Abstract
Objective:
To report the development of CNS vasculitis in a patient with multiple sclerosis (MS) treated with daclizumab.
Methods:
This report includes clinical, MRI, immunologic, and pathology data and CSF analysis.
Results:
After completing a phase II daclizumab monotherapy study with an optimal response as evidenced by significant decrease in MRI disease activity and stable clinical examinations, the patient elected to continue daclizumab therapy outside of NIH study. Daclizumab was discontinued after 21 doses due to the onset of new clinical symptoms and evidence of a vascular pattern of contrast enhancement on brain and spine MRI. Because of continued clinical deterioration, stereotactic brain biopsy was performed, showing small-vessel CNS vasculitis. Treatment was initiated with IV methylprednisolone followed by a regimen of cyclophosphamide. Immunologic studies suggest that unexpected lack of expansion of CD56bright NK cells and predictable decline in FoxP3+ T-regs combined with a transient interruption in daclizumab dosing may have contributed to this serious side effect.
Conclusions:
Only safety data from larger phase III studies and potentially postmarketing experience will define the exact risk of daclizumab-induced immunopathologies. Nevertheless, our case provides plausible hypothesis and potential biomarker that may be used to screen susceptible patients and implement preventive safety measures during potentially vulnerable periods.
Daclizumab is a humanized monoclonal antibody specific for interleukin (IL)–2Rα receptor (CD25) and has been approved for the prevention of allograft rejection in solid organ transplantation. Multiple phase II studies of daclizumab in multiple sclerosis (MS) have reported significant efficacy in reducing contrast-enhancing lesions (CEL) and clinical disability.1–6 Clinical efficacy of daclizumab treatment in MS has been linked to the expansion of CD56bright NK cells,3,6,7 which can regulate adaptive immunity by killing activated autologous T cells.7 We describe a 42-year-old Caucasian woman (ZAP10) with a 5-year history of relapsing-remitting MS (RRMS) who completed a phase II clinical trial of daclizumab monotherapy in MS.2
METHODS
Magnetic resonance images were obtained at the height of the patient's symptoms using described methodology.2 CSF was collected during pretreatment baseline, month 1.5, and month 6.5 on daclizumab therapy on NIH protocol, and during clinical deterioration on daclizumab therapy outside of NIH clinical trial. CSF was spun within 30 minutes of collection and cell-free supernatants were cryopreserved until analysis. CSF supernatants were concentrated (up to 10-fold) by centrifugation through Millipore Amicon Ultra 3 kDa filters and analyzed in multiplex for IL-12p40, CCL19, and interferon-γ as described.2 CXCL13 was measured by ELISA (R&D Systems, Minneapolis, MN; Catalogue number DY801).
Standard protocol approvals, registrations, and patient consents
The study was approved by the NIH/CNS Institutional Review Board. Written informed consent was obtained from the patient.
RESULTS
ZAP10 initially had excellent therapeutic response to daclizumab as judged by significant inhibition of CELs (average of 17.5 CEL/month before initiation of daclizumab to 0.5 CEL/month during treatment) on brain MRI, stable or improving Expanded Disability Status Scale (EDSS) score (figure 1; highlighted in green), and inhibition of markers of intrathecal inflammation, such as CSF IL-12p40, CXCL13, and CCL19 (figure 2A, Dac Mo1.5 and Mo6.5).
Figure 1. Clinical course of ZAP10.
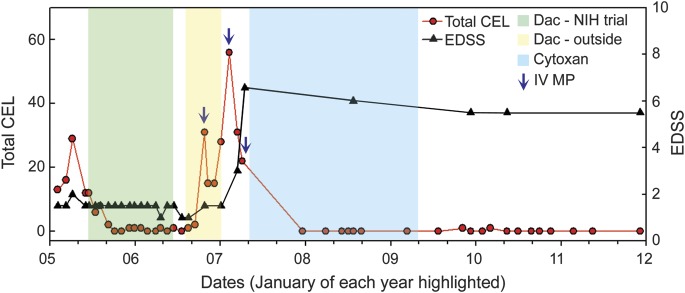
Periods of daclizumab therapy are highlighted in green (NIH clinical trial) and yellow (off-label therapy), while 5-day courses of IV methylprednisolone (MP) are highlighted by blue arrows. Therapy of CNS vasculitis with IV cyclophosphamide (Cytoxan) is highlighted in blue. Longitudinal measurements of Expanded Disability Status Scale (EDSS) (black triangles) and total number of contrast-enhancing lesions (CEL) on brain MRI (red circles) are depicted for a period of 8 years.
