Abstract
Current criteria for the clinical diagnosis of pathologically confirmed corticobasal degeneration (CBD) no longer reflect the expanding understanding of this disease and its clinicopathologic correlations. An international consortium of behavioral neurology, neuropsychology, and movement disorders specialists developed new criteria based on consensus and a systematic literature review. Clinical diagnoses (early or late) were identified for 267 nonoverlapping pathologically confirmed CBD cases from published reports and brain banks. Combined with consensus, 4 CBD phenotypes emerged: corticobasal syndrome (CBS), frontal behavioral-spatial syndrome (FBS), nonfluent/agrammatic variant of primary progressive aphasia (naPPA), and progressive supranuclear palsy syndrome (PSPS). Clinical features of CBD cases were extracted from descriptions of 209 brain bank and published patients, providing a comprehensive description of CBD and correcting common misconceptions. Clinical CBD phenotypes and features were combined to create 2 sets of criteria: more specific clinical research criteria for probable CBD and broader criteria for possible CBD that are more inclusive but have a higher chance to detect other tau-based pathologies. Probable CBD criteria require insidious onset and gradual progression for at least 1 year, age at onset ≥50 years, no similar family history or known tau mutations, and a clinical phenotype of probable CBS or either FBS or naPPA with at least 1 CBS feature. The possible CBD category uses similar criteria but has no restrictions on age or family history, allows tau mutations, permits less rigorous phenotype fulfillment, and includes a PSPS phenotype. Future validation and refinement of the proposed criteria are needed.
When first described, “corticodentatonigral degeneration with neuronal achromasia” was considered a distinct clinicopathologic entity,1 eventually termed corticobasal degeneration (CBD).2 Clinicopathologic studies have since revealed that the originally described clinical features of CBD, now called corticobasal syndrome (CBS), are often due to other pathologies. As a pathologic diagnosis, CBD is characterized by widespread deposition of hyperphosphorylated 4-repeat tau in neurons and glia, the latter as astrocytic plaques, in specific topographic areas.3 Despite various clinical diagnostic criteria (table e-1 on the Neurology® Web site at www.neurology.org),4–10 the pathology of CBD is predicted antemortem in only 25% to 56% of cases.11–17 Additionally, while these clinical criteria continue to be widely applied and cited, they reflect CBS alone and not the more recently recognized behavioral presentations of CBD.
Definition and standardization of clinical diagnostic criteria for CBD are critical, especially as potential neuroprotective therapies for tauopathies emerge. In light of advances in the understanding of CBD, we used specialist consensus, brain bank cases, and a critical literature review to develop new diagnostic criteria. During this process, however, it became clear that clinicopathologic heterogeneity of CBD confounds the development of specific criteria, unlike what has been accomplished for other neurodegenerative diseases. Thus, we propose 2 sets of criteria: a narrower, more specific one for probable CBD and a broader set for possible CBD that has less specificity for CBD pathology while still representing probable tau-based pathology.
METHODS
Previous CBD clinical diagnostic criteria were reviewed. Invited international specialists in behavioral neurology, neuropsychology, and movement disorders met on October 14–15, 2009, and based on participants' experience and literature reviews, clinical phenotypes were identified and CBD criteria were drafted.
Subsequently, a systematic literature search and later update using MEDLINE (1950 to April 2012) and EMBASE (1980 to April 2012) identified English-language pathologically proven CBD series. Search terms included “corticobasal,” “corticobasal degeneration,” and “CBD” text word searches paired with “pathology” as a MeSH search term and text word search. Inclusion criteria were 1) a minimum of 5 pathologically proven CBD cases (chosen a priori to avoid the bias toward atypical cases from case reports) and 2) extractable data for clinical phenotype, symptoms/features, or both. Only patients with pathologically proven CBD were included. Inclusion criteria were intentionally broad to enable a large sample size and decrease the impact of ascertainment bias and variations in CBD feature reporting. Information was entered into a database including commonly reported features and those deemed relevant by the panel. Publication authors and institutions were cross-checked to prevent case duplication. Additionally, 5 centers with CBD brain bank cases provided data on published and unpublished cases. When available, the original brain bank data were abstracted rather than using the less comprehensive information from brain bank–related publications. Two overlapping sets of cases were developed: cases for which features of CBD could be extracted and cases for which information was available regarding clinical diagnosis or phenotype.
Clinical features were recorded at 2 time parameters, at presentation and “ever” (during the disease course), variables for which the most consistent data could be abstracted. Presentation was a mean of 3.0 (SD 1.9) years after symptom onset in one series.18 When data were abstracted, features were considered as present or absent only if specifically described. While this approach carries the risk of overestimating the frequency of each feature, it was thought suitable due to the complexity of CBD and the varying degrees of detail in the retrospective data. Because of this approach, the denominator for calculating the frequency of any given clinical feature is less than the total number of cases identified, reflecting the number of cases for which that feature was reported. Finally, a literature search was conducted for clinicopathologic correlation articles including CBD subjects and cases with other proven pathologies to identify specific clinical features that might improve the accuracy of proposed CBD criteria vs other pathologic diagnoses. Results of the specialist panel, case reviews, and clinicopathologic studies were integrated into the proposed criteria. A glossary of terms is available in appendix e-1.
