Abstract
Parametric and semiparametric competing risks methods were used to estimate proportions, timing, and predictors of acquired immune deficiency syndrome (AIDS)-related and non-AIDS-related mortality among individuals both positive and negative for the human immunodeficiency syndrome (HIV) in the Multicenter AIDS Cohort Study (MACS) and Women's Interagency HIV Study (WIHS) from 1984 to 2008 and 1996 to 2008, respectively. Among HIV-positive MACS participants, the proportion of deaths unrelated to AIDS increased from 6% before the introduction of highly active antiretroviral therapy (HAART) (before 1996) to 53% in the HAART era (P < 0.01); the median age of persons who died from non-AIDS-related causes after age 35 years increased from 49.0 to 66.0 years (P < 0.01). In both cohorts during the HAART era, median ages at time of non-AIDS-related death were younger for HIV-positive individuals than for comparable HIV-negative individuals (8.7 years younger in MACS (P < 0.01) and 7.6 years younger in WIHS (P < 0.01)). In a multivariate proportional cause-specific hazards model, unemployment (for non-AIDS death, hazard ratio (HR) = 1.8; for AIDS death, HR = 2.3), depression (for non-AIDS death, HR = 1.4; for AIDS death, HR = 1.4), and hepatitis B or C infection (for non-AIDS death, HR = 1.8, for AIDS death; HR = 1.4) were significantly (P < 0.05) associated with higher hazards of both non-AIDS and AIDS mortality among HIV-positive individuals in the HAART era, independent of study cohort. The results illuminate the changing face of mortality among the growing population infected with HIV.
Keywords: acquired immunodeficiency syndrome; antiretroviral therapy, highly active; cohort studies; competing risks; HIV; mixture model; mortality; proportional hazards models
Editor's note: An invited commentary on this article appears on page 126, and the authors' response appears on page 129.
The arrival of highly active antiretroviral therapy (HAART) in the mid-1990s dramatically improved the prognosis for human immunodeficiency virus (HIV)-positive individuals (1–3). As the risk of acquired immune deficiency syndrome (AIDS) has declined and survival times have increased, death from non-AIDS causes has become more common in persons with the disease (4–9). However, evidence has suggested that HAART lowers the risks of several non-AIDS morbidities and causes of mortality (10–12), despite links between some forms of antiretroviral therapy and hepatotoxicity, renal disease, and myocardial infarction (13, 14).
Most studies comparing mortality rates between HIV-positive and HIV-negative individuals have used external reference populations, and only a few studies, such as those from the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) collaboration (5, 15), have used competing risks methods to disaggregate mortality. In studies of HIV-related mortality in which such methods have been used, 2 approaches have predominated: comparison of subdistribution hazards as per Fine and Gray (16) and extension of Cox methods for proportional cause-specific hazards (17). Parametric survival estimates have been rare, and few of these analyses have formally compared the impact of a covariate across outcomes. In general, the shift toward non-AIDS-related mortality in the HAART era has not been characterized in detail, and a complete understanding of the determinants of the distribution and timing of cause-specific mortality among HIV-positive individuals is still lacking.
MATERIALS AND METHODS
Study cohorts
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective cohort study of HIV infection in men who have sex with men. The study recruited men from 4 sites (Baltimore, Maryland; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania) during 3 phases (1984–1985, 1987–1991, and 2001–2003) for a total of 6,972 enrollees since 1984 (18). The Women's Interagency HIV Study (WIHS) is an ongoing prospective cohort study of HIV infection in women. The study recruited women from 6 sites (Bronx, New York; Brooklyn, New York; Chicago, Illinois; Los Angeles, California; San Francisco, California; and Washington, DC) during 2 phases (1994–1995 and 2001–2002) for a total of 3,766 enrollees since 1993 (19). Both cohorts include HIV-negative and HIV-positive person-time. Studies were approved by the committees of human research of participating institutions.
Criteria for enrolling HIV-negative and HIV-positive individuals into MACS and WIHS were identical except for HIV status. Men were recruited to MACS via active outreach to the gay population through the use of media and interactions with community leaders, social gatherings, and clinic populations. Participants had to be men who had sex with men, were 18 years of age or older, and were free of an AIDS-defining illness before enrollment. In the 2001–2003 recruitment cycle, enrollment targeted 3 groups of men: HIV-negative; HIV-infected and HAART-naive; and HIV-infected and HAART-exposed with known date of initiation.
WIHS recruitment occurred in venues including HIV testing and primary care clinics; research, drug rehabilitation, and hospital-based programs; community outreach sites, women's support groups; and referrals from enrolled participants. During the 2001–2002 enrollment cycle, women with clinical AIDS were excluded; HAART-naive women and women with a known therapy start date were preferentially enrolled. Details of recruitment and enrollment for MACS and WIHS have been described previously (18–21).
Definition of outcomes
AIDS-related death and non-AIDS-related death were the primary outcomes. Cause of death was ascertained from death certificates and the National Death Index. Mortality in MACS was classified as AIDS-related if AIDS or an AIDS-defining illness was listed as a contributing cause of death on the death certificate or in the National Death Index. Deaths in the WIHS were classified using a review by 2 physicians. We defined deaths classified as “pneumonia/infection” among HIV-positive WIHS participants as AIDS-related.
Definition of exposures
A major strength of both cohorts is the inclusion of HIV-negative individuals with similar risk behaviors drawn from the same population as the HIV-positive individuals. In MACS, the bulk of the study participants were recruited before the identification of HIV as the causal agent of AIDS. Because some HIV-negative individuals acquired HIV during follow-up, we treated HIV infection as a time-varying exposure. We used calendar period to compare therapy eras, with January 1, 1996, defined as the division between the pre-HAART and HAART eras. Because WIHS began recruitment in the mid-1990s, we restricted comparison of therapy eras to MACS. For comparisons within the HAART era, we considered both the MACS and WIHS cohorts. All such comparisons were made within each cohort.
