Abstract
Objective:
There are few detailed data on cognition in patients undergoing dialysis. We evaluated the frequency of and risk factors for poor cognitive performance using detailed neurocognitive testing.
Methods:
In this cross-sectional cohort study, 314 hemodialysis patients from 6 Boston-area hemodialysis units underwent detailed cognitive assessment. The neuropsychological battery assessed a broad range of functions, with established age-, sex-, and education-matched normative scores. Principal component analysis was used to derive composite scores for memory and executive function domains. Risk factors for each domain were evaluated using linear regression adjusting for age, sex, race, and education status. Analyses were repeated in those with Mini-Mental State Examination (MMSE) score ≥24.
Results:
Compared with population norms, patients on dialysis had significantly poorer executive function but not memory performance, a finding that persisted in the subgroup with MMSE score ≥24. In adjusted analyses, vascular risk factors and vascular disease were associated with lower executive function (p < 0.01).
Conclusions:
There is a high frequency of poor cognitive performance in hemodialysis patients, primarily affecting executive function. Risk factors for worse executive function include vascular risk factors as well as vascular disease. Normal performance on the MMSE does not preclude impaired cognitive function, because individuals with MMSE score ≥24 also have a high frequency of poor cognitive performance.
Cognitive impairment in dialysis is an increasingly important public health problem given the aging end-stage renal disease (ESRD) population and the increasing prevalence of diabetes and vascular disease. In older studies in hemodialysis patients, cognitive impairment, defined by poor performance on the Mini-Mental State Examination (MMSE), was present in 40% to 60%.1–3 Despite the fact that the MMSE remains the most frequently used screening tool for cognitive impairment, it focuses on memory and largely neglects other cognitive domains such as executive function; accordingly, the MMSE may not be sufficient to detect more subtle degrees of cognitive impairment.
The major causes of dementia in the general population are Alzheimer disease, which initially manifests with memory loss with later involvement of other cognitive domains, and vascular dementia, which primarily manifests with impairment in executive function.4–7 Although it is likely that patients undergoing dialysis have similar causes for cognitive impairment as the general population, there are few studies that have attempted to distinguish the prevalence of and risk factors for each type of cognitive impairment in this population.
The goals of this study were therefore to evaluate the frequency of and risk factors for poor cognitive performance in hemodialysis patients using detailed measures of multiple cognitive domains, to compare these data with general population norms, and, in patients with MMSE score ≥24, to assess the proportion with cognitive impairment using more detailed testing.
METHODS
Outpatients older than 18 years receiving chronic in-center hemodialysis at 5 Dialysis Clinic Inc. (DCI) units and 1 hospital-based unit (St. Elizabeth's Medical Center) in the greater Boston area were screened for the Cognition and Dialysis Study. Reflecting the nature of the cognitive battery, eligibility criteria included English fluency as well as sufficient visual and hearing acuity to complete cognitive testing. To minimize cognitive testing floor effects and reflecting inability to provide consent, individuals with MMSE score ≤10 and/or advanced dementia based on medical record review were excluded. Nonaccess-related hospitalization within 1 month, receipt of hemodialysis for <1 month, and single-pool Kt/V (a measure of dialysis dose) <1.0 were temporary exclusion criteria.
Standard protocol approvals, registrations, and patient consents
The Tufts Medical Center/Tufts University Institutional Review Board approved the study and all participants who completed the detailed cognitive testing signed informed consent.
Neuropsychological assessment
Participants were administered a battery of cognitive tests by research assistants after training and direct observation by the study neuropsychologist (Dr. Scott). To maintain quality and interrater reliability, testing was observed by the study neuropsychologist at 3- to 6-month intervals. To limit subject fatigue, all testing was completed during the first hour of hemodialysis. When possible, we also performed neurocognitive testing in a private room or in as quiet an environment as possible. The neuropsychological battery included well-validated frequently used cognitive tests that possess high inter- and intrarater reliability and have established age-, sex-, and/or education-matched normative scores. The MMSE8 was used as a screening test, and the North American Adult Reading Test served as a measure of premorbid verbal IQ.9 The neurocognitive battery consisted of the Wechsler Memory Scale-III Word List Learning Subtest,10 the Wechsler Adult Intelligence Scale-III Block Design10 and Digit Symbol-Coding Subtests,10 and Trail Making Tests A and B11 (Trails A and B) (table e-1 on the Neurology® Web site at www.neurology.org). During the last 2 years of the study, the cognitive panel was expanded to include additional verbal tests assessing both memory and executive functions, including Digit Span (forwards and backwards),10 the Mental Alternation Test,12 and the Controlled Oral Word Association Test (COWAT).13 The overall battery assesses a broad range of functioning including global ability, supraspan learning, auditory retention, visual retention, attention/mental processing speed, visual construction/fluid reasoning, and motor speed. We also evaluated depression using the Center for Epidemiological Studies Depression Scale (CESD).14
Factors associated with cognitive impairment
Demographic, clinical, and laboratory factors were ascertained at the time of cognitive testing. Demographic data (age, sex, and race) were obtained via participant report, medical charts, and the DCI and St. Elizabeth's Medical Center databases. Education (<12th grade, high school graduate, and ≥2 years of college) was obtained via patient questionnaire. Medical history, including myocardial infarction and coronary revascularization (which were used to define coronary disease), peripheral vascular disease, stroke, heart failure, presence of diabetes, hypertension, and smoking, were defined by patient history or documentation in the patient's electronic or paper chart. Additionally, DCI electronic medical records and paper records were reviewed for a history of these conditions with specific focus on problem lists, hospital discharge summaries, cardiac testing results, and procedure results. Cause of ESRD and dialysis vintage were obtained from the DCI or St. Elizabeth's electronic record as were physical examination findings of mean monthly systolic and diastolic blood pressures and body mass index. Predialysis blood tests included albumin, hematocrit, phosphorus, intact parathyroid hormone, white blood cell (WBC) count, C-reactive protein, and single-pool Kt/V. All DCI laboratory tests were measured in a central laboratory in Nashville, TN.