RESULTS
Previous clinical diagnostic criteria
While self-described as criteria for CBD, previous diagnostic criteria (table e-1)4–10 outline the clinical features now labeled CBS, reflecting an asymmetric movement disorders presentation combined with lateralized higher cortical features. Consideration of the role of dementia in diagnostic criteria exemplifies changes in our understanding of CBS and CBD. Previous clinical criteria excluded “early dementia” to increase diagnostic specificity,19 but dementia is now recognized as a presenting and predominant feature in many cases of CBD.13,15,20
Systematic literature review
Of 808 nonoverlapping articles identified in the systematic literature search, 37 met inclusion criteria. Clinical features were available for 103 published13,15,16,18,21–24 and 106 brain bank nonoverlapping CBD cases. Brain bank case information was provided by Mayo Clinic Rochester (22 patients, K. Josephs, personal communication, 2011), University of Western Ontario (8 patients, A. Kertesz and P. McMonagle, personal communication, 2011), University of California San Francisco (20 patients, S.E. Lee, personal communication, 2011), and Mayo Clinic Jacksonville (53 patients, D.W. Dickson, personal communication, 2011). Information on 3 unpublished cases was provided by University of Pennsylvania (P. Moore and M. Grossman, personal communication, 2011), supplementing their 15 published cases.15
Clinical features of CBD
Motor features
The motor features of CBD emerged from early case series with incomplete pathologic follow-up in which the CBS presentation predominated, manifesting with asymmetric onset of levodopa-resistant parkinsonism, dystonia, and myoclonus. Seventy-three percent (72/99) of reviewed patients with CBD with parkinsonism had documented asymmetry. Limb rigidity (85%) and bradykinesia (76%) were the most common motor findings (table 1); 57% had limb rigidity and 48% had bradykinesia at presentation (where described). Although often characterized as severe, the nature of limb rigidity was rarely described and may relate to parkinsonism, dystonia, or gegenhalten/paratonia. Axial rigidity was reported in 27% at presentation and 69% at some time during the disease course. CBD series emphasizing cognitive or behavioral presentations show low rates of early parkinsonism.13,15,20
Table 1.
Frequency of motor features in available brain banks and studies with ≥5 pathologically confirmed corticobasal degeneration casesa

Thirty-nine percent of CBD cases had tremor documented during the disease course (table 1), representing a mix of resting, postural and action, and undefined tremors. Tremor phenotype in CBD is poorly characterized, but a CBS series suggests that it differs from the typical rest tremor of Parkinson disease (PD).25 Low-amplitude action myoclonus may resemble tremor.
Gait abnormalities (often poorly characterized) were described in 73% overall, but only in 33% at onset, with postural instability and falls occurring at similar frequencies (table 1). The timing of falls was rarely described.
Sustained levodopa responsiveness—related to parkinsonism—is an exclusion criterion in prior diagnostic schemes.4,5,9,10,26 Patients with CBD may demonstrate transient mild to moderate improvement with levodopa therapy and rarely develop levodopa-induced dyskinesias,16 but a sustained response is rare. The presence or absence of a levodopa response was seldom described in compiled cases.
Dystonia is described in 59%–71% of patients in series mixing CBS and CBD7,25,27; however, only 38% of compiled CBD cases ever had limb dystonia (table 1) and only 20% presented with limb dystonia.
Clinical series describe myoclonus in 55%–93% of patients with CBS,7,25,27,28 but myoclonus occurred in only 27% of compiled CBD cases (table 1). Myoclonus in CBD may be superimposed on dystonia18 and is most commonly described in the upper extremities, but can also be present in the face.13,18 Descriptions include “focal myoclonus,”18 “stimulus-sensitive myoclonus,”13,16 and “action myoclonus.”16 Studies of myoclonus in CBS suggest that a very short latency may be characteristic,29,30 but it is unclear if this is true in CBD.
Higher cortical features
Higher cortical features described in CBD include apraxia, alien limb phenomena, cortical sensory loss, cognitive impairment, behavioral changes, and aphasia.
Apraxia is core to all previous diagnostic criteria (table e-1). Limb apraxia was found in 57% of compiled CBD cases (table 2). Ideomotor apraxia is the most commonly described apraxia in CBD15,16,18 with one series also describing limb-kinetic apraxia.24 The presence of limb dystonia, bradykinesia, and rigidity can make assessing ideomotor apraxia challenging and diagnosing limb-kinetic apraxia impossible. Patients with CBD may also have orobuccal apraxia or “apraxia of eyelid opening,”13,15,16,18,21,24 though the latter is often a misnomer representing pretarsal blepharospasm rather than true apraxia.31
Table 2.
