Abstract
Circulating 25-hydroxyvitamin D (25(OH)D), a marker for vitamin D status, is associated with bone health and possibly cancers and other diseases; yet, the determinants of 25(OH)D status, particularly ultraviolet radiation (UVR) exposure, are poorly understood. Determinants of 25(OH)D were analyzed in a subcohort of 1,500 participants of the US Radiologic Technologists (USRT) Study that included whites (n = 842), blacks (n = 646), and people of other races/ethnicities (n = 12). Participants were recruited monthly (2008–2009) across age, sex, race, and ambient UVR level groups. Questionnaires addressing UVR and other exposures were generally completed within 9 days of blood collection. The relation between potential determinants and 25(OH)D levels was examined through regression analysis in a random two-thirds sample and validated in the remaining one third. In the regression model for the full study population, age, race, body mass index, some seasons, hours outdoors being physically active, and vitamin D supplement use were associated with 25(OH)D levels. In whites, generally, the same factors were explanatory. In blacks, only age and vitamin D supplement use predicted 25(OH)D concentrations. In the full population, determinants accounted for 25% of circulating 25(OH)D variability, with similar correlations for subgroups. Despite detailed data on UVR and other factors near the time of blood collection, the ability to explain 25(OH)D was modest.
Keywords: dietary supplements, 25-hydroxyvitamin D, race, seasons, sex, sunlight, ultraviolet rays, vitamin D
Vitamin D plays an established protective role in bone development and health (1–4) and may influence the risk of other major diseases, including some cancers, cardiovascular disease, autoimmune conditions, and diabetes (1). Because of the known and potential benefits of vitamin D, there is considerable interest in understanding the determinants of vitamin D status (1, 5–7).
Circulating 25-hydroxyvitamin D (25(OH)D) is considered a marker for vitamin D status (8). A key source of circulating 25(OH)D is thought to be ultraviolet radiation (UVR), which interacts with cutaneous 7-dehydrocholesterol; casual UVR exposure reportedly accounts for as much as 90% of vitamin D requirements (9). Other sources include dietary and supplemental vitamin D intake (8). Understanding how various demographic, dietary, behavioral, and environmental factors contribute to 25(OH)D levels could help identify individuals at risk of low status, as well as facilitate modeling of circulating 25(OH)D, which could be less costly than assaying vitamin D for epidemiologic studies.
Investigators in many studies have examined the potential determinants of 25(OH)D variability in different populations (10–41). Very few studies, however, have been designed to examine factors within the relevant time period, especially regarding the key determinant, personal UVR exposure. Circulating 25(OH)D is thought to have a half-life measured in weeks (8, 42, 43), but many studies do not have data on individual UVR exposure just before blood collection (11, 17, 25, 27, 30, 34). Instead, studies have often relied on geographic indicators of UVR (e.g., latitude, regional residence) (11, 17, 25, 27, 30) that are neither individually specific nor close to the date of blood collection for the measurement of vitamin D. Many studies also do not inquire about food and supplement intake during the period immediately preceding the blood draw (11, 21, 25). In addition, numerous studies examined single-sex (12–15, 18, 24, 27, 30) or largely white (11–13, 15, 19, 20, 24, 28) populations, so limited data are available on differences by sex and race.
In the present study, we examined personal UVR exposure and other potential determinants of circulating 25(OH)D within the US Radiologic Technologists (USRT) Study. The study population selected was geographically diverse and included both sexes, as well as substantial numbers of whites and blacks, and the study was designed to identify determinants close to the date circulating 25(OH)D was measured.
MATERIALS AND METHODS
The USRT Study, which began in 1983, comprises a nationwide cohort of US radiological technologists who were certified by the American Registry of Radiological Technologists during 1926–1980 (44). The USRT Study has been approved annually by the human subjects review boards at the University of Minnesota and the National Cancer Institute, and all participants provided written informed consent.
Study population
We targeted participants from a random sample of 10,752 technologists (representative group recruited for multiple purposes) that was enriched by inclusion of all blacks who were not in the random sample (supplementary group, n = 2,593) because the black technologists constitute a small percentage of the USRT Study population. Of these, 9,141 technologists from the representative group and 2,374 from the supplementary group were eligible because they were still living and had not previously refused blood collections.
Participants were approached for blood collection and questionnaire administration between August 2008 and December 2009. Each month, random samples from the representative and supplementary groups were selected within strata defined by sex, age, and ambient UVR exposure and asked to participate in the study. Ambient UVR (erythemal) exposure estimates were derived from linking participants’ residences to the Total Ozone Mapping Spectrometer (TOMS) database (45). A total of 4,321 respondents (3,707 from the representative group and 614 from the supplementary group) provided blood samples and self-administered surveys for this and other studies (Table 1). Among persons eligible for the study, participation rates were 41% in the representative sample and 26% in the sample of black participants. We also recruited a subset of participants (from each of the 2 groups) to provide a second sample approximately 6 months later to examine changes in vitamin D levels over time, which is the subject of another article (46).
Table 1.
Distribution of Blood Samples Among 4,321 Participantsa in the US Radiologic Technologists’ Study of Determinants of Circulating 25-Hydroxyvitamin D, 2008–2009
| Distribution by Racial/Ethnic Group | No. of Participants |
||
|---|---|---|---|
| Provided 1 Sample | Provided 2 Samples | Total | |
| White (n = 3,530) | |||
| Eligible | 3,049b | 329c | |
| Selected | 513d | 329 | 842 |
| Noneligible | 152 | N/A | |
| Black (n = 691)e | |||
| Eligible | 449b | 197c | |
| Selected | 449 | 197 | 646 |
| Noneligible | 45 | N/A | |
| Other (n = 100) | |||
| Eligible | 81b | 12c | |
| Selected | 0 | 12 | 12 |
| Noneligible | 7 | N/A | |
Abbreviation: N/A, not applicable.
a There were 1,500 total participants selected for 25(OH)D assays.
b Nine or fewer days between blood collection and questionnaire administration.
c No restrictive criteria.
d Random stratified sample by sex, age group, ambient UVR region, and season.
e From both reference and supplementary US Radiologic Technologists’ Study samples.
Because of limited resources, 25(OH)D assays were performed on a subset of 2,038 samples from 1,500 individuals, which included samples from participants who had provided 2 samples and single samples from white and black participants who provided blood within 9 days of questionnaire completion (Table 1). We restricted the time between the blood collection and questionnaire to 9 days to assure a short time between questionnaire and blood draw. Of the 1,500 participants, 842 were white, 646 were black, and 12 were of other races/ethnicities.
