger et al., 2009; Morgan et al., 2003) or in conjunction with exposure therapy (Cukor et al., 2010; Ressler and Mayberg, 2007). Despite the existence of behavioral and pharmacological treatments, many individuals continue to display symptoms of PTSD several years after an initial diagnosis (Kessler et al., 1995). Therefore it is critical to study the mechanisms underlying the persistence of memories for trauma.
Norepinephrine has been implicated in normal and pathological fear and anxiety (Bremner et al., 1996a, 1996b; Krystal and Neumeister, 2009; Southwick et al., 1997, 1999, 2002; Aston-Jones et al., 1999, McGaugh 2004). Animal and human studies indicate the involvement of norepinephrine in fear learning and memory (Cahill, 1997; McGaugh, 2000, 2004, Roozendaal et al., 2009, Grillon et al., 1996, 2004). More specifically, norepinephrine has been found to enhance memory consolidation processes by which new learning, initially labile and susceptible to disruption, is transformed into long-term memories (McGaugh, 2004; Prager and Johnson, 2009, Rodrigues et al., 2009). Increased noradrenergic activity during trauma has also been implicated in the enhancement of encoding of the memory for traumatic event (Pitman, 1989; O’Donnell et al., 2004). Further, pilot studies show that blockade of noradrenergic transmission by administration of the beta-adrenergic receptor antagonist propranolol following trauma decreases the risk of PTSD (Pitman et al., 2002; Vaiva et al., 2003). However, clinical research indicates that the persistence and severity of PTSD symptoms is also associated with increased noradrenergic activity long after the traumatic event (Geracioti et al., 2001; Nemeroff et al., 2006; Southwick et al., 1997, 1999; Strawn and Geracioti, 2008). It is possible that norepinephrine is involved not only in the original encoding but also in the maintenance and exacerbation of symptoms associated with traumatic memories.
We addressed this question using a rodent model of fear learning, Pavlovian fear conditioning. Although, PTSD is manifested by complex cognitive, emotional and behavioral alterations, and many of these symptoms are unique for humans, ample evidence demonstrates that fear learning contributes significantly to many anxiety pathologies including PTSD (Dollard and Miller, 1950; Eysenck, 1979; Bandura, 1969; Rosen and Schulkin; 1998; Rasmusson and Charney, 1997; Milad et al., 2006). One of the most commonly used models of fear learning is fear conditioning, the neural basis of which is well understood (Lang et al., 2000; LeDoux, 2007). In fear conditioning, a neutral event (conditioned stimulus, CS), such as tone, is paired with a noxious event (unconditioned stimulus, US), such as electric shock to the foot pads. A key structure involved in fear conditioning is the lateral nucleus of the amygdala (LA), a site where the information about the CS and the US converges (LeDoux, 2007, Maren, 2005). Previous studies from our lab indicate that norepinephrine is a major modulator of fear conditioning in the LA. Thus, norepinephrine in LA is involved in the acquisition, extinction and reconsolidation of auditory fear conditioning (Dębiec and LeDoux, 2004; Bush et al., 2010; Lazzaro et al., 2010). Here we investigated the role of norepinephrine in memory reconsolidation. Reconsolidation is a process by which well-established (consolidated) memories are rendered labile and susceptible to modification (Nader et al., 2000; Doyere et al., 2007, Dębiec et al., 2002, 2006, 2010; Sara, 2000). Reconsolidation has been demonstrated in a variety of species using different learning systems and a wide range of learning tasks (Tronson and Taylor, 2007). In contrast to extinction, where attenuation of learned responding requires prolonged exposure to a learning cue, reconsolidation is triggered by a single exposure to the CS. In a previous study we found that post-retrieval beta-adrenergic receptor blockade impairs reconsolidation of auditory fear conditioning in the LA (Dębiec and LeDoux, 2004). Here we examined whether enhancing beta-adrenergic transmission augments reconsolidation of auditory fear conditioning.