Frequency of higher cortical features in available brain banks and studies with ≥5 pathologically confirmed corticobasal degeneration casesa
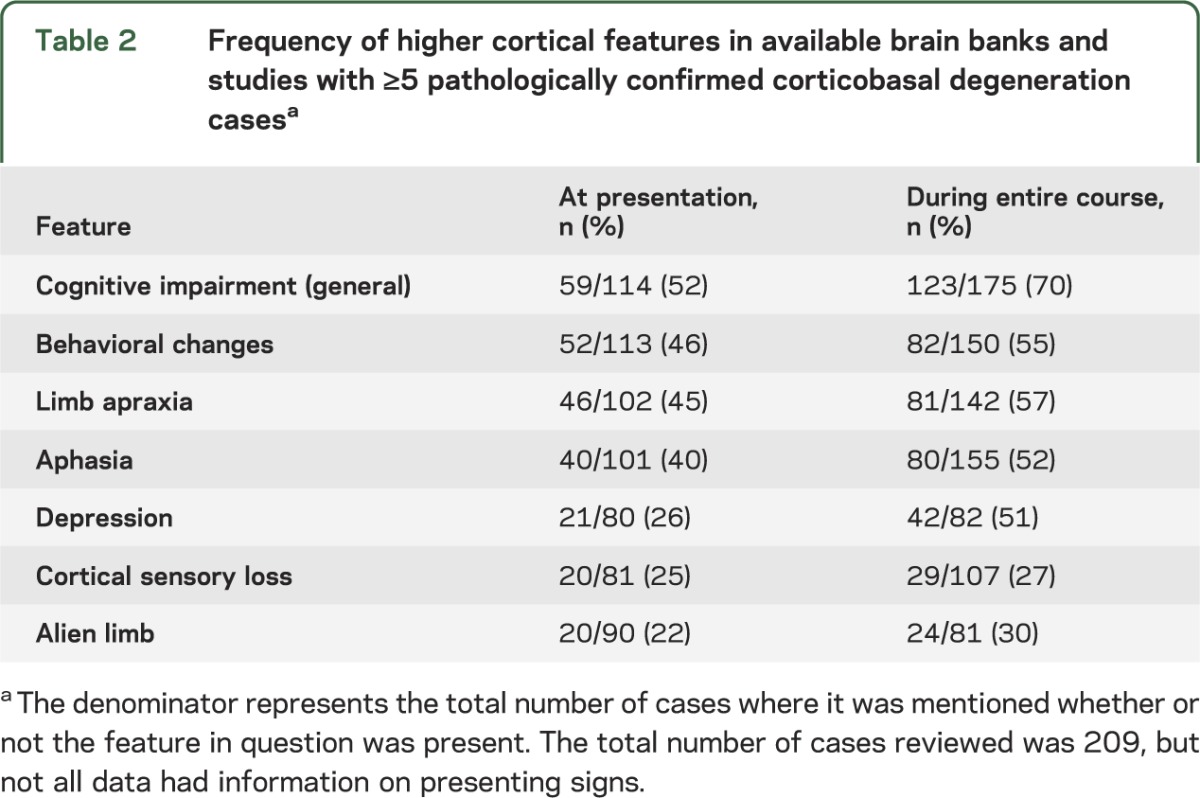
Alien limb phenomena are included in prior criteria (table e-1), yet what behaviors constitute alien limb phenomena remains a matter of debate.26 Alien limb phenomena (including complex unintentional limb movements interfering with normal tasks18 and the sensation that a limb was foreign or had a will of its own16) were described in 30% of compiled CBD cases (table 2).
When reported (less than half of compiled CBD cases), cortical sensory loss was present in approximately a quarter of cases (table 2). Visual neglect occurs in CBD,15,18,20 but also in Alzheimer disease (AD).20
Language impairments are now recognized as a common and frequently presenting feature of CBD. Aphasia occurred in 40% of compiled CBD cases at presentation and in 52% over the disease course (table 2). Reports most commonly categorized the aphasia as primary progressive aphasia (PPA), progressive aphasia, or progressive nonfluent aphasia (PNFA),15,20,32–34 though these terms overlap and reflect older terminology that has recently been revised.35 Aphasic patients with CBD may progress to mutism.13,15,36 Apraxia of speech (AOS) has also been described in CBD on its own and coexisting with aphasia,20,32 though challenges in diagnosing AOS limit efforts to estimate its frequency. Some consider AOS a speech disorder rather than language dysfunction37; consensus has been elusive. Speech abnormalities in general were described in 53% of cases (23% at presentation) (table 3). Some patients had dysarthria,18 but for others, few details were provided.13,15,21
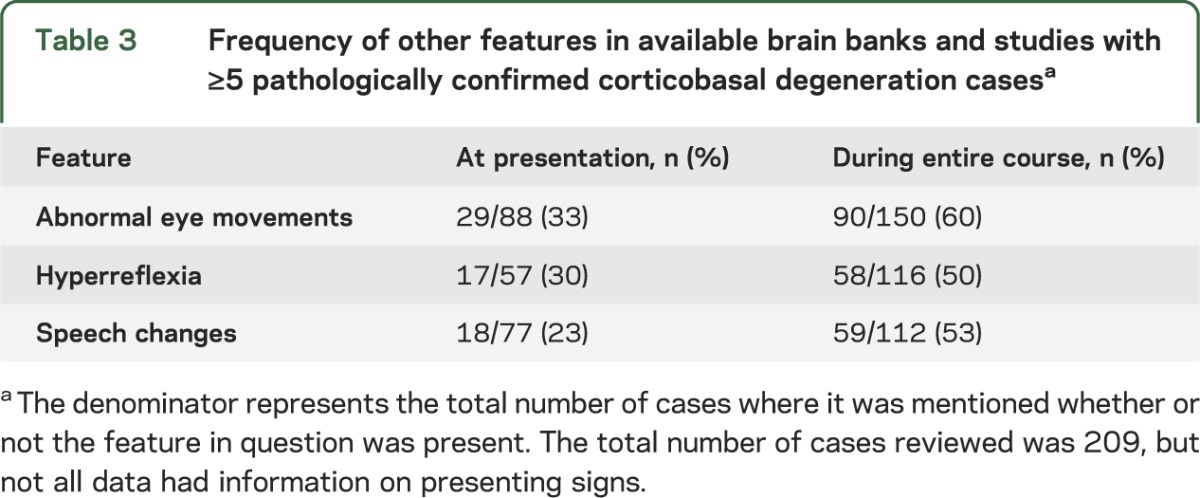
Table 3.