Data collection
Each participant was mailed a blood collection kit together with a questionnaire and asked to complete questionnaires the same day as the blood draws. Questionnaires were returned to the study office and blood samples were shipped overnight with temperature stabilizing packs to the processing laboratory in Frederick, Maryland. The questionnaire, which included 4 pages on potential determinants of vitamin D status, requested that participants provide information about behaviors during the past 30 days, as described below.
Circulating 25(OH)D measurements
The 25(OH)D plasma samples were assayed in 23 batches by Heartland Assays, Inc. (Ames, Iowa) using CLIA/LIASON in commercially available kits from DiaSorin (Stillwater, Minnesota). We selected quality controls (5 per batch) from study samples in a run-in to represent low, medium, and high concentrations, which averaged 42.1 nmol/L, 69.8 nmol/L, and 101.2 nmol/L, respectively. Quality control samples were randomly distributed across and within batches by race, sex, geographic UVR region, and season. The total coefficients of variation were 8.3%, 7.2%, and 5.8%, for low, medium, and high circulating 25(OH)D, respectively, with an overall average of 7.1%.
Statistical analysis
The following questionnaire-derived variables were considered as potential determinants of circulating 25(OH)D levels: age (continuous); sex; race (white, black, or other (based on self-classification)); season of blood draw (January–March, April–June, July–September, or October–December); latitude of residence (continuous); average total number of hours outdoors each day between sunrise and sunset; hours outside in time blocks (e.g., 10 am–2 pm); percentage of time outside when participant was moderately/strenuously physically active (0; <25%, 25%–74%, ≥75%); frequency of various protective behaviors (i.e., wearing a hat, long-sleeved shirt, or long pants, moving into shady areas, or wearing sunscreen when in the sun); sun protection factor if sunscreen was used; number of days in sun for more than 1 hour if more than 200 miles from home on a trip; tanning bed use (ever vs. never); complexion (light, medium, or dark); tan levels (comparing inner upper arm with back of hand); body mass index (BMI; weight (kg)/height (m2); continuous); current smoking (yes vs. no); current hormone replacement therapy use (yes vs. no); and inflammatory bowel disease (yes vs. no).
Mean UVR erythemal ambient exposures were estimated for the month of questionnaire administration based on residential linkage to the TOMS database, with data from 1978–1993 averaged. The TOMS database, maintained by the National Aeronautics and Space Administration, provides daily estimates of ground-level erythemal exposures (which incorporate part of the UVR spectrum contributing to 25(OH)D production, particularly UVB) on a 1°-latitude-by-1.25°-longitude grid.
Dietary intake of vitamin D in international units per day was calculated based on self-reported consumption of salmon, tuna, fortified cereal, milk, eggs, margarine, and fortified juice, using frequency and serving size categories, with international units per food portion derived from the Office of Dietary Supplements Fact Sheet (47). Vitamin D supplement intake (IU/day) was based on multivitamin, vitamin D, and cod liver oil consumption (using frequency per week, reported dose for vitamin D and cod liver oil supplements, and name brands for multivitamins to permit dose estimates). When the name brands of multivitamins were not reported or could not be linked to a dose, we imputed a dose of 400 IU, the most common formulation.
Participant characteristics were analyzed by cutpoints for circulating 25(OH)D that correspond to very low levels (<25 nmol/L) (25); alternative thresholds for potential insufficiency, 50 nmol/L (1) and 75 nmol/L (48); and a common upper cutpoint, 100 nmol/L (25). The plot to examine normality showed a slightly skewed deviation of 25(OH)D from normality. The Box-Cox procedure (49) suggested that the square root transformation of 25(OH)D was closer to a normal distribution. However, because the transformed and untransformed results were similar, we present the untransformed results. We examined each potential determinant using multivariate linear regression adjusted for age, sex, and race. Analyses were repeated within sex, race, and season subgroups. Many key variables had no missing data (e.g., age, race, season, latitude, mean UVR exposure), and most had less than 5% missing data. Missing information was included in the model by coding “missing” to separate dummy variables.
Backwards stepwise linear regression analysis was used to select determinants in a random sample of two thirds of the participants (n = 1,000, model construction sample) and within sex, race, and season subgroups. If a participant provided 2 samples, the first was used. Additional factors entered into the models were season of blood draw, BMI, ambient UVR quintiles, time outdoors, physically active time outdoors, frequency of wearing long-sleeved shirts, dietary intake of vitamin D, supplement intake of vitamin D, tan level, smoking status, hormone replacement therapy use, and number of days in the sun away from residence. We excluded some factors for which a similar factor explained more variance in the single-factor regression analysis (e.g., total time outdoors vs. time blocks, long sleeves vs. long pants). The backwards stepwise regression procedure selected variables with 1 or more categorical level with a P value ≤ 0.05, fixing age, sex, and race to remain in the model. We applied the resulting beta coefficients to the remaining one-third validation sample, that is, those not in the model construction sample (n = 500), and within the subgroups, then correlated the predicted and actual values for the validation sample. Because the Pearson correlation coefficients for the validation sample was reasonably good (r = 0.50, P < 0.0001), we applied the models developed in the model construction sample to the full study population (n = 1,500) and subgroups (27). Aside from age, sex, and race, which were fixed in all models, only variables with 1 or more categorical levels that were statistically significant in the model construction sample were presented in the final models.