We hypothesized that enhancing noradrenergic signaling immediately following retrieval of conditioned fear will augment the memory and make it resistant to extinction. We tested the effects of isoproterenol on reconsolidation using multiple cue presentations (extinction test) instead of an exposure to a single or few CSs in order to investigate the response of the memory to extinction treatments.
Most of the reconsolidation studies so far used a single reactivation approach to modify the memory (Dudai, 2006; Nader et al., 2000; Alberini et al., 2006). If each retrieval leads to the release of norepinephrine and the further reconsolidation of the memory, it would help explain why traumatic memories are so persistent. It would also suggest that reconsolidation blockade may be an effective treatment.
We thus further hypothesize that after multiple reconsolidation events it is still possible to block reconsolidation and impair fear memory. In life, as opposed to controlled experimental settings, memories are being constantly retrieved and processed. It is thus important to investigate whether reconsolidating memories may be altered more than once. In other words, it is important to determine the effects of multiple retrievals, each under a different (drug) condition, on a memory. This is especially important if reconsolidation is to be applied to a better understanding of lasting traumatic memories, such as those in PTSD, as PTSD is characterized by recurrent intrusive recollections. Using two distinct memory retrieval sessions separated in time, we asked whether effects of treatments applied during first round of reconsolidation (first retrieval session) could be reversed upon the subsequent memory retrieval.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (Hilltop Laboratories, Scottdale, PA, USA) weighing 275-300g at the beginning of the procedures were housed individually in clear plastic Nalgene cages in a thermally controlled colony room. Rats were placed on a 12/12 hr light/dark cycle, and food and water were provided ad libitum throughout the duration of the experiment. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the New York University Animal Care and Use Committee.
Surgery and Histology
Surgery and histology procedures have been the same as in previous study (Dębiec et al, 2010). Under Nembutal anaesthesia (45 mg/kg; i.p.), animals were implanted bilaterally with 22-gauge stainless guide cannulae (Plastics One Inc, Roanoke, VA) aimed at the lateral nuclei of the amygdala (LA) or 2mm dorsal to the LA. All coordinates were taken from Paxinos and Watson (1986). Coordinates for intra-LA were: 3.0 mm posterior to Bregma, 5.3 mm lateral to the midline and 8.0 mm ventral to the skull surface. For cannulae implanted 2 mm dorsal to the LA, anterior-posterior and lateral coordinates were the same as for intra-LA implants and the ventral coordinate was 6mm. The guide cannulae were fixed into the skull with screws and acrylic dental cement. A dummy cannula was inserted into each guide cannula to prevent clogging. Postsurgical analgesics (2 mg/kg ketoprofen) were given daily for 3 days after all surgeries. Rats had at least one week to recover before the start of behavioural procedures.
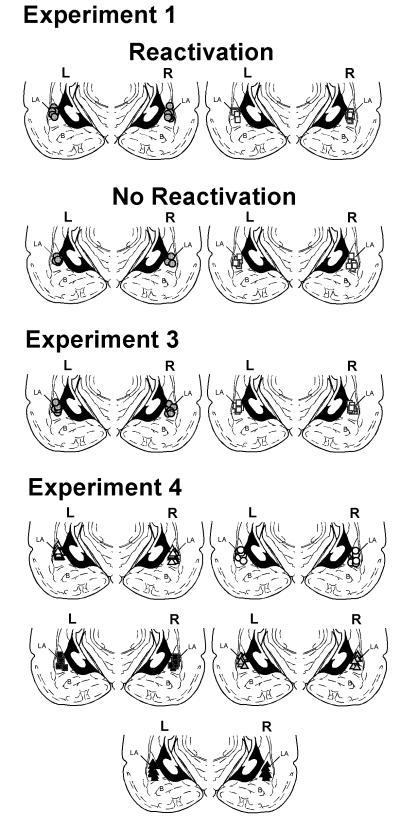
At the completion of the experiment, rats were euthanized by an overdose of chloral hydrate (600 mg/kg) and perfused with 10% buffered formalin. Their brains were sectioned at 50μm thickness. The sections were stained using Cresyl violet and examined with light microscopy for cannula placement. After histological verification, only animals that had placements for both cannulae into the LA (or 2mm dorsal to the LA) were included in the present report (Figure 1).