Statistical analysis
Continuous variables were presented as mean (SD) or median (interquartile range) as appropriate and categorical variables as proportions. The study population was compared with eligible but nonrecruited dialysis patients using χ2 tests for categorical data and t tests and the nonparametric Wilcoxon test for continuous data. Test scores in participants with and without history of stroke were standardized for age, sex, and education where appropriate before comparison with population-based normative data. The percentage of dialysis patients with test scores >1, >1.5, and >2 SDs below expected population norms was reported. A 1-sample t test was used to evaluate differences between dialysis patients and normative data. For the Mental Alternation Test and the COWAT, normal values were extrapolated from published data, with impairment defined as a score <15 on the Mental Alternation Test15–17 and below the age- and education-adjusted 25th percentile fluency scores for the COWAT.15,18
Principal component analysis (PCA) with varimax rotation was used as a data-reduction technique to derive composite scores for separate cognitive domains in the entire study population.19 Two principal components with eigenvalues >2 were obtained, and the resulting component scores were subsequently used as a dependent variable in linear regression analyses. The first component was primarily comprised of Word List Learning Recall and Recognition and was interpreted to reflect memory (table e-1). The second component was interpreted to reflect executive functioning, attention, and processing speed (referred to as executive function within the Results section), with Trails A and B, Block Design, and Digit Symbol-Coding tests contributing substantially. Digit Span, Mental Alternation Test, and the COWAT were not used in calculation of the PCA because of the smaller number of individuals who completed these tests. There were 274 individuals with complete testing on Trails A and B, Blocks, Digit Symbol, and components of the Word List Learning Subtest. For 18 individuals who were missing results on 1 cognitive test (or 2 results if derived from the same test), single-item imputation was performed using multivariable linear regression models based on performance on other tests in the cognitive battery. These imputations results were incorporated to derive the PCA but were not used for evaluating performance on individual cognitive tests. The total number for the PCA analysis was therefore 292. The relationship between risk factors and cognitive domains was assessed in multivariable linear regression models adjusted for age, sex, race, and education. All analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC). Differences were considered statistically significant at p values <0.05.
Sensitivity analyses
Analyses were repeated excluding patients with a history of stroke and excluding patients with MMSE score <24. Because some cognitive testing may be dependent on manual dexterity that is hindered during hemodialysis because of arteriovenous access in the dominant arm, we performed a sensitivity analysis limiting the study population to those individuals who performed testing using their dominant hand in an unencumbered manner. In additional analyses, we adjusted for depression (CESD) because it may have an effect on cognitive function. Furthermore, we evaluated the shape of the relationship of dialysis vintage with cognitive function and adjusted for nonlinear effects in multivariable analyses.