Figure 2. Intrathecal inflammatory biomarkers, MRI, and pathology at the time of CNS vasculitis.
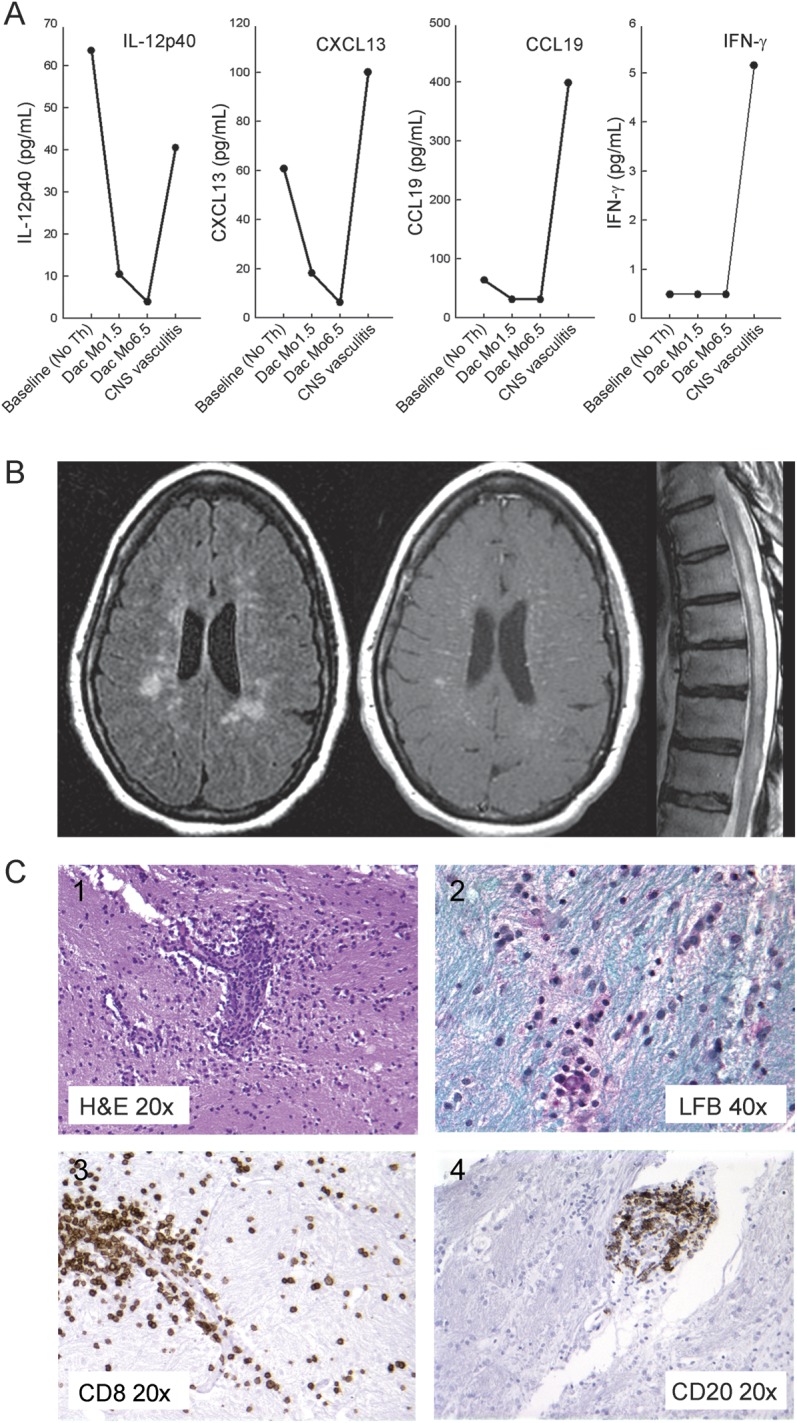
(A) CSF analysis of soluble inflammatory biomarkers. (B) Representative MRI: fluid-attenuation inversion recovery, T1-weighted image obtained postinjection of gadopentetate dimeglumine (Magnevist 0.1 mmol/kg, Bayer Healthcare Pharmaceuticals, Wayne, NJ) of the brain, and T2-weighted image of the thoracic spinal cord. (C) Histologic analysis of brain biopsy: hematoxylin & eosin (H&E) stain (C1) demonstrated lymphocytic infiltration of small vessels with structural damage to the vessel wall; Luxol fast blue (LFB) (C2) failed to reveal clear areas of demyelination in sampled biopsy tissue. Lymphocytic infiltrate had abundance of CD8 T cells and B cells as evidenced by immunohistochemistry for CD8+T cells (C3) and CD20+ B cells (C4). IFN = interferon; IL = interleukin.
Following completion of trial2 the patient elected to continue monthly IV daclizumab treatments with a private neurologist. There was an 8-week interim between last study dose and first off-label dose of daclizumab. The patient had a mild clinical relapse following third off-label dosing of daclizumab (figure 1; highlighted in yellow). IV methylprednisolone (1 g/day × 5 days) was administered, with transient improvement. However, the patient continued to deteriorate with headaches, fevers, weight loss, and arthralgia, and eventually demonstrated diffuse weakness, ataxia, and gait difficulty (EDSS 6). Numerous focal T2-weighted lesions and striking linear contrast enhancement in the deep medullary veins were observed on brain MRI (figure 2B). Despite stopping daclizumab treatment and initiating second treatment with IV methylprednisolone, repeat MRI showed persistent CELs remarkable for their “vascular” and leptomeningeal enhancement (not shown). MRI of the spinal cord showed diffuse intramedullary cord abnormalities with cord swelling, edema (figure 2B), and numerous petechial foci of enhancement (not shown).