All statistical tests were 2-sided, with P ≤ 0.05 reflecting statistical significance. Statistical analyses were performed with SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
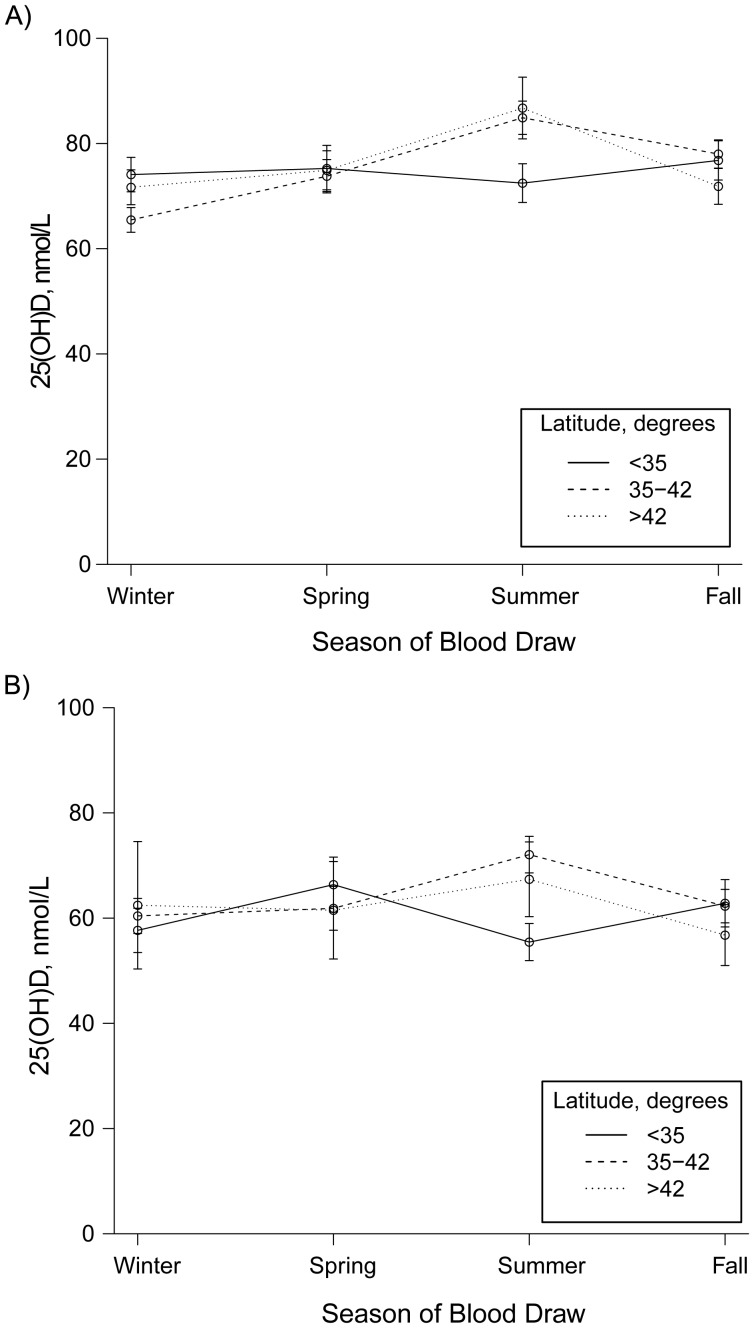
In the present study, black and white participants were similar in age, but black participants included a higher percentage of women and residents of lower latitudes (i.e., closer to the equator) (Table 2). Figure 1 illustrates the unadjusted distribution of circulating 25(OH)D levels by season of blood draw in the total study population. In both whites (Figure 1A) and blacks (Figure 1B), the highest mean 25(OH)D concentrations occurred in summer in the northern part of the United states, that is, in latitudes at or above 35°. The distribution of study participant characteristics by categories of 25(OH)D status is shown in Table 3.
Table 2.
Means and Proportion of Selected Characteristics, by Racial Groupa, in the US Radiologic Technologists’ Study, 2008–2009
| Characteristic | Whites (n = 842) |
Blacks (n = 646) |
||
|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | |
| 25(OH)D, nmol/L | 74.2 (28.8) | 62.7 (33.3) | ||
| Women | 51.7 | 65.8 | ||
| Age, years | 63.5 (9.5) | 62.5 (8.1) | ||
| Range, years | 48.0–93.0 | 49.0–92.0 | ||
| Season of blood receiptb | ||||
| Winter | 36.6 | 24.6 | ||
| Spring | 23.0 | 23.7 | ||
| Summer | 13.4 | 24.3 | ||
| Fall | 27.0 | 27.4 | ||
| Latitude | ||||
| <35° | 25.4 | 34.5 | ||
| 35°–42° | 48.8 | 56.2 | ||
| >42° | 25.8 | 9.3 | ||
| Quintile of mean annual UVR exposurec | ||||
| 1 | 23.4 | 15.8 | ||
| 2 | 22.1 | 16.7 | ||
| 3 | 19.6 | 20.7 | ||
| 4 | 19.2 | 21.1 | ||
| 5 | 15.7 | 25.7 | ||
| Minutes outside per week | 84.8 (81.8) | 75.5 (74.7) | ||
| Long sleeves never or rarely worn outside | 18.3 | 28.0 | ||
| Dietary intake, IU/day | 270.4 (256.7) | 365.9 (470.8) | ||
| Took supplements | 58.9 | 48.3 | ||
| Supplement intake, IU/day | 523.3 (490.7) | 452.8 (482.1) | ||
| ≥1,000 IU/day | 14.3 | 11.2 | ||
| Current smoker | 9.3 | 9.6 | ||
| Body mass indexd | 28.2 (5.7) | 30.1 (6.3) | ||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; SD, standard deviation; UVR, ultraviolet radiation.
a Characteristics describe all whites and blacks contributing to the analysis based on first (or only) samples. “Whites” refers to non-Hispanic whites and “blacks” to non-Hispanic blacks. Twelve participants who were not white or black are not included in this table. However, all 12 provided 2 samples and are included in the study analyses presented in subsequent tables.
b The seasons were defined as winter (January–March), spring (April–June), summer (July–September), and fall (October–December).
c Mean UVR doses were estimated for the month of questionnaire administration based on residential linkage for the Total Ozone Mapping Spectrometer database, with data averaged from 1978 to 1993. Quintile 1 was lowest; quintile 5 was highest.
d Weight (kg)/height (m)2.
Figure 1.
Mean circulating 25-hydroxyvitamin D (25(OH)D) concentrations by season and latitude in the US Radiologic Technologists’ Study, 2008–2009. Circles represent mean values and bars, standard errors for the means. A) 25(OH)D concentrations in whites (n = 842). B) 25(OH)D concentrations in blacks (n = 646).
Table 3.