RESULTS
Baseline information
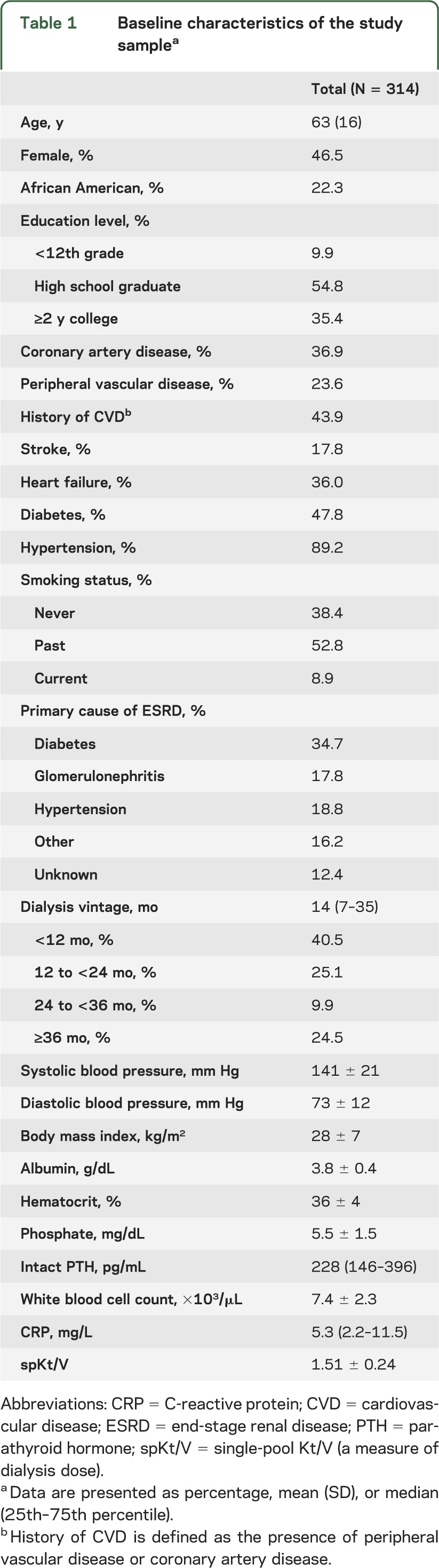
Among 929 patients screened, 414 were ineligible for complete cognitive testing (figure). Of the remaining 515 individuals, 314 underwent more detailed cognitive testing. Patients who were eligible but did not enroll were slightly older (aged 66 vs 63 years, p = 0.05) and had slightly lower serum albumin (3.7 vs 3.8 g/dL, p = 0.01), but otherwise had similar demographic and clinical characteristics. The mean age of study participants was 63 years; 22% were African American, 46% were women, and 90% had a high school or higher education level (table 1).
Figure. Flow diagram of enrolled patients.
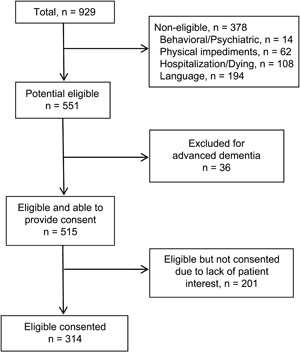
Table 1.
Baseline characteristics of the study samplea
Comparisons with normative data
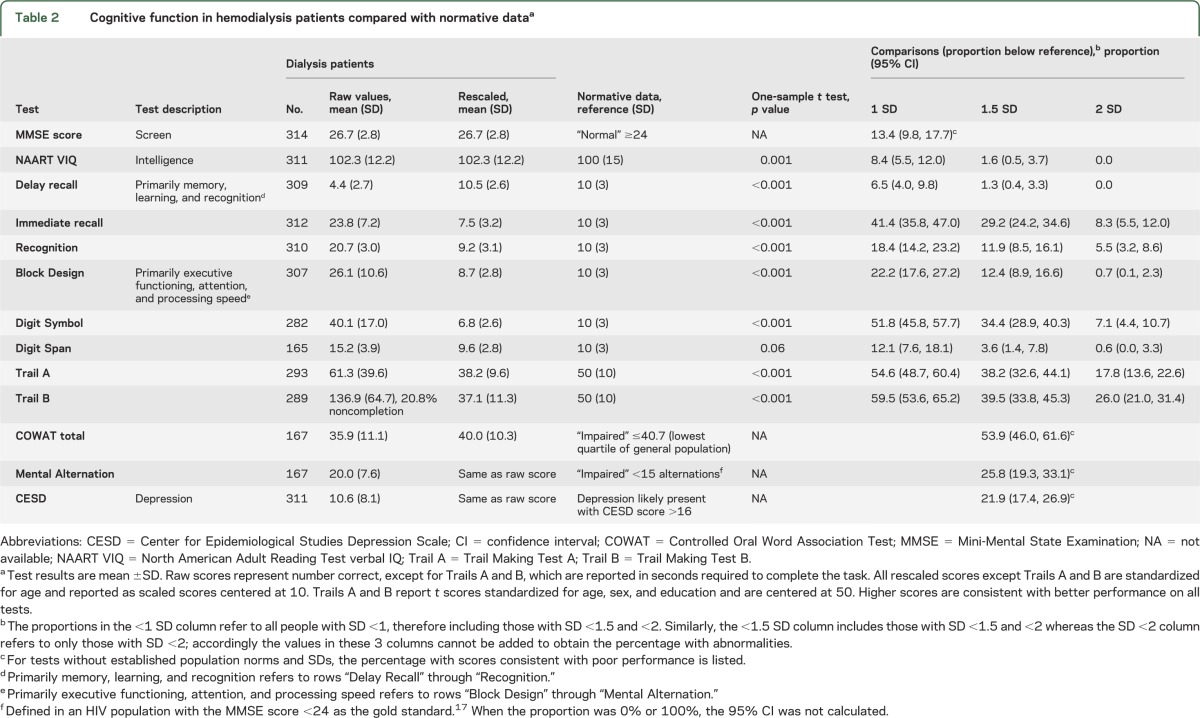
Mean (SD) MMSE score was 26.7 (2.8), with 42 participants (13.4%) scoring <24. Of these, 17 had MMSE scores ≤21 and 6 had MMSE scores ≤18. Because an additional 36 dialysis patients were excluded based on advanced dementia, a total of 22% (36 + 42 of 350) of potentially eligible hemodialysis patients therefore had cognitive impairment defined by MMSE score <24.