We defined each individual's baseline as the time of entry into the analysis, specific to HIV infection category and calendar period. We defined baseline values for exposure variables relevant to both HIV-positive and HIV-negative individuals that have known associations with death: educational level, employment, smoking, alcohol use, injection drug use (IDU), any nonprescribed drug use, depression, and chronic infection with the hepatitis B virus (HBV) or hepatitis C virus (HCV) (8, 22–28). Although each exposure may vary over time, we were interested specifically in using groups defined by time-fixed covariates at baseline to measure associations with mortality rather than examining acute effects of contemporaneous exposure.
All sociodemographic and behavioral variables were measured by self-report and dichotomized. We defined drug use and smoking as ever/never at baseline, whereas employment, drinking, and depression were defined by the measured value at baseline. Chronic hepatitis infection was ascertained at enrollment via tests for hepatitis B surface antigen and hepatitis C viral RNA. Depression was defined as having a Center for Epidemiologic Studies Depression score higher than 16 (29). We dichotomized race as white (non-Hispanic) and nonwhite. We used cohort membership in the analysis as a proxy to address other differences between the 2 cohorts.
Time scale
The time scale for survival analysis was age. Making comparisons between the HIV-negative population and the HIV-positive population precluded the use of time scales specific to the HIV-positive group, such as seroconversion and immune status. Using age as the time scale also permitted the best possible control of a powerful predictor of mortality and allowed estimation of life expectancy among adults. To prevent sparse events from having undue influence on model results, we only considered person-time and events between the ages of 35 and 70 years, anchoring the time origin at age 35 years. We used late entry methods, left-truncating data at time of entry and appropriately aligning person-time on the age scale, to accommodate individuals who entered the study after 35 years of age (30, 31). We administratively censored events and person-time after December 31, 2008.
Mixture models
We used parametric methods to estimate cause-specific survival times using mixture models. In the study population, each HIV-positive individual was observed to either: 1) die of non-AIDS causes, 2) die of AIDS, 3) die of unknown causes, or 4) exit the analytical period alive. If π is the proportion of individuals dying of non-AIDS-related causes by the age of 100 years (defined as the upper limit of age), then (1 − π) is the proportion of individuals dying of AIDS. Let S1(t) be the survival function of the times specific to the π% of the study population dying of non-AIDS, S2(t) be the survival function for the (1 − π)% dying of AIDS, w be entry time, and t be exit time. If f1(t) and f2(t) denote the density functions associated with S1(t) and S2(t), respectively, the contributions to the likelihood for observations of each type are as follows (32): 1) πf1(t)/S(w); 2) (1 − π)f2(t)/S(w); 3) [πf1(t) + (1 − π)f2(t)]/S(w); and 4) [πS1(t) + (1 − π)S2(t)]/S(w), where S(w) = πS1(w) + (1 − π)S2(w).
A large proportion of individuals in the study population exited the analytical period alive (type 4). In such a situation, a parametric model must use a relatively small number of event times to estimate the survival function. The resulting estimate may not necessarily reflect limits of the aging process. Instead of expression 4 above, we used the following likelihood contribution, with age 35 years as the time origin and the upper limit of age defined as 100: π[S1(t) − S1(100 − 35)] + (1 − π)[S2(t) − S2(100 − 35)]/S(w).
Here, we forced estimated event times to fall between our specified lower and upper limits rather than allowing the likelihood function to estimate unbounded and unrealistically late ages at death. In doing so, we converted right-censored observations into interval-censored observations and thereby ensured that estimated survival functions reach zero by the age of 100, imparting a degree of realism into the analysis.
We fitted generalized gamma distributions to survival times for the full study population, and estimates for the shape parameters indicated that Weibull distributions were appropriate; this was confirmed by comparisons with Nelson-Aalen nonparametric cumulative incidence estimates. To balance parsimony with flexibility, the mixture models used Weibull distributions of survival times with location β and scale σ such that the pth percentile is exp(β + σ(log(−log(1−p)))) for each cause of death. We allowed location, scale, and mixture (π) parameters to vary by subgroup.
Proportional cause-specific hazards models
Infrequency of mortality during the HAART era precluded fitting multivariate mixture models, so we used multivariate cause-specific hazards models to identify and compare predictors of each type of mortality in the HAART era. We used stratified Cox regression to estimate cause-specific hazards ratios for each exposure, which allowed formal comparisons of whether the effects of covariates differ across causes of death (17).
We constructed multivariate proportional cause-specific hazards models starting from models that included only cohort membership (MACS/WIHS). We added one variable at a time, chosen by the greatest divergence between the likelihoods of the new model and the more parsimonious model, until no likelihood ratio test statistic was statistically significant (α = 0.05). Both cohorts included HIV-positive deaths from unknown causes because of missing or equivocal information from death certificates or the National Death Index; these cannot be apportioned to AIDS and non-AIDS deaths via the likelihood function, as in the mixture model. As a consequence, we multiply imputed unknown deaths to these categories. We used the cohort-stratified mixture model to estimate probability density functions for each cause of death. For each unique event time, we used these density functions to define the probability of non-AIDS death, as follows: πf1(t)/[πf1(t) + (1 − π]f2(t)]. We apportioned events to each category via a random draw from this event time-specific probability. We averaged coefficient values across 10 imputations and appropriately adjusted standard errors (33). We conducted sensitivity analyses in which all unknown deaths were apportioned to either AIDS-related or non-AIDS-related causes.
We focused on cause-specific hazards rather than the subhazards of the cumulative incidences because it was of interest to formally compare the hazard ratios associated with each exposure across event types. This is unattainable in proportional subhazards models because a single parameter governs the relation between an exposure and each outcome (34). We used SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina) for all analyses.