Figure 1. Cannualae placements in the lateral nucleus of the amygdala.
Images show cannulae placements for rats included in Experiments 1, 3 and 4. Symbols indicate injection sites. White squares indicate saline (SAL) groups and grey circles indicate isoproterenol (ISO) groups in Experiments 1 and 3. White triangles indicate saline-saline (SAL-SAL) group, white circles indicate isoproterenol-saline (ISO-SAL) group, black squares indicate propranolol-saline (PRO-SAL), grey triangles indicate isoproterenol-saline (ISO-SAL) group and black triangles indicate propranolol-isoproterenol (PRO-ISO) group in Experiment 4. LA – lateral nucleus of the amygdala; B – basal nucleus of the amygdala. All sections depicted at around 3.8 mm posterior to Bregma.
Drugs and infusions
Isoproterenol [ISO; R(−)-isoproterenol (+)-bitartrate; Sigma-Aldrich, St. Louis, MO] and propranolol [PRO; DL-Propranolol; Sigma-Aldrich, St. Louis, MO] were both dissolved in saline (SAL) (6.25μg/μl). Drugs were slowly infused through infusion cannulae at 0.25 μl/min using a pump. A total volume of 0.2 μl of isoproterenol (or propranolol) solution or an equivalent amount of saline vehicle was infused bilaterally into the LA (or 2 mm dorsal to the LA as in the Experiment 2). We have previously shown that the same 1.25 μg dose of propranolol infused bilaterally into the LA impairs reconsolidation of auditory fear conditioning (Dębiec and LeDoux, 2004). Infusion cannulae were left in place for an additional minute to allow the solution to diffuse from the cannulae tips.
Apparatus and stimuli
Auditory fear conditioning was conducted in chamber A; memory reactivation and testing took place in chamber B. Chamber A was a Plexiglas chamber with a metal grid floor (Model E10-10, Coulbourn Instruments, Leigh Valley, PA, USA) that was dimly illuminated by a single house light and enclosed within a sound attenuating chamber (Model E10-20). Chamber B consisted of a distinct conditioning Plexiglas chamber (ENV-001; MedAssociates, Inc. Georgia, VT, USA) and was located in a different room. Chamber B was brightly illuminated by three lights and contained a flat Formica floor that had been scented with peppermint soap. In our previous study we have shown that this testing environment is distinct enough to minimize generalization from the training environment (Dębiec and LeDoux, 2004). The conditioned stimulus (CS) was a 30 s, 5 kHz, 75 dB tone. The unconditioned stimulus (US) was 1.0 mA, 1 s footshock. Behavior during training and testing was videotaped with a camera installed at the top of the chamber.
Behavioral procedures
General procedures
On the day before conditioning, rats were habituated for 2 min to handling and for 10 min to conditioning chamber A. For conditioning, after a 2 min acclimatizing period, rats were given a single conditioning trial consisting of one CS that coterminated with the US. After completion of all the procedures, rats were returned to the colony. Memory retrieval. Memory retrieval was triggered on the day following fear conditioning (in Experiment 4, the second memory retrieval trial took place 48 hours following the first memory retrieval). After a 2-min acclimatization period in chamber B, a single CS was given. The freezing response during the CS presentation was used to measure the conditioned fear response (Blanchard and Blanchard, 1969). Freezing was videotaped and scored by a blind experimenter, and then used for analysis. Memory extinction test. 48 hours (or 3 hours for Experiment 3) following memory reactivation (or following the second memory reactivation session for Experiment 4) rats received nine CS presentations. The mean inter-trial interval was 120 sec. Freezing during each CS presentation was videotaped and scored by an experimenter who was blind to the experiment condition. An average of the nine scores for each CS for each rat was subsequently used for the analysis (an average of nine scores was used as there was not any significant treatment x trial interaction). Freezing in all experiments was expressed as a percentage of the total duration of the tone presentation.