When considering cognitive tests that associate primarily with memory processes, it was noted that performance on delayed recall was slightly higher in hemodialysis patients in comparison with general population norms (table 2), whereas performance on immediate recall and recognition was significantly lower in hemodialysis patients. When considering cognitive tests that associate primarily with executive processes, results on all tests were significantly lower in hemodialysis patients compared with general population norms, with as many as 30% to 40% of dialysis patients having performance on the Digit Symbol Substitution Test and Trails A and B >1.5 SDs below general population norms (table 2).
Table 2.
Cognitive function in hemodialysis patients compared with normative dataa
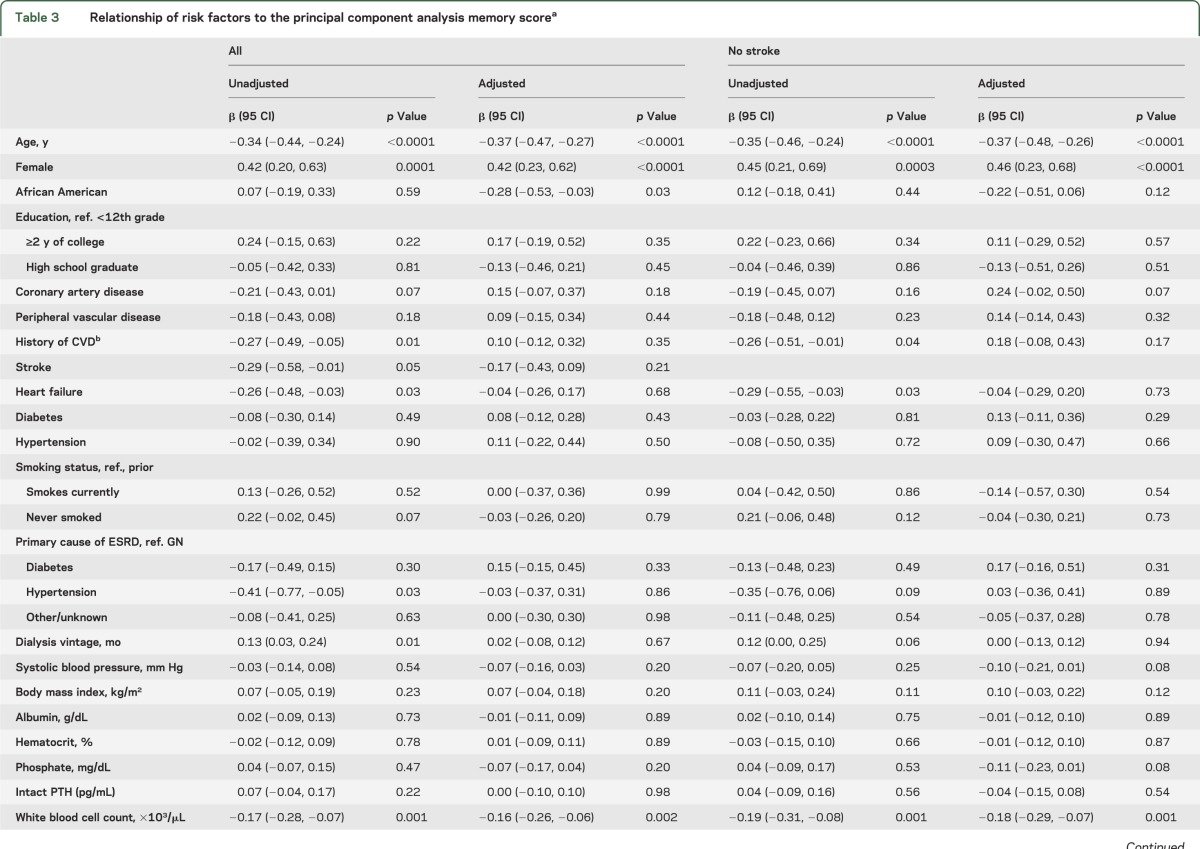
Factors associated with poorer memory defined by PCA
In univariate analyses, older age, male sex, different forms of vascular disease, and higher WBC count were associated with lower levels of memory by PCA score. After adjustment for age, sex, race, and education, only higher WBC count and more depression symptoms as assessed by the CESD were significantly associated with lower memory by PCA score (table 3).
Table 3.