Distribution of Participant Characteristicsa by Circulating 25-Hydroxyvitamin D Category, US Radiologic Technologists’ Study, 2008–2009
| Characteristic | No. of Participants | Circulating 25(OH)D Level |
||||
|---|---|---|---|---|---|---|
| <25 (n = 81) | 25 to <50 (n = 352) | 50 to <75 (n = 499) | 75 to <100 (n = 348) | ≥100 (n = 220) | ||
| Age at blood draw, yearsb | ||||||
| <60 | 704 | 6.0 | 25.3 | 34.9 | 22.0 | 11.8 |
| ≥60 | 796 | 4.9 | 21.9 | 31.8 | 24.3 | 17.2 |
| Sex | ||||||
| Women | 863 | 6.5 | 24.0 | 29.6 | 24.6 | 15.4 |
| Men | 637 | 3.9 | 22.8 | 38.3 | 21.4 | 13.7 |
| Race | ||||||
| Black | 646 | 9.0 | 31.9 | 29.4 | 17.5 | 12.2 |
| White | 842 | 2.4 | 17.0 | 36.0 | 27.9 | 16.8 |
| Season of blood draw | ||||||
| Winter | 476 | 6.3 | 27.3 | 31.9 | 22.7 | 11.8 |
| Spring | 347 | 4.6 | 24.8 | 32.9 | 22.5 | 15.3 |
| Summer | 271 | 5.5 | 17.0 | 36.2 | 24.0 | 17.3 |
| Fall | 406 | 4.9 | 22.2 | 33.3 | 23.9 | 15.8 |
| Body mass indexc | ||||||
| <25 | 338 | 3.6 | 14.8 | 29.3 | 30.8 | 21.6 |
| 25 to <30 | 588 | 4.9 | 22.3 | 35.4 | 21.9 | 15.5 |
| 30 to <35 | 274 | 4.7 | 30.7 | 35.0 | 19.7 | 9.9 |
| ≥35 | 210 | 11.0 | 32.4 | 29.5 | 17.1 | 10.0 |
| Latituded | ||||||
| <35° | 445 | 5.8 | 25.6 | 33.3 | 22.5 | 12.8 |
| 35°–42° | 776 | 5.5 | 23.6 | 32.6 | 22.4 | 15.9 |
| >42° | 279 | 4.3 | 19.7 | 35.1 | 26.5 | 14.3 |
| Quintile of mean UVR exposuree | ||||||
| 1 | 300 | 4.7 | 27.0 | 34.7 | 20.0 | 13.7 |
| 2 | 299 | 6.0 | 26.1 | 31.1 | 23.8 | 13.0 |
| 3 | 301 | 7.3 | 31.1 | 33.2 | 25.9 | 13.6 |
| 4 | 300 | 4.0 | 23.0 | 33.7 | 22.7 | 16.7 |
| 5 | 300 | 5.0 | 21.3 | 33.7 | 23.7 | 16.3 |
| Quintile of mean UVR exposuref (only whites) | ||||||
| 1 | 197 | 3.1 | 21.3 | 36.6 | 22.3 | 13.7 |
| 2 | 186 | 1.6 | 12.0 | 32.3 | 29.6 | 15.6 |
| 3 | 165 | 4.9 | 14.6 | 33.9 | 30.9 | 15.8 |
| 4 | 162 | 1.2 | 14.8 | 37.7 | 25.9 | 20.4 |
| 5 | 132 | 0.8 | 10.6 | 36.4 | 32.6 | 19.7 |
| Time spent outdoors, hours/day | ||||||
| <0.25 | 198 | 6.6 | 26.8 | 27.8 | 22.2 | 16.7 |
| 0.25–0.5 | 285 | 8.4 | 22.1 | 34.7 | 21.8 | 13.0 |
| 0.5–1 | 333 | 5.7 | 25.5 | 32.1 | 21.3 | 15.3 |
| 1–2 | 353 | 4.3 | 23.8 | 36.0 | 22.4 | 13.6 |
| ≥2 | 268 | 2.6 | 20.2 | 32.1 | 30.6 | 14.6 |
| Time spent being physically active outdoors | ||||||
| <1.7 hours/week | 619 | 7.1 | 27.3 | 32.5 | 18.7 | 14.4 |
| ≥1.7 hours/week | 658 | 3.7 | 19.9 | 34.5 | 27.2 | 14.7 |
| Wear long sleeves | ||||||
| Never | 354 | 2.6 | 17.4 | 35.2 | 27.0 | 17.9 |
| Rarely | 266 | 4.5 | 20.3 | 33.1 | 26.3 | 15.8 |
| Sometimes | 313 | 4.8 | 21.4 | 37.4 | 24.3 | 12.1 |
| Usually | 202 | 6.9 | 26.7 | 31.7 | 21.8 | 12.9 |
| Almost always | 338 | 6.2 | 23.1 | 34.6 | 20.4 | 15.7 |
| Use sun protection factor | ||||||
| Never | 791 | 6.0 | 25.6 | 34.9 | 20.5 | 13.0 |
| Rarely | 262 | 6.1 | 19.5 | 37.8 | 22.1 | 14.5 |
| Sometimes | 151 | 5.3 | 19.2 | 31.1 | 30.5 | 13.9 |
| Usually | 129 | 0 | 14.0 | 34.1 | 30.2 | 21.7 |
| Almost always | 137 | 4.4 | 19.7 | 28.5 | 29.9 | 17.5 |
| Dietary vitamin D intake, IU/dayg | ||||||
| <201.1 | 741 | 6.6 | 24.6 | 32.1 | 22.9 | 13.8 |
| ≥201.1 | 741 | 4.3 | 22.1 | 34.3 | 23.8 | 15.5 |
| Supplement intake, IU/dayh | ||||||
| <400 | 438 | 10.7 | 40.4 | 31.3 | 12.8 | 4.8 |
| ≥400 | 774 | 2.3 | 16.4 | 34.8 | 27.8 | 18.7 |
| Tan leveli | ||||||
| Same | 427 | 7.7 | 23.7 | 31.9 | 23.2 | 13.6 |
| Slightly darker | 631 | 4.1 | 24.9 | 33.6 | 22.5 | 14.9 |
| Moderately darker | 279 | 5.0 | 20.8 | 34.4 | 26.5 | 13.3 |
| Much darker | 80 | 3.8 | 17.5 | 31.3 | 22.5 | 25.0 |
| Redder | 9 | 0 | 22.2 | 33.3 | 11.1 | 33.3 |
| Current smoker | ||||||
| Yes | 141 | 8.5 | 31.9 | 24.1 | 21.3 | 14.2 |
| No | 1,309 | 5.0 | 22.6 | 33.8 | 23.5 | 15.1 |
| Current HRT use (women only) | ||||||
| Yes | 120 | 4.2 | 22.5 | 30.0 | 27.5 | 15.8 |
| No | 342 | 5.0 | 19.3 | 29.5 | 27.2 | 19.0 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; HRT, hormone replacement therapy; UVR, ultraviolet radiation.
a Characteristics of respondents based on the 30 days before survey response. Totals do not add up to 1,500 if there are missing responses.
b Age at time of blood collection.
c Weight (kg)/height (m)2.
d At the place of residence.
e Mean ambient UVR (based on erythemal dose) at residence at month of blood collection averaged from 1978 to 1993. The UVR variable combines whites and blacks in the study. Quintile 1 was lowest; quintile 5 was highest.
f The UVR quintiles are presented for whites alone. There was little variation across 25(OH)D categories by UVR quintile in blacks, and the results are not presented here.
g Sum of international units from dietary vitamin D sources (salmon, tuna, fortified cereal, milk, eggs, margarine, and fortified juice). Median = 201.1 IU.
h Sum of international units of vitamin D from multivitamins, vitamin D supplements, and cod liver oil.
i Comparing the skin color on back of hand to the inside of upper arm.