Experiment 1
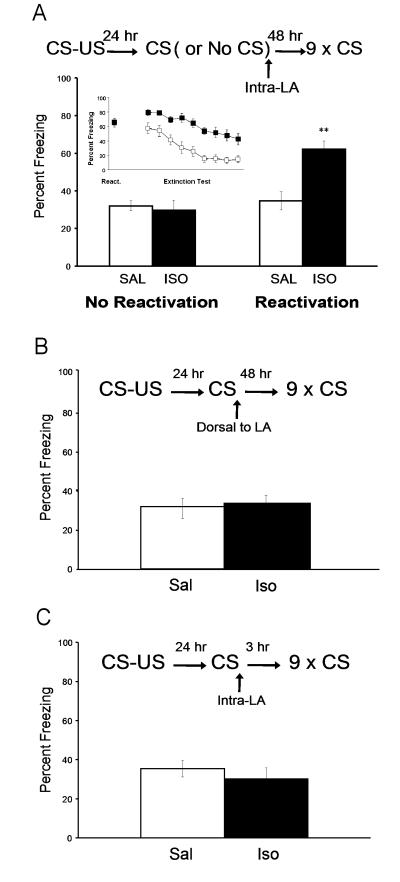
On the day following conditioning, rats were divided into two groups. One group of rats received a memory retrieval (Reactivation group) trial followed by intra-LA infusions with ISO (n = 9) or SAL (n = 11). Another group of rats received either intra-LA infusions of ISO (n = 5) or SAL (n = 6), without explicit memory retrieval (No Reactivation group) (Figure 2A). On the following day all animals received an extinction test.
Figure 2. Isoproterenol enhances reconsolidation of cue conditioned fear.
(A) Post-reactivation isoproterenol infusions into the lateral nucleus of the amygdala (LA) enhance fear memory and impair extinction processes forty-eight hours later. The behavioral procedure (top) and freezing to the CS (bottom) during extinction test. (B) Post-reactivation isoproterenol infusions two millimeters dorsal to the LA have no effect on fear memory. The behavioral procedure (top) and freezing to the CS (bottom) during extinction test. (C) Post-reactivation isoproterenol infusions into the lateral nucleus of the amygdala (LA) have no effect on memory three hours later. The behavioral procedure (top) and freezing to the CS (bottom) during extinction test. (ISO: isoproterenol; SAL: saline; ** indicates different from all other groups Tukey’s HSD p < .01)
Experiment 2
Procedures were the same as in Experiment 1 except that rats were implanted with cannulae outside of the LA and received drug (or vehicle) infusions 2 mm dorsal to the LA (ISO: n = 7; SAL: n = 7) (Figure 2B).
Experiment 3
Procedures were the same as in Experiment 1 except that memory extinction test was performed 3 hours following drug (or vehicle) infusions (ISO: n = 7; SAL: n = 7) (Figure 2C).