Relationship of risk factors to the principal component analysis memory scorea

Factors associated with poorer executive function defined by PCA
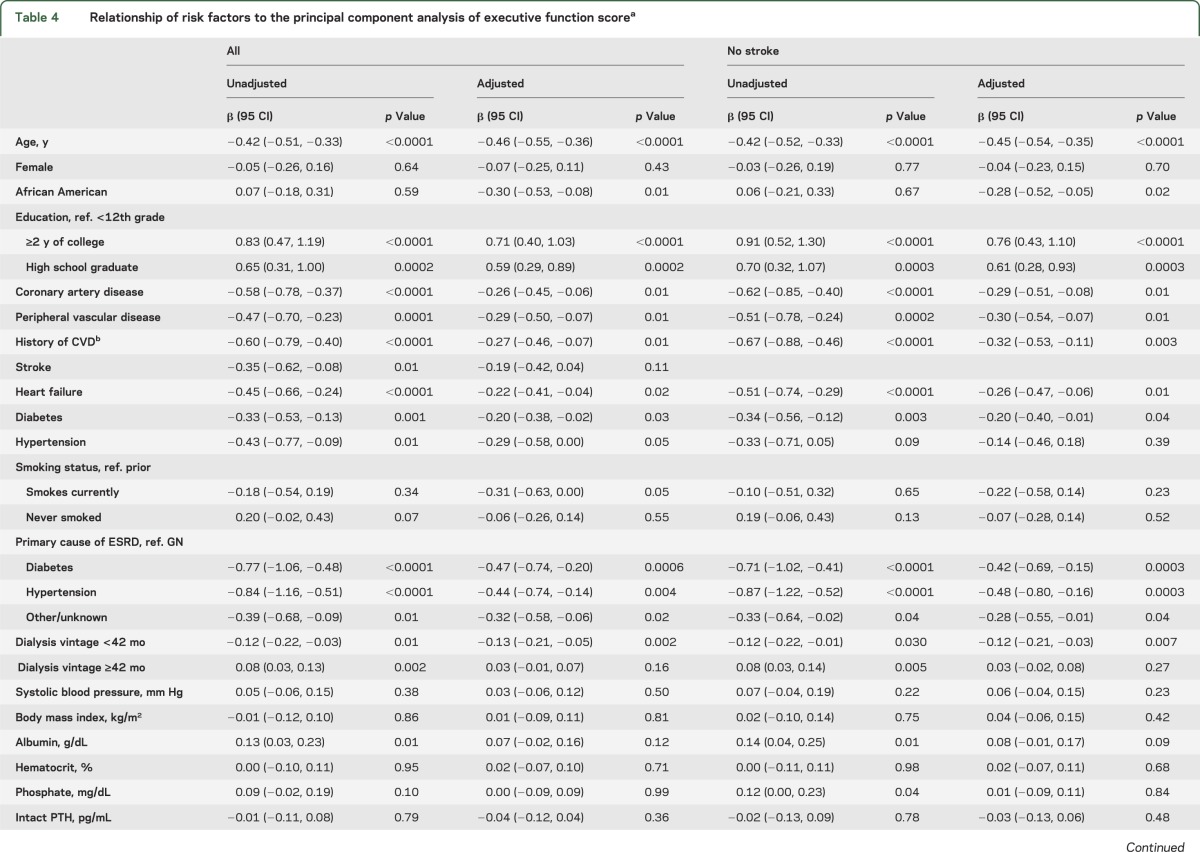
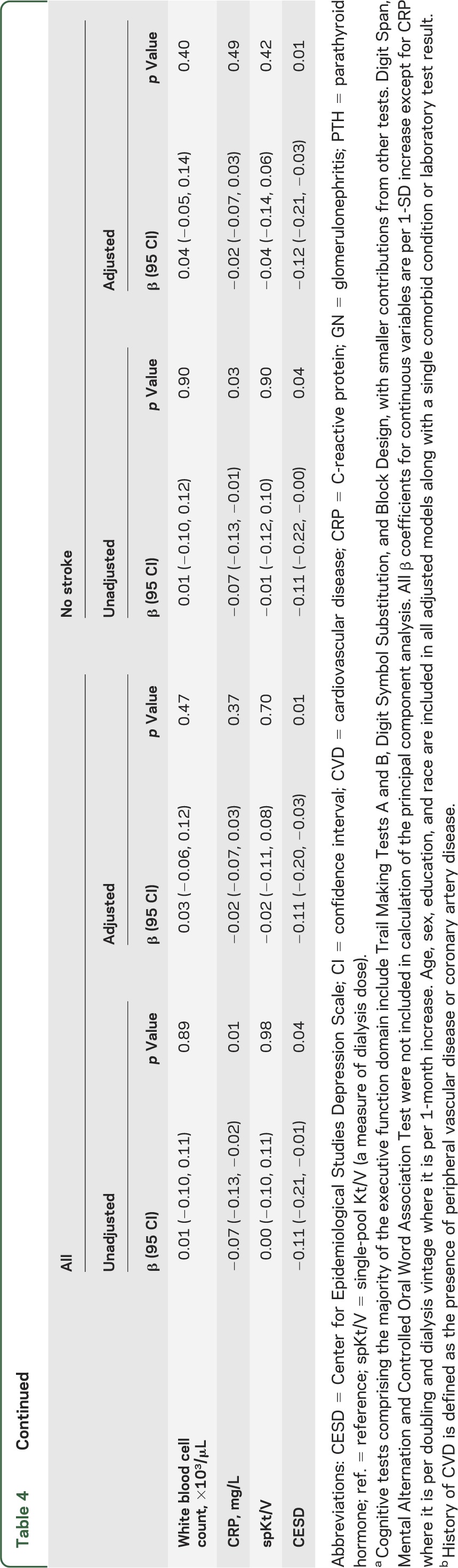
In univariate analyses, older age, lower levels of education, presence of diabetes, either diabetes or hypertension as the cause of ESRD, lower serum albumin, and various forms of vascular disease were associated with decreased performance on tests of executive function. In analyses adjusting for age, sex, race, and education, a history of diabetes, having diabetes or hypertension as the cause of ESRD, peripheral vascular disease, coronary artery disease, heart failure, and more depression symptoms were all associated with lower executive function (table 4).