Frequency of other features in available brain banks and studies with ≥5 pathologically confirmed corticobasal degeneration casesa
Over half of compiled CBD cases had cognitive impairment at onset and 70% during the disease course (table 2). While this represented patients' subjective memory concerns in some series, memory complaints may relate to amnestic or nonamnestic (e.g., executive, language) cognitive disturbances. Neuropsychological testing in one study revealed that patients with CBD had difficulties with learning tasks, word fluency, verbal comprehension, perceptual organization, and cognitive flexibility.38 Another study showed impairments in executive, language, and visuospatial domains with relatively preserved episodic memory.15 In contrast, prominent memory loss was a presenting symptom in one series without neuropsychological testing.13 The presence of memory impairment in CBD is underscored by numerous series in which the clinical diagnoses of AD or atypical AD proved to be CBD.12,13,15,16,21,23,39 Acalculia and visuospatial difficulties (with limited details) are rarely described in CBD.15 Behavioral changes and executive dysfunction are common in CBD, underscored by many patients presenting with a behavioral variant frontotemporal dementia (FTD) syndrome.13,15,16,20,33,39–41 Symptoms include apathy, bizarre or antisocial behavior, personality changes, irritability, disinhibition, and hypersexuality.13,15,16,39 Fifty-five percent of reviewed CBD cases had behavioral changes, often at presentation (table 2). Clinical depression (rather than formal diagnosis) was described in 51% of patients where mood was recorded (table 2), but this information was provided in less than half of cases. Only a single published patient had hallucinations, coincident with levodopa treatment,16 but hallucinations not associated with levodopa were also rarely described in brain bank subjects (S.E. Lee and D.W. Dickson, personal communications, 2011).
Other features
Eye movement abnormalities may be present in CBD, but details are rarely provided and terminology is often ambiguous. Sixty percent of compiled cases had eye movement abnormalities. At onset, 33% showed such abnormalities (table 3). Studies of patients with CBS describe increased saccadic latency,42–45 but this was not confirmed in a publication including oculographic measurements in 4 subjects with CBD, where 3 had visually guided saccades that were indistinguishable from normal controls. Antisaccade performance was abnormal in all cases.46 Upper motor neuron signs are another feature described in CBD cases (table 3), but since they also occur in other atypical parkinsonisms, they are unlikely to be a helpful distinguishing sign.
CBD phenotypes
Whereas clinical features were identified and extracted for the 209 CBD cases described above, 267 CBD cases in the published literature and available brain banks had information regarding clinical diagnosis or phenotype. Of these 267 cases, data regarding final diagnosis were available for 210 patients; 129 cases included information regarding initial diagnosis.
The 210 cases in brain banks and the literature13,15,16,21,23,39,41,47,48 with final clinical diagnoses reported suggest 5 phenotypes associated with CBD pathology, capturing 87.1% (183/210) of cases: CBS (37.1%, 78/210), progressive supranuclear palsy syndrome (PSPS, also called Richardson syndrome) (23.3%, 49/210, 2 described as atypical), FTD (13.8%, 29/210), AD-like dementia (8.1%, 17/210 with 13 AD, 2 dementia, and 2 atypical dementia), and aphasia (typically categorized as PPA or PNFA, 4.8%, 10/210). An additional 5.7% (12/210) were mixed diagnoses involving these phenotypes. PD was diagnosed in 3.8% (8/210, 1 described as atypical). Two patients were diagnosed with dementia with Lewy bodies (DLB) (D.W. Dickson, personal communication, 2011) and 3 patients received other diagnoses16 (K. Josephs, personal communication, 2011). Two patients received no syndromic diagnosis.
In the 129 patients in the brain banks and literature12,16,21,49,50 with initial clinical diagnoses described, CBS was the most common presenting diagnosis (27.1%, 35/129), followed by FTD and PD or atypical PD (each 15.5%, 20/129), aphasia (14.7%, 19/129), AD/dementia (9.3%, 12/129), and PSPS (6.2%, 8/129). The finding of PD as an early clinical diagnosis and the difference in frequency between early and late clinical diagnoses underscores the challenge in making an accurate early diagnosis and the changing phenomenology over time.
Having identified CBD phenotypes, a literature search was performed to identify clinical features from clinicopathologic series that could help predict underlying pathology given that the identified phenotypes also have known associations with non-CBD pathologies.
Age
The combined mean (SD) age at CBD symptom onset was 63.7 (7.0) years. Age at onset ranged from 45 to 77.2 years.15,16,18,51
Disease duration
Mean CBD disease duration was 6.6 years (SD 2.4, range 2.0–12.5).15,18 In a series of patients with rapidly progressive dementia, neither of the patients with CBD died in the first year, in contrast to the 8 patients with Creutzfeldt-Jakob disease (CJD) (of 22 cases) who died within 12 months of onset.52
Family history
Most patients have no family history of CBD. One series, however, reported a family history of an FTD spectrum disorder in 2 of 14 patients with CBD.15 Additionally, the tau mutation N296N has resulted in pathology similar to CBD.53 Whether such cases should be included with typical sporadic CBD remains unclear. Currently, these rare familial CBD-like disorders are the exception rather than the rule. Familial CBS may be associated with granulin (GRN) mutations and frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions (FTLD-TDP) (i.e., non-tau) pathology rather than with CBD.54–56
Comparison of phenotypes
Studies comparing the different CBD phenotypes are described in the supplemental text. No study conclusively identified clinical features or imaging characteristics distinguishing CBD from other pathologies. Potential differentiating features are described in the supplemental text but require validation with larger sample sizes.