CSF studies showed lymphocytic pleocytosis of 179 leukocytes (98% lymphocytes), 2 erythrocytes, 100 mg/dL protein, and 48/dL glucose. Intrathecal inflammatory markers (CSF IL-12p40, CXCL13, CCL19, and IFN-γ) rose at or above daclizumab–pretreatment baseline (figure 2A). Extensive CSF virology studies were negative. CSF was negative for malignancy by cytology and flow cytometry. Antinuclear antibodies, anticardiolipin antibodies immunoglobulin G, extractable nuclear antigen, and rheumatoid factor were negative.
MRI-guided diagnostic brain biopsy (targeting apparent vasculitic lesions) showed abundant lymphocytic infiltration of cerebral white matter. Intense perivascular cuffing was associated with marked structural damage to small vessels indicative of vasculitis (figure 2C). Damage to small vessels was evident on hematoxylin and eosin stains and confirmed by immunohistochemistry for CD31 and CD34 (not shown). CD8+ T cells were more numerous compared to CD4+ T cells. B (CD20+) lymphocytes were confined to perivascular cuffs. There was no evidence of demyelination on Luxol fast blue stains. Diffusely scattered microglia/macrophages were identified by CD68 staining but no lipid-laden cells were present. Special stains for bacteria, fungi, and acid-fast bacilli were negative. Immunohistochemistry for cytomegalovirus, Toxoplasma, herpes simplex virus, simian virus 40, and in situ hybridization for Epstein-Barr virus were negative.
The patient was treated with high-dose pulsed methylprednisolone followed by a regimen of cyclophosphamide for CNS vasculitis (figure 1; highlighted in blue). She responded with substantial clinical recovery and significant resolution of brain MRI abnormalities. At 33 months after last cyclophosphamide dose, the patient maintains EDSS of 5 and brain MRI shows no CELs and minimal increase of T2 lesion load.