RESULTS
Characteristics of the study population
A total of 8,771 individuals (5,843 in MACS and 2,928 in WIHS) contributed person-time to the analysis; individuals could contribute person-time to more than 1 calendar period and HIV-infection category. Ages at initial recruitment were similar between the cohorts, but at entry into the HAART era, MACS men—many of whom had been recruited during the 1980s—were older than WIHS women (see Web Table 1, available at http://aje.oxfordjournals.org/).
Table 1 shows characteristics of the study population, stratified by HAART era, cohort, and HIV status. Nearly all of the HIV-positive person-time in the pre-HAART era was accrued before individual HAART exposure, whereas 70% of the HIV-positive person-time in the HAART era was accrued after HAART initiation.
Table 1.
Characteristics of Multicenter AIDS Cohort Study and Women's Interagency HIV Study Participants Stratified by Human Immunodeficiency Virus Status and Highly Active Antiretroviral Therapy Eraa, 1984–2008
| Characteristic | MACS (1984–1995) |
MACS (1996–2008) |
WIHS (1996–2008) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-Negative |
HIV-Positive |
HIV-Negative |
HIV-Positive |
HIV-Negative |
HIV-Positive |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Individuals | 2,581 | 2,119 | 2,034 | 1,741 | 634 | 2,228 | ||||||
| Person-years | 19,117 | 11,826 | 17,262 | 13,161 | 4,677 | 16,359 | ||||||
| Person-years before HAART initiation | N/A | N/A | 11,826 | 100 | N/A | N/A | 3,820 | 29 | N/A | N/A | 4,904 | 30 |
| Person-years after HAART initiation | N/A | N/A | 0.45 | 0 | N/A | N/A | 9,340 | 71 | N/A | N/A | 11,455 | 70 |
| Alive at exit of analysis | 2,530 | 98 | 994 | 47 | 1,954 | 96 | 1,367 | 79 | 581 | 92 | 1,548 | 69 |
| AIDS deaths | N/A | N/A | 1,039 | 49 | N/A | N/A | 202 | 12 | N/A | N/A | 372 | 17 |
| Non-AIDS deaths | 51 | 2 | 58 | 3 | 80 | 4 | 84 | 5 | 53 | 8 | 241 | 11 |
| Deaths of unknown cause | N/A | N/A | 28 | 1 | N/A | N/A | 88 | 5 | N/A | N/A | 67 | 3 |
| All-cause death rateb | 267 | 9,513 | 463 | 2,842 | 1,133 | 4,157 | ||||||
| White, non-Hispanic | 2,313 | 90 | 1,734 | 82 | 1,544 | 76 | 1,066 | 61 | 86 | 14 | 346 | 16 |
| High school educationc | 2,517 | 99 | 2,048 | 98 | 1,927 | 96 | 1,596 | 94 | 420 | 67 | 1,402 | 63 |
| College educationc | 1,695 | 66 | 1,174 | 56 | 1,190 | 59 | 785 | 46 | 56 | 9 | 162 | 7 |
| Employedc | 2,351 | 91 | 1,816 | 86 | 1,678 | 83 | 1,144 | 66 | 252 | 40 | 598 | 27 |
| Smoking historyc | 1,540 | 60 | 1,372 | 65 | 1,388 | 69 | 1,262 | 73 | 492 | 78 | 1,632 | 74 |
| >13 alcoholic drinks/weekc | 360 | 15 | 302 | 15 | 195 | 10 | 145 | 8 | 61 | 10 | 142 | 6 |
| Injection drug use historyc | 97 | 4 | 270 | 13 | 158 | 8 | 290 | 17 | 189 | 30 | 828 | 37 |
| Any drug use historyc | 2,192 | 85 | 1,988 | 94 | 1,714 | 84 | 1,505 | 86 | 535 | 84 | 1,783 | 80 |
| Depressive symptomsc | 504 | 20 | 477 | 23 | 488 | 24 | 557 | 32 | 259 | 41 | 1,092 | 49 |
| HBV at study entry | 107 | 4 | 152 | 7 | 61 | 3 | 118 | 7 | 3 | 0 | 60 | 3 |
| HCV at study entry | 2 | 0 | 16 | 1 | 39 | 2 | 83 | 5 | 111 | 18 | 657 | 29 |
| HBV or HCV at study entry | 109 | 4 | 168 | 8 | 100 | 5 | 195 | 11 | 114 | 18 | 701 | 31 |
Abbreviations: AIDS, acquired immune deficiency syndrome; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; N/A, not applicable; WIHS, Women's Interagency HIV Study.
a Pre-HAART era: 1984–1995; HAART era: 1996–2008.
b All-cause death rate per 100,000 person-years.
c Values at baseline, defined as earliest study visit after age 35 years within the calendar period with specified HIV status.
The majority of MACS participants were white non-Hispanic men, whereas the majority of WIHS participants were black non-Hispanic women. Although more MACS men reported heavy consumption of alcohol, WIHS women had higher proportions of IDU, smoking history, unemployment, low educational attainment, depression, and hepatitis infection.
Among individuals who were HIV-positive during the study period, 1,613 died of AIDS, 383 died of non-AIDS-related causes, and 183 died of unknown causes. There were 184 deaths among HIV-negative individuals. Cardiovascular disease (21%) and injury or poisoning (21%) were the leading causes of non-AIDS-related death, followed by non-AIDS cancer (16%) and liver disease (11%); see Web Table 2 for details.