Experiment 4
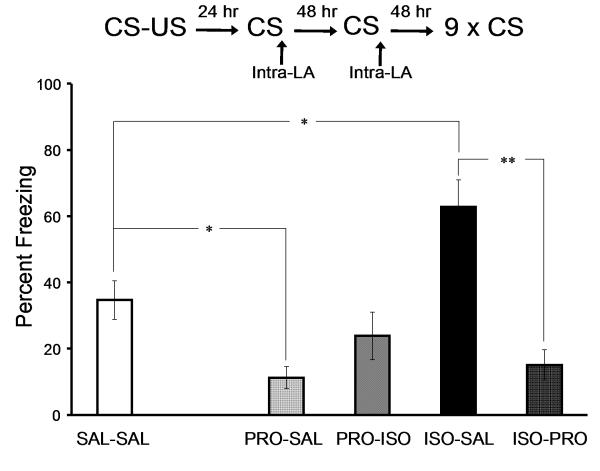
On the day following training, rats received a memory retrieval trial followed by intra-LA infusions of ISO (n = 19), PRO (n = 19), or SAL (n = 10). Forty-eight hours later, all rats received a second retrieval trial followed by intra-LA drug (or vehicle) infusions. All rats that received SAL infusions during the first retrieval trial were infused with saline again (SAL-SAL: n = 10). Half of the rats that had been infused with ISO following the first memory retrieval trial were infused with SAL (ISO-SAL: n = 9), whereas the other half were infused with PRO (ISO-PRO: n = 10). Rats that received PRO infusions following the first retrieval trial were also divided into two groups following the second memory retrieval. One group was infused with SAL (PRO-SAL: n = 10), whereas the other group was infused with ISO (PRO-ISO; n = 9).
Data analysis
Data were analyzed using Student’s t-test for independent samples, or a two -way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc test, and significance levels were set at p<0.05.
RESULTS
Fear conditioned rats received bilateral intra-LA infusions of ISO or SAL (Experiment 1; Figure 2A). One group of rats was presented with a single CS in order to reactivate the memory (Reactivation group) while the other group received infusions without memory reactivation (No Reactivation group). During memory reactivation, both SAL and ISO groups expressed comparable levels of freezing (SAL = 63.9%, ± 5.5; ISO = 65.9%, ± 5; p > .05). Immediately after, rats received bilaterally intra-LA infusions of either ISO or SAL. Forty-eight hours later, all rats received an extinction test. Freezing during the CS presentation was measured and scored for analysis. The results from the ANOVA indicated a significant Reactivation x Drug interaction (F(1,33)=10.76, p < .01). Post hoc mean comparisons with Tukey’s HSD indicated that the freezing scores from the Reactivation-ISO group were significantly different (higher) than all other groups (p < .01), and none of the other groups were significantly different from one another. Our data demonstrate that post-retrieval intra-LA infusions of the beta-adrenergic receptor agonist isoproterenol enhance a well consolidated auditory conditioned fear memory making it resistant to extinction treatments. The results also confirm that a single reactivation trial alone does not affect fear extinction learning 48 hours later.
In order to verify whether the lateral amygdala (LA) was a site of action for ISO on conditioned fear as observed in previous experiment, another group of rats received post-retrieval drug or vehicle infusions outside (2 mm dorsal) of the LA (Experiment 2; Figure 2B). During memory reactivation, both groups showed comparable levels of freezing (SAL = 75.2%, ± 5.1; ISO = 73.8%, ± 6.6; p > .05). There was no statistically significant difference in freezing responding between SAL and ISO groups 48 hours later during the extinction test (p > .05) (Figure 2B).
It is possible that exogenous treatments may affect reconsolidation by producing nonspecific effects. In order to control for this condition it is important to demonstrate that the amygdala is functional following drug infusions before the reconsolidation window is closed (typically few hours following memory recall) (Nader et al., 2000; Tronson and Taylor, 2007). In order to determine whether post-reactivation isoproterenol affects fear memory shortly after reactivation, before reconsolidation processes are completed (Nader et al., 2000), another group of rats received an extinction test 3 hours following memory retrieval with post-reactivation drug or vehicle infusions (Experiment 3; Figure 2C). During memory reactivation, both groups showed comparable levels of freezing (SAL = 62.4%, ± 4.4; ISO = 62.3%, ± 5.4; p > .05). There was no statistically significant difference in freezing between SAL and ISO groups (p = 0.47) on freezing during the extinction test 3 hours later. Thus post-retrieval exogenous beta-adrenergic stimulation has no effect on the retrieval of auditory conditioned fear three hours later.
Experiments described above together with our earlier studies (Dębiec and LeDoux, 2004; 2006) demonstrate that well consolidated auditory fear memories following a single memory retrieval are susceptible to bidirectional modulation through interference or enhancement of beta-adrenergic signaling in the LA.
