Abstract
Objective:
Our aim was to investigate the relationship of carotid structure and function with MRI markers of cerebral ischemic small-vessel disease.
Methods:
The study comprised 1,800 participants (aged 72.5 ± 4.1 years, 59.4% women) from the 3C-Dijon Study, a population-based, prospective cohort study, who had undergone quantitative brain MRI and carotid ultrasound. We used multivariable logistic and linear regression adjusted for age, sex, and vascular risk factors.
Results:
Presence of carotid plaque and increasing carotid lumen diameter (but not common carotid artery intima-media thickness) were associated with higher prevalence of lacunar infarcts: odds ratio (OR) = 1.60 (95% confidence interval [CI]: 1.09–2.35), p = 0.02 and OR = 1.24 (95% CI: 1.02–1.50), p = 0.03 (by SD increase). Carotid plaque was also associated with large white matter hyperintensity volume (WMHV) (age-specific top quartile of WMHV distribution): OR = 1.32 (95% CI: 1.04–1.67), p = 0.02, independently of vascular risk factors. Increasing Young elastic modulus and higher circumferential wall stress, reflecting augmented carotid stiffness, were associated with increasing WMHV (effect estimate [β] ± standard error: 0.0003 ± 0.0001, p = 0.024; β ± standard error: 0.005 ± 0.002, p = 0.008). Large WMHV was also associated with increasing Young elastic modulus (OR = 1.22 [95% CI: 1.04–1.42], p = 0.01) and with decreasing distensibility coefficient (OR = 0.83 [95% CI: 0.69–0.99], p = 0.04), independently of vascular risk factors. Associations of carotid lumen diameter with lacunar infarcts and of carotid stiffness markers with WMHV were independent of carotid plaque.
Conclusions:
In addition to and independently of carotid plaque, increasing carotid lumen diameter and markers of carotid stiffness were associated with increasing prevalence of lacunar infarcts and increasing WMHV, respectively.
Brain imaging studies have shown that the impact of ischemic brain injury, a major cause of disability, dementia, and mortality in adults,1 extends much beyond that of acute clinical events such as stroke. Vascular lesions, including MRI-defined brain infarcts (BIs) and white matter hyperintensities (WMH), are frequently observed in older community-dwelling individuals. The prevalence of BIs ranges between 8% and 28%2,3; most are small and located in subcortical brain regions, referred to as lacunar infarcts (LIs). WMH volume (WMHV) is estimated between 1.5 and >10 cm3 in population-based cohorts on average,4 and >90% of subjects aged 80 years and older have some degree of white matter damage.5 LIs and WMH are believed to result mostly from cerebral ischemic small-vessel disease (SVD),6 and predict an increased risk of stroke, dementia, and death.5
Age and high blood pressure are major risk factors for MRI markers of ischemic SVD,1,5 but underlying mechanisms are not fully understood. Although an association of increasing carotid intima-media thickness (IMT) and number or characteristics of carotid plaques with LI and WMHV was suggested, results are partly controversial.7–9 Besides, little is known about the relationship of large-artery geometry and stiffness with cerebral ischemic SVD.
We aimed to examine the relationship of ultrasound markers for carotid structure and function with MRI markers of cerebral ischemic SVD in a large community-based sample. This could improve our understanding of the pathophysiology underlying this highly prevalent cerebrovascular disorder and guide clinicians in the workup and management of patients at risk of cerebrovascular disease.
METHODS
Population and study design.
The 3C-Dijon Study is a prospective cohort study whose design has been described in detail elsewhere.10,11 Briefly, 4,931 noninstitutionalized persons aged 65 years and older were recruited from the electoral rolls of Dijon, France, between March 1999 and March 2001. Participants enrolled between June 1999 and September 2000, younger than 80 years, and who were able to come to the examination center (n = 2,763) were invited to undergo a brain MRI. Although 2,285 subjects agreed to participate, because of financial limitations, only 1,924 MRI scans were performed. A carotid ultrasound examination with evaluation of carotid structure was proposed to all participants younger than 85 years (n = 4,580) who were able to come to the examination center. Because of logistic concerns, this examination was not proposed during the last 6 months of subject recruitment and was finally performed on 3,323 participants. Estimation of carotid wall mechanics (carotid function) was provided by a special protocol introduced between December 1999 and December 2000, and thus proposed to the 2,391 subjects who underwent carotid ultrasound examination during this period. Carotid function could be measured in 76.5% of them (n = 1,830). In total, 1,800 participants had both measures of carotid structure and a brain MRI scan with evaluation of lacunar BIs (1,742 with quantitative WMHV measurement); 912 participants had both measures of carotid function and a brain MRI scan with evaluation of BIs (879 with quantitative WMHV measurement). Among participants with brain MRI measurements, those with measures for carotid structure were slightly older than participants with measures for both carotid structure and function, but had similar vascular risk factor profiles (table e-1 on the Neurology® Web site at www.neurology.org).
Standard protocol approvals, registrations, and patient consents.
The Ethics Committee of Kremlin-Bicêtre University Hospital approved the study protocol, and each participant signed an informed consent.
Brain MRI.