Comparison of therapy eras
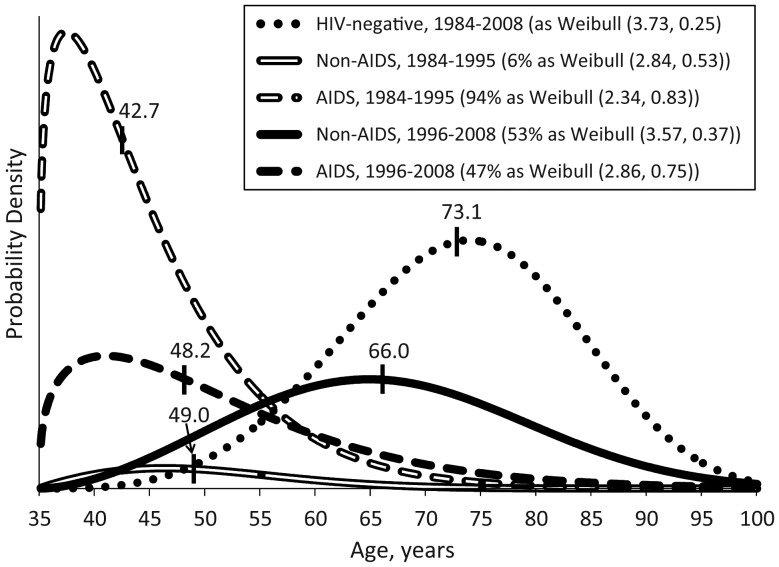
Figure 1 displays estimated probability density functions for cause-specific mortality in each therapy era from a mixture model. Table 2 reports estimated parameters from the models.
Figure 1.
Estimated probability density functions for cause-specific mortality after 35 years of age by highly active antiretroviral therapy era in the Multicenter AIDS Cohort Study, 1984–2008. Vertical hash marks and text labels indicate estimated median ages at death. Percentages in legend indicate the final estimated proportion of all-cause mortality along with the estimated location and scale parameters from Weibull models.
Table 2.
Estimated Proportions of Mortality, Location Parameters, Scale Parameters, and Median Ages at Death From Weibull Mixture Models Comparing the Pre- and Post-Highly Active Antiretroviral Therapy Erasa Among Multicenter AIDS Cohort Study Participantsb, 1984–2008
| Mortality Category | Proportion of Mortality, % | 95% CI | Location Parameter | 95% CI | Scale Parameter | 95% CI | Median Age, yearsb |
|---|---|---|---|---|---|---|---|
| HIV-negative, 1984–2008 | N/A | N/A | 3.73 | 3.71, 3.75 | 0.25 | 0.24, 0.26 | 73.1 |
| Pre-HAART era (reference) | |||||||
| Non-AIDS mortality | 6 | 5, 8 | 2.84 | 2.63, 3.04 | 0.53 | 0.41, 0.66 | 49.0 |
| AIDS mortality | 94 | 92, 95 | 2.34 | 2.29, 2.40 | 0.83 | 0.79, 0.87 | 42.7 |
| HAART era | |||||||
| Non-AIDS mortality | 53* | 48, 59 | 3.57* | 3.52, 3.62 | 0.37* | 0.34, 0.40 | 66.0* |
| AIDS mortality | 47* | 41, 52 | 2.86* | 2.70, 3.01 | 0.75 | 0.68, 0.83 | 48.2* |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; N/A, not applicable.
* P < 0.05 for difference relative to pre-HAART era.
a Pre-HAART era: 1984–1995; HAART era: 1996–2008.
b Time origin is age 35 years. For HIV-negative individuals, survival times were modeled as Weibull (β, σ). For HIV-positive individuals, survival times were modeled as a mixture with the proportion who died of non-AIDS causes (π) as Weibull (location (β)1, scale (σ)1) and the complement (1 − π) who died of AIDS as Weibull (β2, σ2).
In both eras, AIDS-related deaths occurred at lower ages than did non-AIDS-related deaths among HIV-positive individuals in MACS. There were dramatic increases in the estimated proportion (from 6% to 53%; P < 0.01) and median age (from 49.0 to 66.0 years; P < 0.01) of HIV-positive non-AIDS-related death in the HAART era. The median age at AIDS death increased by 5.5 years, from 42.7 to 48.2 years (P < 0.01). In the pre-HAART era, HIV-positive individuals who died from non-AIDS causes did so far earlier than did their HIV-negative counterparts, with a more than 2-decade gap between median survival times (49.0 vs. 70.9 years; P < 0.01). After 1996, the gap shrank to only 8.7 years, although the median age at non-AIDS-related death for HIV-positive individuals was still earlier than for HIV-negative individuals (66.0 vs. 74.7 years; P < 0.01). In a multivariate proportional cause-specific hazards model (Web Table 3), the HAART era was associated with a 33% lower hazard of non-AIDS death and a 90% lower hazard of AIDS death relative to the pre-HAART era.
Results from the combined MACS and WIHS cohorts in the HAART era
Results from mixture models
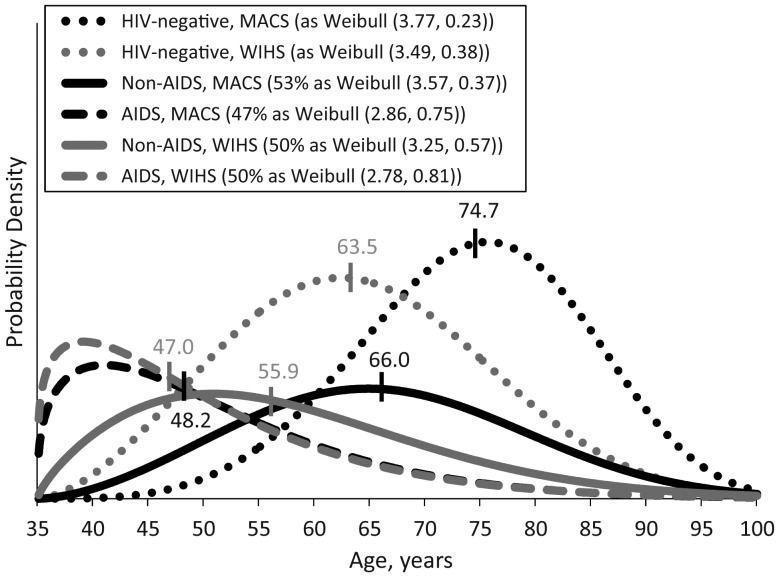
Figure 2 displays estimated probability density functions from a mixture model comparing MACS participants with WIHS participants in the HAART era. Table 3 displays estimated parameters from this model. The estimated proportion of AIDS-related mortality was similar for WIHS women and MACS men in the HAART era (50% vs. 47%; P = 0.38), and median AIDS-related life expectancies were similar between WIHS and MACS participants (47.0 vs. 48.2 years; P = 0.37). However, life expectancies for HIV-positive WIHS participants who died of non-AIDS-related causes were significantly lower than those for HIV-positive MACS participants (median 55.9 vs. 66.0 years; P < 0.01).