In the overall population, many single factors were associated with 25(OH)D levels, such as age, race, season of blood draw, BMI, milk intake, dietary intake (borderline statistically significant), supplement intake, and several indicators of UVR exposure, including high levels of mean UVR, number of hours outdoors (overall hours outdoors; hours outdoors 10 am–2 pm (borderline statistically significant); 8 am–10 am combined with 2 pm–5 pm), physically active hours outdoors, certain UVR protective behaviors (i.e., wearing a hat, long sleeves, or long pants, standing in shade, wearing sunscreen and sunscreen protection factor), tanning bed use, and tan level (Web Table 1, available at http://aje.oxfordjournals.org/). However, age, sex, and race together accounted for only 6% of the 25(OH)D variability, and other factors generally explained little of the 25(OH)D variability, with the exception of supplementation with vitamin D, which explained another 15% in variability. Sex, latitude, days in the sun away from residence, early morning and evening hours outside, indoor hours physically active, complexion (beyond that signified by race), smoking status, current hormone replacement therapy use, and inflammatory bowel disease were not related to 25(OH)D levels.
Findings for whites were similar to those for the total population (Web Table 1). For blacks, sun exposure variables (e.g., time outdoors, ambient UVR exposure, and sun protective behaviors) were generally not related to vitamin D levels. The determinants for men were similar to those for the overall population, whereas for women, UVR exposure variables explained little 25(OH)D variability.
Winter samples had the greatest number of significant determinants, particularly for UVR- related factors (Web Table 2). Neither hours outdoors nor most sun protective factors were significantly predictive of 25(OH)D in summer or fall.
In the stepwise regression analyses, factors that were most strongly associated with higher circulating 25(OH)D levels in the overall population were increased age, summer and fall blood collection (compared with winter collection), hours outdoors being physically active, and vitamin D supplement intake level (Table 4). Factors most strongly related to lower 25(OH)D were race (black compared with white) and BMI above 25. These factors combined accounted for 25% of the 25(OH)D variability in the overall population.
Table 4.
Predictors of Circulating 25-Hydroxyvitamin D Levelsa, Overall and by Sex and Race, US Radiologic Technologists’ Study, 2008–2009
| Characteristic | Overall (R2 = 0.25) |
Men (R2 = 0.30) |
Women (R2 = 0.27) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | β Coefficient (SE) | P Value | No. of Participants | β Coefficient (SE) | P Value | No. of Participants | β Coefficient (SE) | P Value | |
| Ageb | 1,037 | 0.21 (0.10) | 0.03 | 459 | 0.40 (0.14) | 0.004 | 663 | 0.13 (0.13) | 0.30 |
| Sex | |||||||||
| Women | 578 | Referent | |||||||
| Men | 459 | 1.99 (1.77) | 0.26 | ||||||
| Race | |||||||||
| White | 611 | Referent | 305 | Referent | 344 | Referent | |||
| Black | 419 | −8.91 (1.75) | <0.0001 | 148 | −11.51 (2.66) | <0.0001 | 317 | −7.94 (2.15) | 0.0002 |
| Other | 7 | −17.19 (10.08) | 0.09 | 6 | −22.63 (10.74) | 0.04 | 2 | 3.88 (19.38) | 0.84 |
| Season | |||||||||
| Winter | 338 | Referent | 145 | Referent | |||||
| Spring | 231 | 2.24 (2.27) | 0.32 | 91 | −5.74 (4.71) | 0.22 | |||
| Summer | 185 | 10.09 (2.45) | <0.0001 | 82 | 2.59 (4.78) | 0.59 | |||
| Fall | 283 | 5.32 (2.13) | 0.01 | 141 | 8.11 (3.17) | 0.01 | |||
| Quintile of mean UVR exposurec | |||||||||
| 1 | 87 | Referent | |||||||
| 2 | 98 | 6.10 (3.85) | 0.11 | ||||||
| 3 | 99 | 5.38 (4.08) | 0.19 | ||||||
| 4 | 102 | 17.53 (4.83) | 0.0003 | ||||||
| 5 | 73 | 12.99 (5.86) | 0.03 | ||||||
| BMId | |||||||||
| <25 | 244 | Referent | 91 | Referent | |||||
| 25 to <30 | 411 | −5.67 (2.15) | 0.009 | 201 | −8.08 (3.24) | 0.01 | |||
| 30 to <35 | 197 | −9.30 (2.56) | 0.003 | 96 | −12.72 (3.80) | 0.0009 | |||
| ≥35 | 164 | −12.93 (2.73) | <0.0001 | 58 | −20.93 (4.34) | <0.0001 | |||
| Time spent being physically active outdoors, hours/week | 1,037 | 0.38 (0.16) | 0.02 | 459 | 0.50 (0.20) | 0.01 | |||
| Wears long sleeves | |||||||||
| Not outside | 22 | Referent | |||||||
| Never | 67 | 11.93 (6.35) | 0.06 | ||||||
| Rarely | 106 | 5.40 (5.90) | 0.36 | ||||||
| Sometimes | 93 | −1.29 (5.87) | 0.83 | ||||||
| Usually | 55 | 3.70 (6.24) | 0.55 | ||||||
| Almost always | 113 | 2.09 (5.69) | 0.71 | ||||||
| Vitamin D supplement intake, IU/day | 1,037 | 0.02 (0.002) | <0.0001 | 459 | 0.02 (0.002) | <0.0001 | 663 | 0.03 (0.002) | <0.0001 |
| Blacks (R2 = 0.26) | Whites (R2 = 0.23) | ||||||||
| No. of Participants | β Coefficient (SE) | P Value | No. of Participants | β Coefficient (SE) | P Value | ||||
| Ageb | 498 | 0.41 (0.17) | 0.02 | 611 | 0.09 (0.11) | 0.43 | |||
| Sex | |||||||||
| Women | 317 | Referent | 306 | Referent | |||||
| Men | 181 | 4.53 (2.74) | 0.10 | 305 | 2.80 (2.27) | 0.22 | |||
| Race | |||||||||
| White | |||||||||
| Black | |||||||||
| Other | |||||||||
| Season | |||||||||
| Winter | 226 | Referent | |||||||
| Spring | 133 | −6.98 (3.86) | 0.07 | ||||||
| Summer | 84 | 2.66 (4.16) | 0.52 | ||||||
| Fall | 168 | 8.32 (2.62) | 0.002 | ||||||
| Quintile of mean UVR exposurec | |||||||||
| 1 | 142 | Referent | |||||||
| 2 | 142 | 6.45 (3.02) | 0.03 | ||||||
| 3 | 116 | 5.52 (3.43) | 0.11 | ||||||
| 4 | 118 | 18.69 (4.14) | <0.0001 | ||||||
| 5 | 93 | 15.03 (4.77) | 0.002 | ||||||
| BMId | |||||||||
| <25 | 170 | Referent | |||||||