Neuroimaging and laboratory markers
Few studies evaluate imaging and laboratory markers in CBD. Studies using clinical cohorts report abnormalities consistent with the topography of clinical findings rather than reflecting specific underlying pathology. Recently described atrophy patterns in CBS associated with CBD, progressive supranuclear palsy (PSP), AD, and FTLD-TDP pathology57 are promising but require prospective validation prior to inclusion in diagnostic criteria. Imaging can help exclude other conditions with CBS presentations, such as CJD. CSF biomarkers hold promise but are not yet adequately studied in CBD. For these reasons we have not currently proposed a laboratory-supported diagnostic category.
CBD diagnostic criteria
Given the complex clinicopathologic correlations and varying phenotypes over time,34,36 it is not surprising that developing CBD diagnostic criteria has proven challenging. Five potential phenotypes were identified above, but while AD was a common clinical misdiagnosis of CBD in compiled cases, few details are available regarding how these diagnoses were made and what features prompted this diagnosis. Given the relative frequencies of AD and CBD, the false-positive diagnosis rate of an AD phenotype for CBD would be high if this phenotype was included; it was thus excluded. The 4 clinical phenotypes thought to be most representative of CBD were CBS, frontal behavioral-spatial syndrome, nonfluent/agrammatic variant of primary progressive aphasia, and PSPS (table 4).
Table 4.
Proposed clinical phenotypes (syndromes) associated with the pathology of corticobasal degenerationa
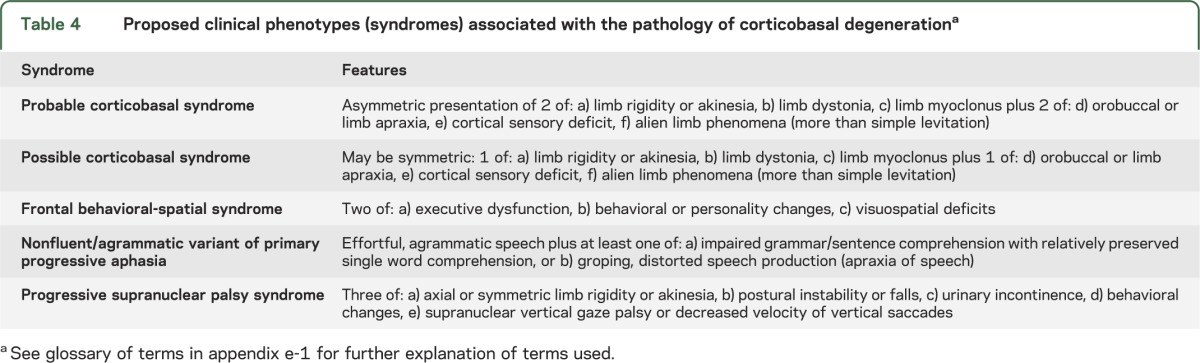
While these 4 phenotypes are frequent clinical presentations of CBD, it was difficult to identify specific features that would consistently predict a CBD diagnosis vs other pathologic diagnoses such as FTLD or PSP (supplemental text). Our deliberations led us to propose 2 diagnostic classifications for CBD (table 5): 1) clinical research criteria for probable CBD (cr-CBD), which attempt to maximize the chance of diagnosing classic CBD without contamination from other pathologies; and 2) possible CBD criteria (p-CBD), which are less restrictive but still emphasize presentations consistent with underlying tau pathology. The p-CBD criteria will catch more CBD cases but will also yield more false-positives, including patients who also meet criteria for other neurodegenerative conditions such as PSP. This approach could be acceptable if research studies, including experimental therapies, were directed at the broader issue of tau-based pathology.
Table 5.