Table 4.
Relationship of risk factors to the principal component analysis of executive function scorea
Sensitivity analyses
Results were essentially unchanged if those with stroke were excluded (tables 3 and 4, table e-2) and if only those with MMSE score ≥24 were included (table e-3). After excluding 27 individuals with dialysis access in their dominant arm and 47 individuals with information on dominant arm missing, results were essentially unchanged in the remaining 240 participants. Although higher CESD levels were associated with worse executive function, additional adjustment for CESD did not significantly change the importance of other risk factors in adjusted analyses (data not shown). Dialysis vintage had no significant relationship with memory, but vintage was associated with executive function in a model incorporating 2 slopes. That is, below 42 months, longer vintage was associated with worse executive function, whereas beyond 42 months no association with vintage was noted (table 4). Adjustment for dialysis vintage using the 2-slope model did not significantly change the importance of other risk factors in adjusted analyses (data not shown).
DISCUSSION
In this study, we demonstrate a high frequency of cognitive impairment in hemodialysis patients in comparison with general population normative data. Impairment is present despite preserved MMSE scores and is particularly manifest in tasks that reflect executive function domains. Although there are few independent risk factors for the memory component of the cognitive function, the presence of vascular disease risk factors and vascular disease is associated with impairment in domains spanning executive functions.
This is one of the largest studies of which we are aware that has evaluated detailed measures of cognitive function in hemodialysis patients. Our results confirm prior studies that have demonstrated a high prevalence of cognitive impairment in this population.3,20–22 In one study, the MMSE was administered to 336 dialysis patients: 22% of subjects had mild impairment (MMSE scores 18–23) and 8% had moderate-severe impairment (MMSE scores 0–17).3 In another study evaluating 338 hemodialysis patients, 14% were classified with mild impairment, 36% with moderate impairment, 37% with severe impairment, and 13% with normal cognition. Cognitive impairment was associated with low education, higher Kt/V, and a history of stroke.22 In the Frequent Hemodialysis Network, impaired executive function, defined by failure to complete the Trail Making B task within 5 minutes, was common among hemodialysis patients but was not strongly associated with patient or dialysis-associated factors.21 Our study substantially adds to these by performing detailed cognitive testing, focusing on both memory and executive function, evaluating a wide range of risk factors for each form of cognitive impairment, and comparing the results to validated normative data. We also demonstrate that, even among those with MMSE score ≥24, there is a high frequency of more subtle degrees of cognitive impairment. Previously, in a pilot study of 25 dialysis patients, we noted that despite preserved MMSE score (≥24), hemodialysis patients had significant cognitive impairment.23 We have now extended this result to a much larger and more generalizable population. Patients on average had preserved premorbid verbal IQ (as ascertained by the North American Adult Reading Test), suggesting that the declines in cognitive function likely occurred through the course of their illness.
This study does not demonstrate that dialysis per se is a risk factor for cognitive impairment, but rather that dialysis patients (even those with preserved MMSE score) have a high frequency of poor performance on cognitive testing. The only way the former question could be addressed is by having a control group of individuals who do not have kidney disease but have a similar prevalence of vascular disease and vascular risk factors as dialysis patients. We are not aware of large studies performing similar cognitive tests that meet these inclusion criteria.