| 25 to <30 | 250 | −9.55 (2.60) | 0.0003 | ||||||
| 30 to <35 | 106 | −16.74 (3.21) | <0.0001 | ||||||
| ≥35 | 73 | −16.63 (3.65) | <0.0001 | ||||||
| Time spent being physically active outdoors, hours/week | 611 | 0.44 (0.19) | 0.02 | ||||||
| Wears long sleeves | |||||||||
| Not outside | |||||||||
| Never | |||||||||
| Rarely | |||||||||
| Sometimes | |||||||||
| Usually | |||||||||
| Almost always | |||||||||
| Vitamin D supplement intake, IU/day | 498 | 0.03 (0.003) | <0.0001 | 611 | 0.02 (0.002) | <0.0001 | |||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; SE, standard error; UVR ultraviolet radiation.
a The final model was built using a sample of 1,500, but only 1,037 were included in the final model because of missing data for some variables. Age, sex, and race were fixed in the model. Variables that were not statistically significant in the model construction sample were removed from the model.
b At the time of blood collection.
c Mean ambient UVR exposure (based on erythemal dose) at residence in month of blood collection averaged from 1978 to 1993. Quintiles were based on the overall population and applied to the overall population and subgroups. Quintile 1 was lowest; quintile 5 was highest.
d Weight (kg)/height (m)2.
Factors most strongly associated with 25(OH)D levels in whites included season of blood collection, ambient UVR exposure, BMI, hours outside being physically active, and supplement intake (Table 4). In blacks, only age and supplement use were associated with 25(OH)D levels. In women, only race and vitamin D supplement level significantly predicted 25(OH)D levels, whereas in men, age, race, season, ambient UVR level, BMI, hours outside physically active, and level of supplement use significantly predicted circulating 25(OH)D levels. The R2 values varied in subgroups between 0.23 (whites) and 0.30 (men).
When stratified by season, race was significantly associated with 25(OH)D only in summer and fall (Web Table 3). Supplement intake was associated with 25(OH)D in every season. The R2 values by season ranged from 0.20 in the spring to 0.29 in the winter.
DISCUSSION
To our knowledge, this is the first large nationwide US study to examine the determinants of 25(OH)D levels by assessing detailed personal UVR and other exposures in the period just before blood collection in both black and white participants. Despite the detailed information collected on a wide variety of potential determinants of circulating 25(OH)D, our ability to explain circulating 25(OH)D levels was modest, accounting for 25% of the variance in 25(OH)D in the total population and similar percentages within racial and sex subgroups. Other studies have had comparably limited ability to explain 25(OH)D variance (14, 17, 29) in part because of the lack of information on individual sun exposure (27), whereas our study specifically evaluated UVR-related factors. Moreover, one study with a relatively high overall R2 of 0.42 (16) could attribute only about 3% of variability to individual sun exposure factors.
Age, sex, and race together explained only 6 percent of the variability in the overall study population. Although increasing age was positively related to 25(OH)D levels, other studies have reported mixed results for age (12, 15, 16, 18, 22, 27, 32, 50). In experimental studies, it has been suggested that aging skin has a declining capacity to produce vitamin D (51, 52), but this limitation may be concentrated in older ages, for example, in persons older than 69 years of age in the study by Need et al. (53). The positive associations with age seen in the present study may reflect the age distribution of USRT participants, who were generally not elderly. Sex did not play a role in accounting for 25(OH)D levels in the total population or within subgroups, a result common to some studies (16, 32) but not others (20, 25).
Race (black compared with white) was significantly associated with lower 25(OH)D levels in both men and women. Race has been consistently related to circulating 25(OH)D levels (8, 16, 25, 27, 29, 30, 32). Some of the racial disparity reflects differences in skin melanin content, as dark skin has a higher threshold for synthesizing detectable pre-vitamin D3 (54). In the present study, however, race accounted for less than 6% of the 25(OH)D variability. The relatively low contribution of race to 25(OH)D variability in this study may partly reflect the shared behavioral patterns in an occupational cohort of people who may have fairly similar lifestyles. Shared behavioral patterns may also contribute to the similarity in levels of 25(OH)D in white and black participants. In the present study, 45% of white participants had 25(OH)D levels of 75 nmol/L of higher compared with 30% of black participants, whereas in the Adventist Health Study-2 (16), 52% of whites compared with 16% of blacks had levels of 75 nmol/L or higher.
BMI was a significant inverse determinant of 25(OH)D level in the total population, although not in women or blacks. The inverse association with BMI has been seen in other studies (8, 12, 16, 25), as has the absence of a relation with BMI (or body fat) observed in many (16, 25, 31, 55), but not all (10), studies of blacks. The inverse association with higher BMI may reflect reduced sun exposure due to less time outdoors, more clothing, and/or vitamin D sequestering in fat (56). It has been hypothesized that the limited association between BMI and 25(OH)D levels in blacks (at least for women) may reflect a higher proportion of lean body mass in black, compared with white, women for any particular BMI (31, 57).