DISCUSSION
The anti-CD25 monoclonal antibody daclizumab has emerged as an effective treatment for MS and is currently in phase III trials of clinical testing. Daclizumab has been generally well tolerated, with the notable exception of skin rashes and transient liver function abnormalities.4,8 This is the first report of CNS vasculitis in a patient treated with daclizumab.
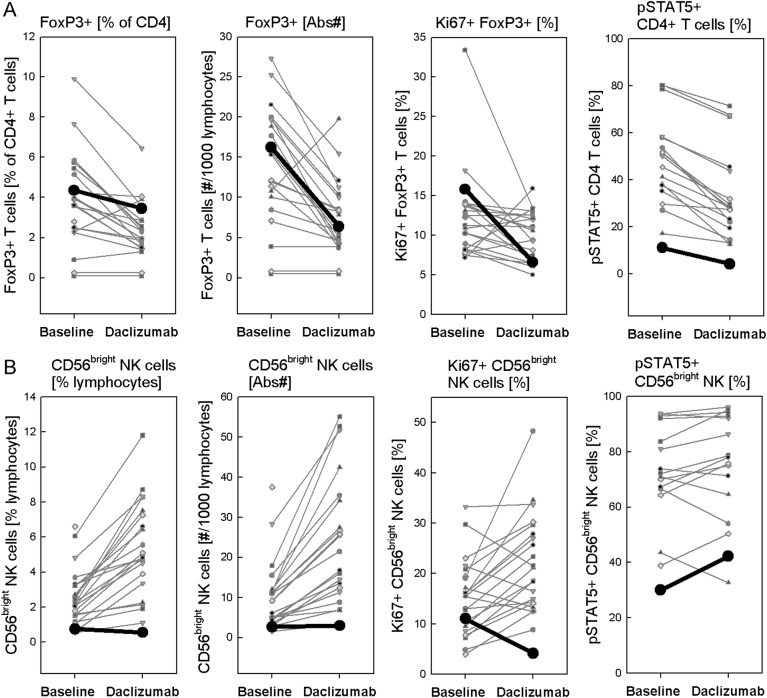
The etiology of CNS vasculitis in this patient is uncertain. Because MS is ultimately a diagnosis of exclusion and the brain biopsy specimen lacked evidence of demyelination, there is a slight possibility that the primary pathology in this patient was neuroinflammatory disorder other than MS. However, extensive diagnostic workup (appendix e-1 on the Neurology® Web site at www.neurology.org) did not reveal any alternative systemic or specific neuroinflammatory disorder. Thus, we conclude that it is much more likely that the induction of vasculitis was linked to daclizumab treatment. Careful immunologic analysis of ZAP10 in relationship to other patients with MS who participated in NIH clinical trials of daclizumab in MS1–3 demonstrated that ZAP10 had comparable daclizumab-induced decrease in proportion, absolute numbers, and proliferation (i.e., Ki67-staining) of FoxP3+ regulatory CD4 T cells (figure 3A). However, in contrast to other trial participants, ZAP10 did not expand immunoregulatory CD56bright NK cells7 during daclizumab treatment (figure 3B). It is likely that lack of expansion of CD56bright NK cells in this patient was genetically determined, because we observed that she had lowest levels of IL-2 signaling as measured by STAT5 phosphorylation in her T cells, but also in CD56bright NK cells at pretreatment baseline (figure 3, A and B, last panels).
Figure 3. Immunologic studies comparing ZAP10 with other daclizumab-treated patients with multiple sclerosis.
(A, B) Immunologic studies were performed at pretreatment baseline and after 6.5 months of daclizumab therapy, as reported perviously.2,10 Data from patient ZAP10 are highlighted as thicker black lines in each panel.