Figure 2.
Estimated probability density functions for cause-specific mortality after 35 years of age in the highly active antiretroviral therapy era (1996–2008), Multicenter AIDS Cohort Study and Women's Interagency HIV Study. Vertical hash marks and text labels indicate estimated median ages at death. Percentages in legend indicate the final estimated proportion of all-cause mortality along with the estimated location and scale parameters from Weibull models.
Table 3.
Estimated Proportions of Mortality, Location Parameters, Scale Parameters, and Median Ages at Death From Weibull Mixture Models Comparing Women From the Women's Interagency HIV Study With Men from the Multicenter AIDS Cohort Study During the Highly Active Antiretroviral Therapy Era, 1996–2008
| Mortality Category and Study | Proportion of Mortality, % | 95% CI | Location Parameter | 95% CI | Scale Parameter | 95% CI | Median Age, yearsa |
|---|---|---|---|---|---|---|---|
| HIV-negative | |||||||
| MACS (reference) | N/A | N/A | 3.77 | 3.74, 3.79 | 0.23 | 0.22, 0.25 | 74.7 |
| WIHS | N/A | N/A | 3.49* | 3.41, 3.56 | 0.38* | 0.33, 0.42 | 63.5* |
| Non-AIDS mortality | |||||||
| MACS (reference) | 53 | 48, 59 | 3.57 | 3.52, 3.62 | 0.37 | 0.34, 0.40 | 66.0 |
| WIHS | 50 | 45, 55 | 3.25* | 3.18, 3.32 | 0.57* | 0.53, 0.61 | 55.9* |
| AIDS mortality | |||||||
| MACS (reference) | 47 | 41, 52 | 2.86 | 2.70, 3.01 | 0.75 | 0.68, 0.83 | 48.2 |
| WIHS | 50 | 45, 55 | 2.78 | 2.66, 2.90 | 0.81 | 0.75, 0.87 | 47.0 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; N/A, not applicable; WIHS, Women's Interagency HIV Study.
* P < 0.05 for difference relative to MACS participants.
a Time origin is age 35. For HIV-negative individuals, survival times were modeled as Weibull (β, σ). For HIV-positive individuals, survival times were modeled as a mixture with the proportion who died of non-AIDS causes (π) as Weibull (location (β)1, scale (σ)1) and the complement (1 − π) who died of AIDS as Weibull (β2, σ2).
Among HIV-negative individuals, the median age at death in WIHS was 11.2 years earlier than in MACS (63.5 vs. 74.7 years; P < 0.01). Within each cohort, the timing of non-AIDS-related death for HIV-positive individuals was substantially earlier than for HIV-negative individuals. Among WIHS participants, HIV-positive individuals died of non-AIDS causes at a median age 7.6 years earlier (P < 0.01) than their HIV-negative counterparts. For MACS participants, the difference in medians was 8.7 years (P < 0.01).
Results from proportional cause-specific hazards models
Results from bivariate proportional cause-specific hazards models estimating the effects of baseline covariates in the HAART era are shown in Table 4. Each model was adjusted for WIHS/MACS membership to address the substantial differences between the 2 cohorts.
Table 4.
Estimated Cause-Specific Hazard Ratios Adjusted for Cohort Membership Among Multicenter AIDS Cohort Study and Women's Interagency HIV Study Participants in the Highly Active Antiretroviral Therapy Era, 1996–2008
| HIV-Negative Death |
HIV-Positive Non-AIDS Death |
HIV-Positive AIDS Death |
||||
|---|---|---|---|---|---|---|
 |
95% CI |  |
95% CI |  |
95% CI | |
| WIHS membership alone | 3.90*,a | 2.70, 5.64 | 2.33*,b | 1.83, 2.96 | 1.24* | 1.06, 1.46 |
| Non-white race | 1.52 | 0.93, 2.47 | 1.16 | 0.88, 1.51 | 1.18 | 0.97, 1.42 |
| Less than a high school education | 1.93* | 1.18, 3.14 | 1.58*,b | 1.24, 2.01 | 1.10 | 0.90, 1.33 |
| Less than a college education | 2.16* | 1.41, 3.30 | 1.75* | 1.23, 2.50 | 1.18 | 0.94, 1.47 |
| Unemployment | 2.81* | 1.86, 4.24 | 2.38* | 1.80, 3.14 | 2.74* | 2.25, 3.33 |
| Smoking history | 1.88* | 1.17, 3.02 | 2.14*,b | 1.59, 2.88 | 1.46* | 1.20, 1.77 |
| >13 alcoholic drinks/week | 2.11* | 1.37, 3.24 | 1.43* | 1.00, 2.05 | 1.29 | 0.98, 1.70 |
| Injection drug use history | 5.17*,a | 3.48, 7.67 | 2.38*,b | 1.90, 2.98 | 1.53* | 1.30, 1.81 |
| Any drug use history | 2.41* | 1.17, 4.93 | 2.72*,b | 1.78, 4.15 | 1.39* | 1.09, 1.76 |
| Depression | 1.69* | 1.19, 2.42 | 1.67* | 1.34, 2.07 | 1.68* | 1.43, 1.97 |
| Hepatitis B or C infection | 4.58*,a | 3.03, 6.91 | 2.60*,b | 2.08, 3.25 | 1.72* | 1.44, 2.04 |
| Years of age at enrollment | 1.03 | 1.00, 1.07 | 1.03* | 1.01, 1.06 | 1.03* | 1.02, 1.05 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus;  , estimated hazard ratio; WIHS, Women's Interagency HIV Study.