We saw no significant contribution to 25(OH)D from dietary sources in the final models for the overall population or subgroups, although some studies have shown a modest positive association (15, 16, 23, 25). In our study, the strongest determinant, including for subgroups, was vitamin D supplement intake. This likely reflects the high vitamin D intake in the USRT participants, a group of health care workers, who reported a mean daily supplement intake of 523 IU in whites and 453 IU in blacks (with 14% and 11%, respectively, taking ≥1,000 IU/day). In contrast, one recent study in the United States reported a mean vitamin D intake of 252 IU in whites and 200 IU in blacks (16), and another showed 400 IU or less taken by about 90% of participants (27).
As noted earlier, UVR exposure comprised ambient and personal UVR exposure factors, including hours outdoors, which varied by duration and time of day, susceptibility factors (e.g., complexion), and protective behaviors. Despite surveying these factors, UVR exposure played a minor role in predicting 25(OH)D levels.
Notably, latitude played no role in explaining 25(OH)D levels, whereas mean erythemal dose for the month of blood draw only modestly explained variation for men and whites. Erythemal dose may not sufficiently represent the ultraviolet B wavelengths relevant to 25(OH)D production. Also, sun avoidance may be so commonplace (mean time outdoors per week was less than 1.5 hours (Table 2)) that geographic solar irradiance is not particularly relevant to personal 25(OH)D dose.
The fact that the number of hours outdoors failed to explain much of the variability in 25(OH)D levels may reflect that cutaneous vitamin D synthesis reaches saturation levels quickly (58), which would suggest that repeated outdoor spans are more relevant, although difficult to estimate. Other studies with personal UVR exposure information have also found that individual UVR exposure factors did not substantially improve explanatory power (12, 14, 16, 23).
That the number of hours being physically active outdoors was a stronger predictor than hours outside also suggests that the character of outside time may be important. Some have suggested that exercise itself, independent of outdoor time, may affect vitamin D levels (34, 59); however, our finding that indoor physical activity was unrelated to vitamin D levels does not support this hypothesis.
For blacks, UVR factors played no significant role in 25(OH)D levels. Personal UVR exposure also was unrelated in blacks in 2 other US studies in which determinants of vitamin D in both blacks and whites were examined (10, 16). Notably, Chan et al. (16) did see a significant role for season and erythemal zone based on ultraviolet index maps in predicting 25(OH)D in black participants, although the contribution of erythemal zone was small in both whites and blacks. Similarly, in a study of elderly whites and blacks, Shea et al. (36) found that time walking, a surrogate for outdoor time, was not related to 25(OH)D levels in blacks.
Strengths of our study include the nationwide distribution of participants, substantial numbers of both whites and blacks, and the detailed questionnaire covering personal/ambient UVR and other potential determinants close to the date of blood collection. In addition, all assays were processed in a laboratory with good quality control. Moreover, as Millen et al. (27) indicated, few studies have validated their models by comparing predicted values with actual measures. Notably, the Pearson correlation coefficient of 0.50 (P < 0.0001) in our validation sample is similar to the correlation (r = 0.45) reported by Millen et al. (27).
Our study is limited by its reliance on self-administered questionnaires. Possibly, 25(OH)D predictability could be improved with more sophisticated measures of adiposity, skin pigmentation, and personal exposure (using UVR dosimeters). Genetic variants may also affect 25(OH)D levels (37, 60). These areas for potential improvement, however, would be inapplicable to deriving questionnaire-based surrogates for 25(OH)D.
In addition, data from US radiologic technologists may not be generalizable to other populations (e.g., persons with lower incomes) with different behavior patterns regarding outdoors activities and supplement use, for example. Also, the low response rate in the USRT supplementary group of blacks (26%) could influence the findings if respondents differed from the larger USRT black population (e.g., in terms of socioeconomic status, which could affect vitamin D supplement use patterns.).
Our study of 25(OH)D determinants in a large study group with detailed information on personal UVR exposures, as well as demographic, lifestyle, and dietary exposures, illustrates the difficulty of predicting circulating vitamin D levels. We could explain only 23% of the 25(OH)D variability in whites and only 26% in blacks. The survey instrument focused on UVR-related behavior, thus suggesting the difficulty in identifying vitamin D-related UVR behavioral factors. Our modest ability to explain 25(OH)D variability further suggests that surveys alone are unlikely to provide adequate surrogates for vitamin D levels. These results also raise questions about findings in which surrogates for vitamin D levels (e.g., latitude, diet) have been interpreted to inform our understanding of vitamin D and disease. Observed relations between surrogates for vitamin D and disease outcomes may provide more insights about the surrogates than about vitamin D.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland (D. Michal Freedman, Elizabeth K. Cahoon, Preetha Rajaraman, Jacqueline M. Major, Michele M. Doody, Barry I. Graubard, Martha S. Linet); Division of Environmental Health Sciences, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Bruce H. Alexander, Richard W. Hoffbeck); and AusSun Research Laboratory, School of Public Health and Social Work, Queensland University of Technology, Brisbane, Australia (Michael Kimlin).
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the US Public Health Service of the Department of Health and Human Services.
The authors thank Jerry Reid of the American Registry of Radiologic Technologists for continued support of the project; Diane Kampa and Allison Iwan of the University of Minnesota for data collection and coordination; Laura Bowen and Li Cheung of Information Management Services, Inc., for biomedical computer assistance; Jackie King of Bioreliance Corp. for plasma sample coordination; and Dr. Ron Horst of Heartland Assays Inc., for performing 25-hydroxvitamin D assays on the plasma samples.
Conflict of interest: none declared.