We next asked whether noradrenergic-dependent reconsolidation of auditory fear conditioning occurs more than once. Five groups of rats implanted with intra-LA cannulae received auditory fear conditioning (Experiment 4, Figure 3). During the first memory reactivation, all groups expressed similar levels of freezing (SAL-SAL = 64%, ± 4.3; PRO-SAL = 60.3, ± 2; PRO-ISO = 60.4%, ± 5; ISO-SAL = 61.5, ± 5.6; ISO-PRO = 62.3, ± 5.5; p > .05). Forty-eight hours later, all the rats received a second reactivation trial. An ANOVA on freezing levels during the second reactivation trial for the drugs below revealed a significant effect of drug group (F(4,43)=11.25, p < .01). Post hoc mean comparisons with Tukey’s HSD showed that the PRO-SAL group expressed significantly less freezing than SAL-SAL group (p < .05) whereas the ISO-SAL group expressed significantly more freezing than SAL-SAL group (p < .05). There was no statistically significant difference between the ISO-PRO and SAL-SAL groups (SAL-SAL = 60.3%, ± 5.7; ISO-PRO = 72.6%, ± 4.6; p = 0.27); nor was there a statistically significant difference between the PRO-ISO and SAL-SAL groups, although there was a trend towards lower freezing in the PRO-ISO group (SAL-SAL = 60.3%, ± 5.7; PRO-ISO = 37.4%, ± 9; p = .11). Forty-eight hours after the second reactivation, all rats received an extinction test. Statistical analysis revealed a significant Reactivation x Drug interaction effect (F(1,33) = 10.76, p < .01). Post hoc mean comparisons with Tukey’s HSD revealed that the PRO-SAL group expressed significantly less freezing than SAL-SAL group (p < .05), and that the ISO-SAL group demonstrated significantly more freezing than the SAL-SAL group (p < .05), whereas the ISO-SAL group showed significantly more freezing than the SAL-SAL group (p < .05) and the ISO-PRO group (p < .01). Noradrenergic-dependent reconsolidation of conditioned fear occurs more than once and enhancement of noradrenergic transmission impairs extinction of fear memory.
Figure 3. Noradrenergic-dependent reconsolidation of conditioned fear occurs more than once.
The behavioral procedure (top) and freezing to the CS (bottom) during extinction test forty-eight hours following the second reactivation trial. (SAL-SAL: saline-saline; PRO-SAL: propranolol-saline; PRO-ISO: propranolol-isoproterenol; ISO-SAL: isoproterenol-saline; ISO-PRO: isoproterenol-propranolol; * indicates Tukey’s HSD p < .05, ** indicates Tukey’s HSD p < .01)
DISCUSSION
In the present study we examined the role of noradrenergic signaling in the amygdala in the reconsolidation of auditory fear conditioning. The major finding of this study was that beta-adrenergic receptor agonist isoproterenol infused into the lateral amygdala following retrieval of a conditioned fear impaired its extinction forty-eight hours later (Figure 2). The failure of ISO in affecting freezing responding without explicit memory retrieval (No Reactivation group), as well as the lack of effect of ISO on memory three hours following reactivation (Figure 2C) is consistent with the memory reconsolidation model which posits that memory modification is contingent upon memory retrieval and that the modification of a memory is typically not observable until reconsolidation processes are completed several hours postretrieval (Nader et al., 2000; Dębiec et al., 2010; Tronson and Taylor, 2007). The augmenting effects of postreactivation ISO on fear conditioning infused into the LA but not outside of the amygdala demonstrate that noradrenergic enhancement of reconsolidation is amygdala-dependent (Figure 2B). These results are consistent with our previous findings (also replicated in this study in Experiment 4) showing that noradrenergic blockade in the amygdala impairs reconsolidation of cue conditioned fear (Dębiec and LeDoux, 2004). Thus, fear memories in the amygdala may be bidirectionally modulated by interference or enhancement with noradrenergic signaling during reconsolidation processes.