Executive function seems to be affected to a greater extent than memory in dialysis patients. The latter is consistent with the hypothesis that vascular disease, whether due to atherosclerosis or arteriosclerosis, may be the primary cause of cognitive impairment in this population. The finding that risk factors for poorer executive function include vascular disease itself and risk factors for vascular disease supports this hypothesis. We had previously demonstrated that vascular disease, as assessed by either coronary disease or peripheral vascular disease, is associated with abnormalities in executive function in hemodialysis patients.24 The current study adds to the latter by including a larger number of individuals, evaluating many potential risk factors for cognitive impairment, providing detailed comparisons with normative data, and incorporating additional cognitive tests that were assessed in nearly half of the study participants.
Our study has several strengths. These include a relatively large cohort focused on cognitive function incorporating detailed ascertainment of both cognitive function as well as its potential risk factors. The study also has several limitations. Although the population is generalizable to the US Renal Data System dialysis population from a demographic, vascular disease prevalence, and laboratory result standpoint,25 it excludes non-English speakers and acutely ill individuals, and includes participants who, overall, are more educated than the US ESRD population.3,26,27 Our cohort consisted of patients who were on dialysis for less time than the US ESRDS population. Although longer vintage was associated with worse executive function in the first 42 months of dialysis, after 42 months there was no significant relationship. The relationship of vintage with cognitive function likely is affected by survival bias. Given the exclusion of acutely ill individuals and those with severe baseline dementia, the frequencies of cognitive impairment in our study likely underestimate the true prevalence of cognitive impairment among hemodialysis patients. Critically, recognition of cognitive impairment and appreciation of the risk factors for early cognitive impairment are important for both future planning as well as implementation of interventions to address cognitive performance in dialysis patients. Although we hypothesize that vascular disease, whether it be macro- or micro-cerebrovascular disease, is the cause of worse performance in executive functioning domains, we do not have imaging studies to confirm this hypothesis. We performed cognitive testing during the dialysis procedure. The dialysis unit can be a noisy environment potentially leading to distractions and the dialysis procedure itself could possibly lead to delirium that affects performance on cognitive tests.28–33 However, we have recently demonstrated that cognitive performance is not affected by the time of testing. In a randomized crossover study of 40 patients, performance on cognitive testing was no different 1 hour before dialysis vs the first hour of the dialysis treatment.34 It is also important to recognize that most patient education and contact with medical practitioners occur during dialysis treatments, making this a critical time for communication. Additionally, reflecting the cross-sectional design, determining cause-and-effect relationships is not possible. Finally, we have not evaluated several nontraditional vascular and nonvascular risk factors that may contribute to cognitive impairment.
There are important implications of our results. Health care providers should be aware of the high prevalence of cognitive impairment when providing care to dialysis patients. Given the high prevalence of cognitive impairment in maintenance hemodialysis patients, if possible, important educational interactions and discussions on end-of-life care should be initiated during the earlier stages of kidney disease. Furthermore, health care proxies should be involved in decision-making. One should be cautious in using an MMSE score <24 to screen for cognitive impairment in dialysis patients because many patients with MMSE score ≥24 also have more subtle degrees of cognitive impairment. Finally, interventions to decrease vascular disease should be evaluated to reduce the prevalence of cognitive impairment in patients undergoing dialysis.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the tremendous assistance of Dialysis Clinic, Inc. and, in particular, the staff and patients at the 5 DCI units in the Boston area and St. Elizabeth's Dialysis Unit without whose cooperation the study would not have been successful.
GLOSSARY
- CESD
Center for Epidemiological Studies Depression Scale
- COWAT
Controlled Oral Word Association Test
- DCI
Dialysis Clinic Inc.
- ESRD
end-stage renal disease
- MMSE
Mini-Mental State Examination
- PCA
principal component analysis
- WBC
white blood cell
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Mark Sarnak: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. Hocine Tighiouart: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Tammy Scott: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Kristina Lou, Eric Sorensen, and Lena Giang: drafting/revising the manuscript, acquisition of data, study supervision. David Drew: drafting/revising the manuscript. Kamran Shaffi: drafting/revising the manuscript, study supervision. James Strom: drafting/revising the manuscript, analysis or interpretation of data, study supervision. Ajay Singh: drafting/revising the manuscript, contribution of vital reagents/tools/patients, study supervision. Daniel Weiner: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision.