REFERENCES
- 1.Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies; 2010. [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 4.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 5.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis CD, Hartmuller V, Freedman DM, et al. Vitamin D and cancer: current dilemmas and future needs. Nutr Rev. 2007;65(8):S71–S74. doi: 10.1301/nr.2007.aug.s71-s74. [DOI] [PubMed] [Google Scholar]
- 7.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin 7. J Clin Endocrinol Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 8.IARC. Vitamin D and Cancer. Geneva, Switzerland: International Association for Research on Cancer; 2008. [Google Scholar]
- 9.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease 2. Am J Clin Nutr. 2004;80(6 suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin A, Moriakova A, Akhter N, et al. Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int. 2009;20(10):1795–1803. doi: 10.1007/s00198-009-0873-6. [DOI] [PubMed] [Google Scholar]
- 11.Brock KE, Graubard BI, Fraser DR, et al. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers in Finland. Eur J Clin Nutr. 2010;64(3):280–288. doi: 10.1038/ejcn.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen R, Molgaard C, Skovgaard LT, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr. 2005;59(4):533–541. doi: 10.1038/sj.ejcn.1602108. [DOI] [PubMed] [Google Scholar]
- 13.Brot C, Vestergaard P, Kolthoff N, et al. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(suppl 1):S97–S103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 14.Brustad M, Alsaker E, Engelsen O, et al. Vitamin D status of middle-aged women at 65–71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004;7(2):327–335. doi: 10.1079/PHN2003536. [DOI] [PubMed] [Google Scholar]
- 15.Burgaz A, Akesson A, Oster A, et al. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am J Clin Nutr. 2007;86(5):1399–1404. doi: 10.1093/ajcn/86.5.1399. [DOI] [PubMed] [Google Scholar]
- 16.Chan J, Jaceldo-Siegl K, Fraser GE. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010;21(4):501–511. doi: 10.1007/s10552-009-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan KM, Signorello LB, Munro HM, et al. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19(5):527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 19.Hill TR, Cotter AA, Mitchell S, et al. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br J Nutr. 2008;99(5):1061–1067. doi: 10.1017/S0007114507842826. [DOI] [PubMed] [Google Scholar]
- 20.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs ET, Alberts DS, Foote JA, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87(3):608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacques PF, Felson DT, Tucker KL, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66(4):929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 23.Lips P, van Ginkel FC, Jongen MJ, et al. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am J Clin Nutr. 1987;46(6):1005–1010. doi: 10.1093/ajcn/46.6.1005. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald HM, Mavroeidi A, Barr RJ, et al. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone. 2008;42(5):996–1003. doi: 10.1016/j.bone.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 25.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeill G, Vyvyan J, Peace H, et al. Predictors of micronutrient status in men and women over 75 years old living in the community. Br J Nutr. 2002;88(5):555–561. doi: 10.1079/BJN2002706. [DOI] [PubMed] [Google Scholar]
- 27.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91(5):1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dam RM, Snijder MB, Dekker JM, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85(3):755–761. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 29.van der Meer IM, Boeke AJ, Lips P, et al. Fatty fish and supplements are the greatest modifiable contributors to the serum 25-hydroxyvitamin D concentration in a multiethnic population. Clin Endocrinol. 2008;68(3):466–472. doi: 10.1111/j.1365-2265.2007.03066.x. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 31.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 32.Harris SS, Soteriades E, Coolidge JA, et al. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 33.Erkal MZ, Wilde J, Bilgin Y, et al. High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk factors. Osteoporos Int. 2006;17(8):1133–1140. doi: 10.1007/s00198-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 34.Brock K, Huang WY, Fraser DR, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121(1–2):462–466. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanri A, Foo LH, Nakamura K, et al. Serum 25-hydroxyvitamin D concentrations and season-specific correlates in Japanese adults. J Epidemiol. 2011;21(5):346–353. doi: 10.2188/jea.JE20100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shea MK, Houston DK, Tooze JA, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc. 2011;59(7):1165–1174. doi: 10.1111/j.1532-5415.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies JR, Chang YM, Snowden H, et al. The determinants of serum vitamin D levels in participants in a melanoma case-control study living in a temperate climate. Cancer Causes Control. 2011;22(10):1471–1482. doi: 10.1007/s10552-011-9827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Choi HS, Oh HJ, Choi H, et al. Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96(3):643–651. doi: 10.1210/jc.2010-2133. [DOI] [PubMed] [Google Scholar]
- 41.Gozdzik A, Barta JL, Wu H, et al. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health. 2008;8:336. doi: 10.1186/1471-2458-8-336. ( doi:10.1186/1471-2458-8-336). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi T, Okano T, Shida S, et al. Variation of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 levels in human plasma obtained from 758 Japanese healthy subjects. J Nutr Sci Vitaminol (Tokyo) 1983;29(3):271–281. doi: 10.3177/jnsv.29.271. [DOI] [PubMed] [Google Scholar]
- 44.Boice JD, Jr, Mandel JS, Doody MM, et al. A health survey of radiologic technologists. Cancer. 1992;69(2):586–598. doi: 10.1002/1097-0142(19920115)69:2<586::aid-cncr2820690251>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.National Aeronautics and Space Administration. TOMS Erythemal UV Exposure. Washington, DC: National Aeronautics and Space Administration; 2005. (http://ozoneaq.gsfc.nasa.gov/TOMSUVExposure.md. ). (Accessed March 4, 2011) [Google Scholar]
- 46.Major JM, Graubard BI, Dodd DW, et al. Variability and reproducibility of circulating vitamin D in a nationwide U.S. population. J Clin Endocrin Metabol. doi: 10.1210/jc.2012-2643. [published onle ahead of print November 8, 2012] ( doi:10:1210/jc.2012-2643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Office of Dietary Supplements. Dietary Supplement Fact Sheet: Vitamin D. Bethesda MD: National Institutes of Health; 2011. (http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ ). (Accessed March 4, 2011) [Google Scholar]
- 48.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B. 1964;26(2):211–252. [Google Scholar]
- 50.Egan KM, Sosman JA, Blot WJ. Sunlight and reduced risk of cancer: is the real story vitamin D? J Natl Cancer Inst. 2005;97(3):161–163. doi: 10.1093/jnci/dji047. [DOI] [PubMed] [Google Scholar]
- 51.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2(8671):1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 53.Need AG, Morris HA, Horowitz M, et al. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58(6):882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 54.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Looker AC. Body fat and vitamin D status in black versus white women 1. J Clin Endocrinol Metab. 2005;90(2):635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 56.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 57.Aloia JF, Vaswani A, Ma R, et al. Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997;129(3):294–299. doi: 10.1016/s0022-2143(97)90177-3. [DOI] [PubMed] [Google Scholar]
- 58.Thieden E, Jorgensen HL, Jorgensen NR, et al. Sunbed radiation provokes cutaneous vitamin D synthesis in humans—a randomized controlled trial. Photochem Photobiol. 2008;84(6):1487–1492. doi: 10.1111/j.1751-1097.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 59.Bell NH, Godsen RN, Henry DP, et al. The effects of muscle-building exercise on vitamin D and mineral metabolism. J Bone Miner Res. 1988;3(4):369–373. doi: 10.1002/jbmr.5650030402. [DOI] [PubMed] [Google Scholar]
- 60.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.