Diagnostic criteria for corticobasal degenerationa
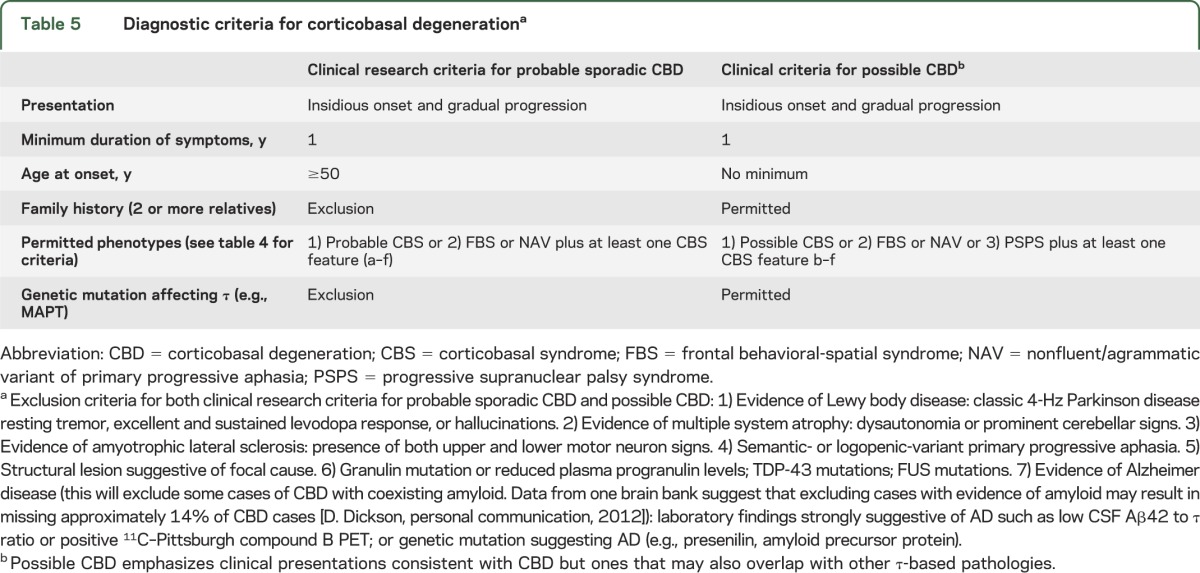
Both sets of criteria require insidious onset and gradual progression with symptom duration of at least 1 year to exclude rapidly progressive conditions more likely to represent other pathologies (e.g., CJD). Age at onset ≥50 years is required for cr-CBD given that this will identify 98% of patients with CBD and exclude pathologies with younger age at onset (e.g., FTLD). No age minimum is set for p-CBD, allowing for young-onset familial cases of CBD related to tau mutations. In addition, a family history (>1 relative) of a similar neurodegenerative disease is an exclusion criterion for cr-CBD but not p-CBD.
Accepted phenotypes are slightly different for the 2 sets of criteria. While PSPS is a CBD phenotype, it is much more likely to represent PSP than CBD.58 Thus, PSPS is an acceptable phenotype only in the p-CBD criteria. Features suggestive of idiopathic PD (classic 4- to 6-Hz resting tremor, excellent and sustained levodopa response) are exclusion criteria for both diagnostic criteria, as are hallucinations, which are much more suggestive of PD or DLB than CBD. Prominent dysautonomia and cerebellar signs are thought to be more suggestive of multiple system atrophy than CBD and thus are exclusion criteria based on consensus; CBD series do not routinely describe these signs. Because of their association with non-tau pathology (supplemental text), the presence of coincident upper and lower motor neuron signs or semantic- or logopenic-variant PPA are exclusion criteria for both cr-CBD and p-CBD.
While tau mutations are allowed for p-CBD, GRN, TDP-43, or FUS mutations are exclusions for both sets of criteria. Because emerging research suggests that amyloid imaging and CSF Aβ42/tau ratio may be useful in diagnosing AD and these biomarkers have been incorporated into the 2011 AD diagnostic criteria,59 results suggestive of AD on these studies are exclusion criteria for CBD, acknowledging that we lack confirmation of the ability of these tests to distinguish AD from CBD. Mutations known to be associated with AD are also exclusion criteria. Similarly, imaging studies suggestive of other pathologies (e.g., CJD) are exclusions for CBD diagnoses, but CBD-supportive imaging is not included as a criterion given the need for further validation.
DISCUSSION
Development of the proposed criteria relied on expert consensus among behavioral neurology, neuropsychology, and movement disorders specialists and a critical review of brain bank and published clinical–pathologic series. Limitations to the use of published and brain bank series include their retrospective nature, often relying on incomplete records, and subspecialty biases in reporting centers. We attempted to minimize these biases by strategically searching for cases, seeking brain bank data where available, and maximizing the sample size of the compiled cohort. Our decision to only consider features as present or absent if specifically described may result in overestimation of the frequency of CBD features. Given the reliance on often incomplete retrospective data from a wide variety of sources, this was thought to incur less bias than marking undescribed features as absent. Our results should be interpreted with this limitation in mind. Furthermore, the lower end of feature frequency can be calculated from the provided tables and the total sample size of 209 cases.
Identifying clinical phenotypes and features specific for CBD is an ongoing struggle limiting the ability to create definitive clinical criteria. This is addressed by proposing 2 sets of CBD criteria. Ultimately, a clear diagnosis of CBD may only be possible once biomarkers and associated genetic mutations are identified. In the meantime, the broader criteria may be useful, since potential disease-modifying agents are likely to deal with tauopathies as a class rather than considering tau-based diagnoses separately.
Our understanding of CBD has grown tremendously since its initial description but challenges with clinical diagnosis remain. We propose new CBD diagnostic criteria based on recent advances and a review of a large number of pathologically proven cases. We expect that these criteria will need continued revisions as our understanding of CBD improves and as imaging studies and biomarkers advance and are validated for distinguishing different phenotypes and diagnoses.
Supplementary Material
ACKNOWLEDGMENT
Brain bank CBD case information was provided by Keith A. Josephs, Paul McMonagle, Andrew Kertesz, Peachie Moore, Murray Grossman, Suzee E. Lee, and Dennis W. Dickson. The authors thank the CBD patients and their families, who must cope with uncertain diagnoses and progressive disease, for their patience as we learn more about this challenging field.