STUDY FUNDING
The study was funded by the NIDDK through grants R21 DK068310 (M.J.S.), R01 DK078204 (M.J.S.), and K23 DK71636 (D.E.W.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci 1995;134:83–88 [DOI] [PubMed] [Google Scholar]
- 2.Kutlay S, Nergizoglu G, Duman N, et al. Recognition of neurocognitive dysfunction in chronic hemodialysis patients. Ren Fail 2001;23:781–787 [DOI] [PubMed] [Google Scholar]
- 3.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 1997;30:41–49 [DOI] [PubMed] [Google Scholar]
- 4.Cannata AP, Alberoni M, Franceschi M, Mariani C. Frontal impairment in subcortical ischemic vascular dementia in comparison to Alzheimer's disease. Dement Geriatr Cogn Disord 2002;13:101–111 [DOI] [PubMed] [Google Scholar]
- 5.Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology 1999;53:670–678 [DOI] [PubMed] [Google Scholar]
- 6.Roman GC. Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc 2003;51:S296–S304 [DOI] [PubMed] [Google Scholar]
- 7.Traykov L, Baudic S, Thibaudet MC, Rigaud AS, Smagghe A, Boller F. Neuropsychological deficit in early subcortical vascular dementia: comparison to Alzheimer's disease. Dement Geriatr Cogn Disord 2002;14:26–32 [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 9.Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychologist 1989;3:129–136 [Google Scholar]
- 10.Tulsky D, Zhu J, Lebetter M. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III), Wechsler Memory Scale-Third Scale (WMS-III): Technical Manual. San Antonio: Harcourt Brace & Company; 1997 [Google Scholar]
- 11.Heaton R, Grant I, Mathews C. Comprehensive Norms for an Expanded Halstead-Reitan Battery. Odessa: Psychological Assessment Resources Inc.; 1991 [Google Scholar]
- 12.Salib E, McCarthy J. Mental Alternation Test (MAT): a rapid and valid screening tool for dementia in primary care. Int J Geriatr Psychiatry 2002;17:1157–1161 [DOI] [PubMed] [Google Scholar]
- 13.Benton A, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1978 [Google Scholar]
- 14.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 15.Sorensen EP, Sarnak MJ, Tighiouart H, et al. The kidney disease quality of life cognitive function subscale and cognitive performance in maintenance hemodialysis patients. Am J Kidney Dis 2012;60:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billick SB, Siedenburg E, Burgert W, III, Bruni-Solhkhah SM. Validation of the Mental Alternation Test with the Mini-Mental State Examination in geriatric psychiatric inpatients and normal controls. Compr Psychiatry 2001;42:202–205 [DOI] [PubMed] [Google Scholar]
- 17.Jones BN, Teng EL, Folstein MF, Harrison KS. A new bedside test of cognition for patients with HIV infection. Ann Intern Med 1993;119:1001–1004 [DOI] [PubMed] [Google Scholar]
- 18.Troyer AK. Normative data for clustering and switching on verbal fluency tasks. J Clin Exp Neuropsychol 2000;22:370–378 [DOI] [PubMed] [Google Scholar]
- 19.Heyer NJ, Bittner AC, Jr, Echeverria D. Analyzing multivariate neurobehavioral outcomes in occupational studies: a comparison of approaches. Neurotoxicol Teratol 1996;18:401–406 [DOI] [PubMed] [Google Scholar]
- 20.Brady CB, Gaziano JM, Cxypoliski RA, et al. Homocysteine lowering and cognition in CKD: the Veterans Affairs homocysteine study. Am J Kidney Dis 2009;54:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol 2011;5:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology 2006;67:216–223 [DOI] [PubMed] [Google Scholar]
- 23.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int 2007;11:309–314 [DOI] [PubMed] [Google Scholar]
- 24.Weiner DE, Scott TM, Giang LM, et al. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 2011;58:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 2010;55:S1–A7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaeffner ES, Mehta J, Winkelmayer WC. Educational level as a determinant of access to and outcomes after kidney transplantation in the United States. Am J Kidney Dis 2008;51:811–818 [DOI] [PubMed] [Google Scholar]
- 27.Khattak M, Sandhu GS, Desilva R, Goldfarb-Rumyantzev AS. Association of education level with dialysis outcome. Hemodial Int 2012;16:82–88 [DOI] [PubMed] [Google Scholar]
- 28.Murray AM, Pederson SL, Tupper DE, et al. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis 2007;50:270–278 [DOI] [PubMed] [Google Scholar]
- 29.Gallai V, Alberti A, Buoncristiani U, Firenze C, Mazzotta G. Changes in auditory P3 event-related potentials in uremic patients undergoing haemodialysis. Electromyogr Clin Neurophysiol 1994;34:397–402 [PubMed] [Google Scholar]
- 30.Lewis EG, O'Neill WM, Dustman RE, Beck EC. Temporal effects of hemodialysis on measures of neural efficiency. Kidney Int 1980;17:357–363 [DOI] [PubMed] [Google Scholar]
- 31.Smith BC, Winslow EH. Cognitive changes in chronic renal patients during hemodialysis. ANNA J 1990;17:283–286 [PubMed] [Google Scholar]
- 32.Tennyson TE, Brown WS, Vaziri ND, Jennison JH. Event-related potential changes during hemodialysis. Int J Artif Organs 1985;8:269–276 [PubMed] [Google Scholar]
- 33.Williams MA, Sklar AH, Burright RG, Donovick PJ. Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis 2004;43:705–711 [DOI] [PubMed] [Google Scholar]
- 34.Drew D, Tighiouart H, Scott T, et al. Cognitive performance before and during hemodialysis: a randomized crossover trial. American Society of Nephrology Annual Meeting, San Diego, 2012, Abstract PO270
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