Most of reconsolidation studies so far focused on a single memory retrieval (for review: Tronson and Taylor, 2007). Few studies reported that reconsolidation may occur more than once (e.g. Rodriguez-Ortiz et al., 2005; Tronson et al., 2006). We demonstrated here that noradrenergic-modulated reconsolidation of cue conditioned fear occurs more than once (Experiment 4). More specifically, we showed that postretrieval ISO-treated fear memory may be ameliorated with PRO upon a subsequent memory reactivation (Figure 3, group ISO-PRO). Interestingly, we did not observe any statistically significant enhancing effects of ISO on memory that had been attenuated by PRO following a previous memory retrieval (Figure 3, group PRO-ISO). One explanation is that treatment with PRO attenuates conditioned fear responding to such a degree that subsequent enhancement by exogenous ISO is not possible. An alternative explanation is that enhancement of previously pharmacologically attenuated memories may require more intense noradrenergic stimulation (higher doses of ISO and/or more retrieval trials). A definite answer to these questions requires further studies.
Blocking reconsolidation by interference with noradrenergic signaling has been proposed by us (Dębiec and LeDoux, 2004; 2006) and by others (Przybyslawski et al., 1999; Brunet et al., 2008) as a possible tool in treatment of anxiety disorders such as PTSD or specific phobias. Although human studies show that postretrieval PRO may disrupt reconsolidation of conditioned fear in humans (Kindt et al., 2009; Soeter and Kindt, 2010) and may ameliorate psychophysiologic responding in PTSD (Brunet et al., 2008), little is known about the role of noradrenergic signaling in reconsolidation, maintenance and possible exacerbation of traumatic memories. Clinical studies suggest that enhanced noradrenergic activity during trauma may augment the encoding of the memory (O’Donnell et al., 2004). It has been thus proposed that the trauma-induced enhancement of memory encoding contributes to the persistence of traumatic memories (overconsolidation hypothesis) (Pitman, 1989). However, clinical research shows that enhanced noradrenergic activity and elevated levels of norepinephrine in the cerebrospinal fluid correlate with severity of symptoms of PTSD (Geracioti et al., 2001; Strawn and Geracioti, 2008). Thus, increased noradrenergic activity may be implied in maintenance of PTSD symptoms. Our study using a rodent model of fear learning is the first to show that the underlying mechanism of the norepinephrine-related persistence of traumatic memories may be reconsolidation-dependent. If confirmed in humans, these findings may not only be useful in developing novel treatments for PTSD but may also help in better understanding and modification of existing therapies, such as an exposure therapy. For example, exposure therapy, especially in its initial stages may cause exacerbation of PTSD symptoms (Foa et al., 2002). Such exacerbation of PTSD symptoms may be likely explained in terms of augmenting reconsolidation of trauma-related memories in the context of associated arousal and an increase in noradrenergic signaling. Noradrenergic enhancement of reconsolidation may also serve as a model for delayed-onset PTSD whereas PTSD symptoms emerge and exacerbate over time (Andrews et al.; 2007; Smid et al., 2009). Memory reconsolidation is a time-dependent process. Existing data from animal studies suggest that memories are susceptible to modification up to few hours following recall. Reconsolidation model may be useful in defining the temporal interval of susceptibility to interference and thus help in a better design of existing therapeutic interventions. Recent studies from our group show that combining reconsolidation and extinction approaches may be more efficient in eliminating learned fear than using extinction alone (Monfils et al., 2009; Schiller et al., 2010). However, additional preclinical and clinical studies are necessary to better understand the translational value of the reconsolidation model in PTSD.
CONCLUSIONS
Noradrenergic augmentation in the amygdala following retrieval of a traumatic memory enhances memory reconsolidation and makes the memory less susceptible to fear extinction. Elevated noradrenergic activity is associated with persistence and severity of PTSD symptoms. It this thus possible that norepinephrine-modulated reconsolidation processes contribute to the maintenance and exacerbation of trauma-related memories in PTSD.