We have recently reported that in addition to immunoregulation via CD56bright NK cells, daclizumab also efficiently inhibits activation of antigen-specific T cells by blocking IL-2 transpresentation by dendritic cells.9 We believe that this latter effect underlies the therapeutic efficacy of daclizumab in ZAP10. For this new mechanism to operate, daclizumab needs to be administered in concentrations that saturate IL-2Rα in the lymph nodes.9 Conversely, when daclizumab concentrations fall below saturating levels in the lymphatic tissues, but still saturate CD25 in the blood, then de novo activated T cells that upregulate CD25 upon antigen-specific stimulation will experience CD25 blockade only during their transit in blood. Such late inhibition of high-affinity IL-2 signaling will make effector T cells resistant to activation-induced cell death, leading to paradoxically greater expansion and survival of antigen-specific T cells.9 It is conceivable that the 8-week interruption in daclizumab dosing in ZAP10 created such a vulnerable situation. Furthermore, the decline in T-regs, experienced in absence of normal expansion of CD56bright NK cells, may have left this patient uniquely defenseless to de novo activation of the adaptive immune responses by unknown trigger. Clearly this explanation, although plausible, remains speculative.
This case report is clinically important, as it demonstrates that in rare individuals, daclizumab administration can lead to inhibition of T-regs without concomitant expansion of immunoregulatory CD56bright NK cells, leaving such individuals potentially vulnerable to novel immunopathologies. While at the moment we do not know how prevalent such complication may be, our analysis of this case provides potential biomarkers for identification of such cases. Indeed, if follow-up studies demonstrate that prevalence of such patients is not negligible, then genetic studies may identify susceptibility alleles that predispose patients to this immunologic complication.
A resulting predictive biomarker would enhance safety of daclizumab treatment by screening susceptible individuals or by implementing preventive therapy (e.g., corticosteroids) during the vulnerable period after conclusion of daclizumab dosing.
Supplementary Material
GLOSSARY
- CEL
contrast-enhancing lesions
- EDSS
Expanded Disability Status Scale
- IL
interleukin
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Mrs. Ohayon drafted/revised the manuscript for content, including medical writing for content. Mrs. Ohayon contributed to study concept. Dr. Oh drafted/revised the manuscript for content, including medical writing for content. Dr. Richert revised the manuscript for content, including medical writing for content. Dr. Richert acquired and interpreted data. Ms. Martin revised the manuscript for content, including medical writing for content. Ms. Martin analyzed and interpreted data. Dr. Vortmeyer revised the manuscript for content, including medical writing for content. Dr. Vortmeyer analyzed and interpreted data. Dr. McFarland revised the manuscript for content, including medical writing for content. Dr. McFarland provided supervision. Dr. Bielekova drafted/revised the manuscript for content, including medical writing for content. Dr. Bielekova analyzed and interpreted data, provided statistical analysis and study supervision.
STUDY FUNDING
Supported by the intramural research program of the National Institute of Neurological Disorders and Stroke, NIH. Study drug was provided to NIH for free by Roche, Inc. NIH CTSA (KL2TR000057 to U.O.).
DISCLOSURE
J. Ohayon and U. Oh report no disclosures. N.D. Richert became an employee of Biogen Idec after study conclusion and after data analysis was finalized. J. Martin and A. Vortmeyer report no disclosures. H. McFarland is coinventor on several NIH patents related to daclizumab and as such has received patent royalty payments from NIH. B. Bielekova is coinventor on several NIH patents related to daclizumab and as such has received patent royalty payments from NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon-beta. Proc Natl Acad Sci USA 2004;101:8705–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielekova B, Richert N, Herman ML, et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology 2011;77:1877–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol 2009;66:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 2007;69:785–789 [DOI] [PubMed] [Google Scholar]
- 5.Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol 2004;56:864–867 [DOI] [PubMed] [Google Scholar]
- 6.Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 2010;9:381–390 [DOI] [PubMed] [Google Scholar]
- 7.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R-alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 2006;103:5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol 2009;66:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med 2011;17:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JF, Perry JS, Jakhete NR, Wang X, Bielekova B. An IL-2 paradox: blocking CD25 on T cells induces IL-2-driven activation of CD56(bright) NK cells. J Immunol 2010;185:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.