, estimated hazard ratio; WIHS, Women's Interagency HIV Study.
*P < 0.05.
a Indicates significant difference (P < 0.05) between HIV-negative hazard ratio and HIV-positive non-AIDS death hazard ratio.
b Indicates significant difference (P < 0.05) between HIV-positive non-AIDS death hazard ratio and AIDS death hazard ratio.
Each of the estimated hazard ratios ( ) in Table 4 was greater than 1, and 29 of 36 were statistically significant. WIHS membership (P < 0.01), high school education (P = 0.03), smoking history (P = 0.04), IDU (P < 0.01), any drug use (P = 0.01), and HBV or HCV infection (P = 0.01) had statistically stronger impacts on the hazard of non-AIDS death than on that of AIDS death. Hazard ratios for WIHS membership (P = 0.02), IDU (P < 0.01), and HBV/HCV infection (P = 0.02) were statistically stronger for death among HIV-negative individuals than for HIV-positive non-AIDS deaths.
) in Table 4 was greater than 1, and 29 of 36 were statistically significant. WIHS membership (P < 0.01), high school education (P = 0.03), smoking history (P = 0.04), IDU (P < 0.01), any drug use (P = 0.01), and HBV or HCV infection (P = 0.01) had statistically stronger impacts on the hazard of non-AIDS death than on that of AIDS death. Hazard ratios for WIHS membership (P = 0.02), IDU (P < 0.01), and HBV/HCV infection (P = 0.02) were statistically stronger for death among HIV-negative individuals than for HIV-positive non-AIDS deaths.
Table 5 shows results from the multivariate model. Here, each  was attenuated by comparison with its counterpart in Table 4, but qualitative results were similar. The strong attenuation of hazard ratios associated with WIHS membership suggests that the other variables capture much of the differences between cohorts.
was attenuated by comparison with its counterpart in Table 4, but qualitative results were similar. The strong attenuation of hazard ratios associated with WIHS membership suggests that the other variables capture much of the differences between cohorts.
Table 5.
Estimated Cause-Specific Hazard Ratios From a Multivariate Model Among Multicenter AIDS Cohort Study and Women's Interagency HIV Study Participants in the Highly Active Antiretroviral Therapy Era, 1996–2008
| HIV-Negative Death |
HIV-Positive Non-AIDS Death |
HIV-Positive AIDS Death |
||||
|---|---|---|---|---|---|---|
 |
95% CI |  |
95% CI |  |
95% CI | |
| WIHS membership | 0.95 | 0.58, 1.57 | 1.16a | 0.88, 1.55 | 0.73* | 0.60, 0.88 |
| Unemployment | 1.75* | 1.12, 2.75 | 1.81* | 1.35, 2.43 | 2.35* | 1.92, 2.88 |
| Smoking history | 1.37 | 0.84, 2.23 | 1.53* | 1.11, 2.11 | 1.18 | 0.96, 1.45 |
| Injection drug use history | 3.27*,b | 2.08, 5.14 | 1.39* | 1.04, 1.84 | 1.09 | 0.89, 1.34 |
| Depression | 1.34 | 0.93, 1.94 | 1.40* | 1.12, 1.75 | 1.38* | 1.17, 1.62 |
| Hepatitis B or C infection | 2.49* | 1.56, 3.97 | 1.82* | 1.38, 2.38 | 1.37* | 1.12, 1.69 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus;  , estimated hazard ratio; WIHS, Women's Interagency HIV Study.
, estimated hazard ratio; WIHS, Women's Interagency HIV Study.
*P < 0.05.
a Indicates significant difference (P < 0.05) between HIV-positive non-AIDS hazard ratio and AIDS hazard ratio.
b Indicates significant difference (P < 0.05) between HIV-negative hazard ratio and HIV-positive non-AIDS hazard ratio.
Hazard ratios associated with unemployment were significantly greater than 1 for each category of death (HIV-negative death 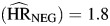 , P = 0.01; HIV-positive non-AIDS death
, P = 0.01; HIV-positive non-AIDS death 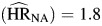 , P < 0.01; AIDS death
, P < 0.01; AIDS death 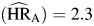 , P < 0.01); there were no statistically significant differences among these hazard ratios. Hazard ratios for depression (
, P < 0.01); there were no statistically significant differences among these hazard ratios. Hazard ratios for depression (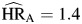 , P = 0.12;
, P = 0.12; 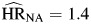 , P < 0.01; and
, P < 0.01; and 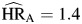 , P < 0.01) followed a similar pattern to those observed for unemployment, as all were above 1 and not statistically different from one another. Hazard ratios for smoking (
, P < 0.01) followed a similar pattern to those observed for unemployment, as all were above 1 and not statistically different from one another. Hazard ratios for smoking (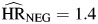 , P = 0.21;
, P = 0.21; 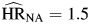 , P = 0.01; and
, P = 0.01; and 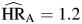 , P = 0.11) also were each above one, but only that for
, P = 0.11) also were each above one, but only that for 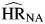 was statistically significant.
was statistically significant.
Inclusion of hepatitis infection into the model resulted in an attenuation of the hazard ratios for IDU (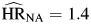 , P < 0.01;
, P < 0.01; 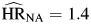 , P = 0.03; and
, P = 0.03; and 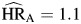 , P = 0.41) because of the strong correlation between the 2 exposures. Nonetheless, IDU was a strong independent predictor of mortality for non-AIDS death regardless of HIV infection status. There was a statistically significant difference (P < 0.01) between hazards of non-AIDS death associated with IDU comparing HIV-negative individuals with HIV-positive individuals.
, P = 0.41) because of the strong correlation between the 2 exposures. Nonetheless, IDU was a strong independent predictor of mortality for non-AIDS death regardless of HIV infection status. There was a statistically significant difference (P < 0.01) between hazards of non-AIDS death associated with IDU comparing HIV-negative individuals with HIV-positive individuals.