ACKNOWLEDGMENTS
We thank Claudia Farb and Nikki Pandya, Lyndon Luk and Ian Fabian for their technical assistance.
This work was supported by the NIH grants MH046516, MH038774 and NSF grant 0920153 to JEL.
REFERENCES
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and destabilization. Cell Mol Life Sci. 2006;63(9):999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319–26. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46(9):1309–20. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Bandura A. Principles of Behavior Modification. Holt; New York: 1969. [Google Scholar]
- Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, Figueira I. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169–80. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23(1):28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23(1):39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–6. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Bush DEA, Caparosa EM, Gekker A, LeDoux JE. Beta-adrenergic receptos in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:1–7. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. The neurobiology of emotionally influenced memory. Implications for understanding traumatic memory. Ann N Y Acad Sci. 1997;821:238–46. doi: 10.1111/j.1749-6632.1997.tb48283.x. [DOI] [PubMed] [Google Scholar]
- Cukor J, Olden M, Lee F, Difede J. Evidence-based treatments for PTSD, new directions, and special challenges. Ann N Y Acad Sci. 2010;1208:82–9. doi: 10.1111/j.1749-6632.2010.05793.x. [DOI] [PubMed] [Google Scholar]
- Dębiec J, Diaz-Mataix L, Bush DE, Doyère V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13(5):536–7. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dębiec J, Doyère V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103(9):3428–33. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dębiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–72. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Dębiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–4. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Dębiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36(3):527–38. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Dollard JC, Miller NE. Personality and Psychopathology. McGraw-Hill; New York: 1950. [Google Scholar]
- Doyère V, Dębiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10(4):414–6. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16(2):174–8. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The conditioning model of neuroses. Behavioral and Brain Sci. 1979;(2):155–99. [Google Scholar]
- Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–5. [PubMed] [Google Scholar]
- Foa EB, Zoellner LA, Feeny NC, Hembree EA, Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? J Consult Clin Psychol. 2002;70(4):1022–8. doi: 10.1037//0022-006x.70.4.1022. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE., Jr. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227–30. doi: 10.1176/appi.ajp.158.8.1227. others. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 2004;175(3):342–52. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1(4):278–97. [PubMed] [Google Scholar]
- Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. (7):CD007316. doi: 10.1002/14651858.CD007316.pub2. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu Rev Clin Psychol. 2006;2:161–97. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–8. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61(3):137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 17(10):489–93. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17(20):R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47(6):783–6. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287(5451):248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–5. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Krystal JH, Southwick SM. Toward early pharmacological posttraumatic stress intervention. Biol Psychiatry. 2003;53(9):834–43. doi: 10.1016/s0006-3223(03)00116-1. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- O’Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50(4):273–83. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26(3):221–3. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal. 2009;2(86):re5. doi: 10.1126/scisignal.286re5. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19(15):6623–8. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Charney DS. Animal models of relevance to PTSD. Ann N Y Acad Sci. 1997;821:332–51. doi: 10.1111/j.1749-6632.1997.tb48290.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, De la Cruz V, Gutierrez R, Bermudez-Rattoni F. Protein synthesis underlies post-retrieval memory consolidation to a restricted degree only when updated information is obtained. Learn Mem. 2005;12(5):533–7. doi: 10.1101/lm.94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–50. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid GE, Mooren TT, van der Mast RC, Gersons BP, Kleber RJ. Delayed posttraumatic stress disorder: systematic review, meta-analysis, and meta-regression analysis of prospective studies. J Clin Psychiatry. 2009;70(11):1572–82. doi: 10.4088/JCP.08r04484. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94(1):30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA, 3rd, Gold PE, Bremner JD, Charney DC. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiatry. 2002;159(8):1420–2. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(8):749–58. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260–71. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–75. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9(2):167–9. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]