GLOSSARY
- AD
Alzheimer disease
- AOS
apraxia of speech
- CBD
corticobasal degeneration
- CBS
corticobasal syndrome
- CJD
Creutzfeldt-Jakob disease
- cr-CBD
clinical research criteria for probable corticobasal degeneration
- DLB
dementia with Lewy bodies
- FTD
frontotemporal dementia
- FTLD-TDP
frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions
- GRN
granulin
- p-CBD
possible corticobasal degeneration criteria
- PD
Parkinson disease
- PNFA
progressive nonfluent aphasia
- PPA
primary progressive aphasia
- PSP
progressive supranuclear palsy
- PSPS
progressive supranuclear palsy syndrome
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Melissa J. Armstrong, MD: analysis or interpretation of the data, drafting the manuscript, revising the manuscript. Irene Litvan, MD: design or conceptualization of the study, analysis or interpretation of the data, drafting the manuscript, revising the manuscript. Anthony E. Lang, MD: analysis or interpretation of the data, drafting the manuscript, revising the manuscript. Thomas H. Bak, MD: analysis or interpretation of the data, revising the manuscript. Kailash P. Bhatia, MD: analysis or interpretation of the data, revising the manuscript. Barbara Borroni, MD: analysis or interpretation of the data, revising the manuscript. Adam L. Boxer, MD, PhD: analysis or interpretation of the data, revising the manuscript. Dennis W. Dickson, MD: analysis or interpretation of the data, revising the manuscript. Murray Grossman, MD: analysis or interpretation of the data, revising the manuscript. Mark Hallett, MD: analysis or interpretation of the data, revising the manuscript. Keith A. Josephs, MD: analysis or interpretation of the data, revising the manuscript. Andrew Kertesz, MD: analysis or interpretation of the data, revising the manuscript. Suzee E. Lee, MD: analysis or interpretation of the data, revising the manuscript. Bruce L. Miller, MD: analysis or interpretation of the data, revising the manuscript. Stephen G. Reich, MD: analysis or interpretation of the data, revising the manuscript. David E. Riley, MD: analysis or interpretation of the data, revising the manuscript. Eduardo Tolosa, MD: analysis or interpretation of the data, revising the manuscript. Alexander I. Tröster, PhD: analysis or interpretation of the data, revising the manuscript. Marie Vidailhet, MD: analysis or interpretation of the data, revising the manuscript. William J. Weiner, MD: analysis or interpretation of the data, revising the manuscript.
STUDY FUNDING
The 1st International CBD Investigators Meeting was funded by a donation from Irene Pollin and the late Abe Pollin and from the Litvan Neurological Research Foundation. Melissa J. Armstrong received support as an Edmond J. Safra fellow at Toronto Western Hospital while working on this project.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia: a progressive disorder in late adult life. Trans Am Neurol Assoc 1967;92:23–26 [PubMed] [Google Scholar]
- 2.Gibb WR, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain 1989;112:1171–1192 [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002;61:935–946 [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Bergeron C, Pollanen M, Lang AE. Cortical-basal ganglionic degeneration. In: Jankovic J, Tolosa E, eds. Parkinson's Disease & Movement Disorders. Baltimore: Williams & Wilkins; 1998:297–316 [Google Scholar]
- 5.Lang AE, Riley DE, Bergeron C. Cortical-basal ganglionic degeneration. In: Calne DB, ed. Neurodegenerative Diseases. Philadelphia: W.B. Saunders; 1994:877–894 [Google Scholar]
- 6.Watts RL, Mirra SS, Richardson EP. Cortical-basal ganglionic degeneration. In: Marsden CD, Fahn S, eds. Movement Disorders, vol 3 Oxford: Butterworth Heinemann; 1994:282–299 [Google Scholar]
- 7.Riley DE, Lang AE, Lewis A, et al. Cortical-basal ganglionic degeneration. Neurology 1990;40:1203–1212 [DOI] [PubMed] [Google Scholar]
- 8.Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998;65:717–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern C, McMillan C, Moore P, Dennis K, Grossman M. Calculation impairment in neurodegenerative diseases. J Neurol Sci 2003;208:31–38 [DOI] [PubMed] [Google Scholar]
- 10.Bak TH, Hodges JR. Corticobasal degeneration: clinical aspects. Handb Clin Neurol 2008;89:509–521 [DOI] [PubMed] [Google Scholar]
- 11.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999;53:795–800 [DOI] [PubMed] [Google Scholar]
- 12.Boeve B. Corticobasal degeneration: the syndrome and the disease. In: Litvan I, ed. Atypical Parkinsonian Disorders: Clinical and Research Aspects. Totowa, NJ: Humana Press; 2005: 309–334 [Google Scholar]
- 13.Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999;53:1969–1974 [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–870 [DOI] [PubMed] [Google Scholar]
- 15.Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology 2007;68:1274–1283 [DOI] [PubMed] [Google Scholar]
- 16.Ling H, O'Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 2010;133:2045–2057 [DOI] [PubMed] [Google Scholar]
- 17.Litvan I, Agid Y, Goetz C, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 1997;48:119–125 [DOI] [PubMed] [Google Scholar]
- 18.Wenning GK, Litvan I, Jankovic J, et al. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 1998;64:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley DE, Lang AE. Clinical diagnostic criteria. Adv Neurol 2000;82:29–34 [PubMed] [Google Scholar]
- 20.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011;70:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 1997;48:959–969 [DOI] [PubMed] [Google Scholar]