Although hazard ratios for hepatitis infection were also attenuated relative to the model adjusted only for cohort membership, persons with HBV or HCV infection still had significantly elevated hazards for each type of mortality (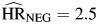 , P < 0.01;
, P < 0.01; 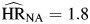 , P < 0.01; and
, P < 0.01; and 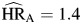 , P < 0.01). Hepatitis hazard ratios for non-AIDS death were independent of HIV infection (P = 0.25). The hazard ratio for hepatitis infection among HIV-positive individuals was higher for non-AIDS death than for AIDS death, but this difference was not statistically significant (P = 0.12).
, P < 0.01). Hepatitis hazard ratios for non-AIDS death were independent of HIV infection (P = 0.25). The hazard ratio for hepatitis infection among HIV-positive individuals was higher for non-AIDS death than for AIDS death, but this difference was not statistically significant (P = 0.12).
Results from sensitivity analyses in which we reclassified deaths from unknown cause as either all AIDS-related or all non-AIDS-related are included in Web Tables 4 and 5 and are similar qualitatively and in magnitude to those presented above.
DISCUSSION
The shift to non-AIDS mortality and predictors of cause-specific mortality
A competing risks approach permits a detailed look at the changes in risks of mortality that have occurred with the wide adoption of HAART and at the remaining gaps between survival among HIV-negative individuals and HIV-positive individuals. On the basis of interpretations from the mixture models, the primary achievement of the HAART era in these cohorts has been a wholesale switch to non-AIDS mortality since the mid-1990s. Despite this tremendous shift, the median age at death of those who died of AIDS in the HAART era was 5.5 years later than in the pre-HAART era, an improvement that is considerably smaller than the 17.0-year shift in median age at death of non-AIDS causes. Persistently low ages at AIDS death may reflect late HAART initiation, drug resistance from prior antiretroviral therapy exposure, or poor adherence.
Because HIV-positive MACS participants in the HAART era died of non-AIDS causes at a median age (66.0 years) similar to that of HIV-negative WIHS participants (63.5 years), it appears that the socioeconomic and behavioral disadvantages of the WIHS participants equate to the deleterious effects of HIV infection among MACS participants. In the multivariate model, there was a nonsignificant elevated hazard of HIV-positive non-AIDS death among WIHS participants, but a lower hazard of AIDS death. This finding is consistent with prior evidence of slower HIV progression among women than among men (35–37).
Those with HBV or HCV infection had a significantly higher hazard for all types of mortality. Even the hazard of AIDS-related death was elevated with hepatitis infection, and this hazard ratio was not significantly lower than that for non-AIDS death. Prior studies have shown only weak evidence for any impact of HCV on HIV progression (38). The results highlight the importance of treating hepatitis in HIV-positive individuals, especially given the high prevalence of HBV and HCV in HIV-infected populations.
IDU is a major transmission pathway for hepatitis infection, particularly HCV. Even after accounting for hepatitis infection, IDU history was independently associated with a higher hazard of HIV-negative mortality. The IDU hazard ratio for HIV-positive non-AIDS mortality was significantly lower than that for HIV-negative death. This attenuation in the HIV-positive group may result from behavior modification leading to lower rates of current IDU or may result from better health care access among HIV-infected injection drug users.
Unemployment and depression were both associated with higher hazards of each type of mortality. The impact of these baseline variables may be mediated by differences in access to health care and quality of health care (for unemployment) and health-seeking behavior (for depression, e.g., adherence to HAART). Smoking is a well-known determinant of mortality among both HIV-negative and HIV-positive individuals (24). Results from the multivariate model are congruent with these known effects, but the lack of statistical significance among HIV-negative individuals may result from insufficient statistical power and the need to better quantify smoking.
Limitations and strengths of the study
The decline of mortality risks in the HAART era testifies to the effectiveness of therapy but limits the ability to assess multiple exposures. This limitation is particularly acute with the mixture models, which require estimation of more additional parameters per exposure than do semiparametric methods. We were also forced to exclude exposures that were measured differently in each cohort (e.g., income, health care access) or measured only in 1 of the 2 cohorts (e.g., domestic violence). We used mixtures of Weibull distributions because they balanced fit with parsimony and were congruent with increasing hazards of mortality with age; richer models, such as the generalized gamma distribution, require richer data.
Deaths of unknown type represented a non-negligible proportion of deaths in each cohort. In the mixture framework, appropriate contributions to the likelihood function were straightforward, but in the proportional cause-specific hazards model, we needed to impute the cause of death using probabilities derived from a mixture model. Misclassification of mortality remains a possible source of bias. However, it is reassuring that we obtained similar results in sensitivity analyses in which all unknown deaths were apportioned to either one outcome type or the other.
The MACS and WIHS cohorts are composed primarily of seroprevalent enrollees, as the date of seroconversion was known with reasonable precision (with a seroconversion window of less than 2 years) for only 355 MACS participants and 16 WIHS participants in the HAART era (368 MACS participants in the pre-HAART era; see Web Tables 6 and 7). This small sample limits possible inferences regarding the expected association of duration of infection and mortality, and such analysis is best suited for a large cohort of seroconverters.
Caution is required when making inference based on these results. One may not infer that differences in survival estimates conditioned on the outcome represent causal effects, as those dying of each cause are highly selected. Differences in the median ages at death from mixture models present a useful picture of what has happened among exposure groups, but explicit causal interpretation is not possible.
An important strength of the study is the inclusion of HIV-negative person-time drawn from the same underlying populations as the HIV-positive person-time. We were also able to directly compare exposure-outcome associations between the HIV-negative and HIV-positive populations. The long duration of follow-up time in both cohorts benefited survival analysis, especially in the MACS cohort, in which we were able to compare the pre-HAART and post-HAART eras. Mortality over the accumulated follow-up period permitted a fully parametric description of cause-specific mortality. Use of age as the time scale also allowed the best possible control of an important determinant of mortality.
The gap in non-AIDS mortality between HIV-negative and HIV-positive individuals
The lower median age at non-AIDS death among HIV-positive individuals represents a significant target for improvement in HIV treatment. Although infrequent mortality in these data limit further disaggregation of non-AIDS mortality, future analyses using more detailed mortality categories, as some analyses of combined cohorts have done (5), could help illuminate reasons for the remaining life expectancy gap.
The outlook for mortality among HIV-positive individuals may be improved with future innovations in HIV therapy that allow for greater adherence, lower toxicity, and improved viral suppression. Moreover, it is likely that the estimates presented here represent lower ages at death than may be expected for a population restricted to those who became HIV-positive in the HAART era. The study population includes individuals who survived suboptimal or nonexistent therapies for several years until HAART became available and who thus may have higher risks of mortality. It may be possible to identify subgroups of HIV-infected individuals for whom non-AIDS mortality risks approach those for comparable HIV-negative individuals.
Beyond technological and demographic changes, however, the results highlight the importance of comprehensive clinical care for HIV-infected patients in lengthening lifespans. Among other components of care, aggressive screening and treatment for hepatitis infection is essential to ameliorate the substantially increased risks of non-AIDS mortality conferred by co-infection. Moreover, social support for HIV-infected patients that includes treatment for depression and substance abuse is likely to pay dividends in the form of lower non-AIDS mortality.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Nikolas Wada, Lisa Jacobson, Alvaro Muñoz); Department of Medicine, John H. Stroger, Jr., Hospital of Cook County, Chicago, Illinois (Mardge Cohen, Audrey French); Department of Medicine, Rush University, Chicago, Illinois (Mardge Cohen, Audrey French); and Feinberg School of Medicine, Division of Infectious Diseases, Northwestern University, Chicago, Illinois (John Phair).
The Multicenter AIDS Cohort Study is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). The Women's Interagency HIV Study Collaborative Study Group is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute and the National Institute on Drug Abuse (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (grant UO1-CH-32632) and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, and MO1-RR-00083).
Conflict of interest: none declared.
REFERENCES
- 1.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315(7117):1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Detels R, Muñoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 3.Schneider MF, Gange SJ, Williams CM, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005;19(17):2009–2018. doi: 10.1097/01.aids.0000189864.90053.22. [DOI] [PubMed] [Google Scholar]
- 4.Cox C, Chu H, Schneider MF, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 5.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20(5):741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 6.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21(9):1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study. Clin Infect Dis. 2007;44(2):287–294. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- 8.French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51(4):399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen M, French AL, Benning L, et al. Causes of death among women with Human Immunodeficiency Virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113(2):91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RD, Gebo KA, Lucas GM, et al. Rate of comorbidities not related to HIV infection or AIDS among HIV-infected patients, by CD4 cell count and HAART use status. Clin Infect Dis. 2008;47(8):1102–1104. doi: 10.1086/592115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grulich AE. Cancer: the effects of HIV and antiretroviral therapy, and implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4(3):183–187. doi: 10.1097/COH.0b013e328329c5b2. [DOI] [PubMed] [Google Scholar]
- 12.Emery S, Neuhaus JA, Phillips A. Major clinical outcomes in antiretroviral (ART) naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 13.Sulkowski MS, Thomas DL, Chaisson RE, et al. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283(1):74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 14.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 15.Babiker A, Darbyshire J, Pezzotti P, et al. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol. 2002;31(5):951–958. doi: 10.1093/ije/31.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 17.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 20.Silvestre AJ, Hylton JB, Johnson LM, et al. Recruiting minority men who have sex with men for HIV research: results from a 4-city campaign. Am J Public Health. 2006;96(6):1020–1027. doi: 10.2105/AJPH.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281(24):2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 23.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Marshall MM, McCormack MC, Kirk GD. Effect of cigarette smoking on HIV acquisition, progression, and mortality. AIDS Educ Prev. 2009;21(3 suppl):28–39. doi: 10.1521/aeap.2009.21.3_supp.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin M. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100(6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipp AM, Desruisseau AJ, Qian HZ. Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J Subst Abuse Treat. 2011;40(4):386–396. doi: 10.1016/j.jsat.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neblett RC, Hutton HE, Lau B, et al. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J Womens Health. 2011;20(2):279–286. doi: 10.1089/jwh.2010.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356(14):1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 30.Lamarca R, Alonso J, Gómez G, et al. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Med Sci. 1998;53(5):M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 31.Samet J, Muñoz A. Evolution of the cohort study. Epidemiol Rev. 1998;20(1):1–14. doi: 10.1093/oxfordjournals.epirev.a017964. [DOI] [PubMed] [Google Scholar]
- 32.Checkley W, Brower RG, Muñoz A. Inference for mutually exclusive competing events through a mixture of generalized gamma distributions. Epidemiology. 2010;21(4):557–565. doi: 10.1097/EDE.0b013e3181e090ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1991. [Google Scholar]
- 34.Grambauer N, Schumacher M, Beyersmann J. Proportional subdistribution hazards modeling offers a summary analysis, even if misspecified. Stat Med. 2010;29(7–8):875–884. doi: 10.1002/sim.3786. [DOI] [PubMed] [Google Scholar]
- 35.Jarrin I, Geskus R, Bhaskaran K, et al. Gender differences in HIV progression to AIDS and death in industrialized countries: slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168(5):532–540. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 36.Nicastri E, Leone S, Angeletti C, et al. Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic review. J Antimicrob Chemother. 2007;60(4):724–732. doi: 10.1093/jac/dkm302. [DOI] [PubMed] [Google Scholar]
- 37.Sterling TR, Vlahov D, Astemborski J, et al. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 38.Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8(1):12–22. doi: 10.1007/s11904-010-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.