- 22.Armstrong RA, Cairns NJ, Lantos PL. A quantitative study of the pathological lesions in the neocortex and hippocampus of twelve patients with corticobasal degeneration. Exp Neurol 2000;163:348–356 [DOI] [PubMed] [Google Scholar]
- 23.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004;56:399–406 [DOI] [PubMed] [Google Scholar]
- 24.Tsuchiya K, Murayama S, Mitani K, et al. Constant and severe involvement of Betz cells in corticobasal degeneration is not consistent with pyramidal signs: a clinicopathological study of ten autopsy cases. Acta Neuropathol 2005;109:353–366 [DOI] [PubMed] [Google Scholar]
- 25.Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998;55:957–961 [DOI] [PubMed] [Google Scholar]
- 26.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003;54:S15–S19 [DOI] [PubMed] [Google Scholar]
- 27.Vanek Z, Jankovic J. Dystonia in corticobasal degeneration. Mov Disord 2001;16:252–257 [DOI] [PubMed] [Google Scholar]
- 28.Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration: a clinical study of 36 cases. Brain 1994;117:1183–1196 [DOI] [PubMed] [Google Scholar]
- 29.Thompson PD, Day BL, Rothwell JC, Brown P, Britton TC, Marsden CD. The myoclonus in corticobasal degeneration. Evidence for two forms of cortical reflex myoclonus. Brain 1994;117:1197–1207 [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Ashby P, Lang AE. Stimulus-sensitive myoclonus in akinetic-rigid syndromes. Brain 1992;115:1875–1888 [DOI] [PubMed] [Google Scholar]
- 31.Ross AH, Elston JS, Marion MH, Malhotra R. Review and update of involuntary facial movement disorders presenting in the ophthalmological setting. Surv Ophthalmol 2011;56:54–67 [DOI] [PubMed] [Google Scholar]
- 32.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMonagle P, Blair M, Kertesz A. Corticobasal degeneration and progressive aphasia. Neurology 2006;67:1444–1451 [DOI] [PubMed] [Google Scholar]
- 34.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005;128:1996–2005 [DOI] [PubMed] [Google Scholar]
- 35.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kertesz A, Blair M, McMonagle P, Munoz DG. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord 2007;21:155–163 [DOI] [PubMed] [Google Scholar]
- 37.Ash S, McMillan C, Gunawardena D, et al. Speech errors in progressive non-fluent aphasia. Brain Lang 2010;113:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanvoorst WA, Greenaway MC, Boeve BF, et al. Neuropsychological findings in clinically atypical autopsy confirmed corticobasal degeneration and progressive supranuclear palsy. Parkinsonism Relat Disord 2008;14:376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman MS, Zhukareva V, Bergeron C, et al. Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol 2002;160:2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000;55:1368–1375 [DOI] [PubMed] [Google Scholar]
- 41.Lladó A, Sánchez-Valle R, Rey MJ, et al. Clinicopathological and genetic correlates of frontotemporal lobar degeneration and corticobasal degeneration. J Neurol 2008;255:488–494 [DOI] [PubMed] [Google Scholar]
- 42.Rivaud-Péchoux S, Vidailhet M, Gallouedec G, Litvan I, Gaymard B, Pierrot-Deseilligny C. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology 2000;54:1029–1032 [DOI] [PubMed] [Google Scholar]
- 43.Rottach KG, Riley DE, DiScenna AO, Zivotofsky AZ, Leigh RJ. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol 1996;39:368–377 [DOI] [PubMed] [Google Scholar]
- 44.Garbutt S, Matlin A, Hellmuth J, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain 2008;131:1268–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidailhet M, Rivaud-Péchoux S. Eye movement disorders in corticobasal degeneration. Adv Neurol 2000;82:161–167 [PubMed] [Google Scholar]
- 46.Boxer AL, Garbutt S, Seeley WW, et al. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer's disease. Arch Neurol 2012;69:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology 2003;61:493–499 [DOI] [PubMed] [Google Scholar]
- 48.Komori T, Arai N, Oda M, et al. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 1998;96:401–408 [DOI] [PubMed] [Google Scholar]
- 49.Hodges JR. Clinico-pathological correlations in frontotemporal dementia: an update on the Cambridge series and review of the literature. Acta Neuropsychol 2011;9:177–191 [Google Scholar]
- 50.Snowden JS, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain 2011;134:2478–2492 [DOI] [PubMed] [Google Scholar]
- 51.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48 [DOI] [PubMed] [Google Scholar]
- 52.Josephs KA, Ahlskog JE, Parisi JE, et al. Rapidly progressive neurodegenerative dementias. Arch Neurol 2009;66:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spillantini MG, Yoshida H, Rizzini C, et al. A novel tau mutation (N296N) in familial dementia with swollen achromatic neurons and corticobasal inclusion bodies. Ann Neurol 2000;48:939–943 [DOI] [PubMed] [Google Scholar]
- 54.López de Munain A, Alzualde A, Gorostidi A, et al. Mutations in progranulin gene: clinical, pathological, and ribonucleic acid expression findings. Biol Psychiatry 2008;63:946–952 [DOI] [PubMed] [Google Scholar]
- 55.Masellis M, Momeni P, Meschino W, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain 2006;129:3115–3123 [DOI] [PubMed] [Google Scholar]
- 56.Spina S, Murrell JR, Huey ED, et al. Corticobasal syndrome associated with the A9D Progranulin mutation. J Neuropathol Exp Neurol 2007;66:892–900 [DOI] [PubMed] [Google Scholar]
- 57.Whitwell JL, Jack CR, Jr, Boeve BF, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 2010;75:1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osaki Y, Ben Shlomo Y, Lees AJ, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord 2004;19:181–189 [DOI] [PubMed] [Google Scholar]
- 59.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