MRI acquisition was performed on a 1.5-T Magnetom scanner (Siemens, Erlangen, Germany) using T1-weighted, T2-weighted, and proton density–weighted sequences. A fast, multislice, double-echo, T2-weighted, 2-dimensional axial acquisition was used, with 4-mm-thick slices, and 0.4 mm between slice spacing. Raw data were transferred for analysis and storage to the MRI study center (Department of Neurofunctional Imaging, Caen). BIs were rated on T1-weighted, T2-weighted, and proton density–weighted images by a single rater, and defined as focal lesions ≥3 mm in diameter, with the same signal characteristics as CSF on all sequences.12 They were discriminated from dilated Virchow-Robin spaces using multiplanar reformatting: lesions with a typical vascular shape and following the orientation of perforating vessels were regarded as dilated Virchow-Robin spaces. LIs were defined as BIs of 3 to 15 mm in size, located in the basal ganglia or in the white matter. Subjects with a non-lacunar BI were excluded from analyses of LIs. Fully automated image-processing software was developed to detect, measure, and localize WMH.13
Carotid ultrasound examination.
Carotid structure.
Ultrasound measurements were performed with the B-mode system (Ultramark 9 High Definition Imaging; Advanced Technology Laboratories) using 5- to 10-MHz sounding, according to a standardized scanning protocol, with standardized central reading at Broussais Hospital, Paris.14 Plaque was defined as localized echo structures encroaching into the vessel lumen for which the distance between the media-adventitia interface and internal side of the lesion was ≥1 mm, on common carotid arteries (CCAs), carotid bifurcations, and internal carotid arteries.14 CCA-IMT was measured at a site free of any discrete plaque along a 10-mm-long segment on the far wall of the CCA, as the distance between lumen-intima and media-adventitia interfaces. On average, 75 measurements were automatically performed on each image and each side. The mean of right and left mean CCA-IMT values was used. Systolic and diastolic CCA lumen diameter was determined for each cardiac cycle and defined as the average of distances between leading edges of far and near wall lumen–intima interfaces along ≥0.5 cm of length using a computerized validated program.14 Diastolic CCA lumen diameter was used for analyses (referred to as carotid lumen diameter throughout the text).
Carotid function.
Ultrasound markers of carotid function are useful surrogates to assess large-artery stiffness. We used the following 4 markers to evaluate elastic properties of the carotid artery (e-Methods): carotid distension, representing the relative stroke change of diameter; cross-sectional distensibility coefficient, determined by stroke change of cross-sectional area relative to pulse pressure,15 which evaluates elastic properties of large arteries as hollow structures16; Young elastic modulus, representing elastic properties of arterial wall material17; circumferential wall stress, corresponding to tensile stress applied in the tangential direction to the arterial wall to enlarge the lumen.16 We used brachial oscillometric blood pressure measured during carotid ultrasonography in calculations. Decreasing carotid distension and distensibility coefficient and increasing Young elastic modulus and circumferential wall stress reflect increasing carotid stiffness.
Covariates.
For covariate definitions, see e-Methods.
Statistics.
Because WMHV and total white matter volume are highly correlated, we studied WMHV as the ratio of total WMHV to total white matter volume. Large WMHV (L-WMHV) was defined as the top age-specific quartile of WMHV distribution (by 5-year age categories). WMHV and triglyceride levels were log-transformed, as they were not normally distributed. We tested the association of vascular risk factors with LIs and L-WMHV using a χ2 test for categorical variables and Student t test for continuous variables.
In our primary analysis, we examined the relationship of MRI markers of cerebral ischemic SVD (WMHV, L-WMHV, LIs) with markers of carotid structure (carotid plaque, CCA-IMT, carotid lumen diameter) and function (carotid distension, cross-sectional distensibility coefficient, circumferential wall stress, Young elastic modulus) using a multivariable linear regression for continuous outcomes and multivariable logistic regression for dichotomous outcomes. We first adjusted for age and sex, then additionally for vascular risk factors associated with BIs or WMH burden (p < 0.05). Odds ratios are expressed by SD increase for continuous independent variables.
To assess the robustness of our findings, we verified whether associations were maintained after adjusting for brachial pulse pressure and antihypertensive drugs instead of hypertension, and after excluding participants with prevalent stroke (n = 42). We also tested whether findings were similar in subgroups stratified by hypertension, body surface area (which is highly correlated with vessel dimensions), age, and sex. Body surface area and age were dichotomized according to the sex-specific mean. To evaluate whether associations with carotid lumen diameter and carotid stiffness markers were independent of carotid plaque, we ran analyses including carotid plaque in the model in addition to carotid lumen diameter or carotid function parameters. We also performed a forward stepwise logistic regression with p = 0.10 as a significance threshold for entering into and staying in the model, to evaluate which parameters best predicted MRI markers of cerebral ischemic SVD. To examine whether markers of carotid structure are associated with severity of cerebral ischemic SVD, we created an “SVD-severity” score ranging from 0 to 2: 0 for participants without LI and without L-WMHV; 1 for participants with ≥1 LI or L-WMHV; 2 for participants with ≥1 LI and L-WMHV. We used multinomial logistic regression (generalized logit model), relating each carotid marker to this score.
Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
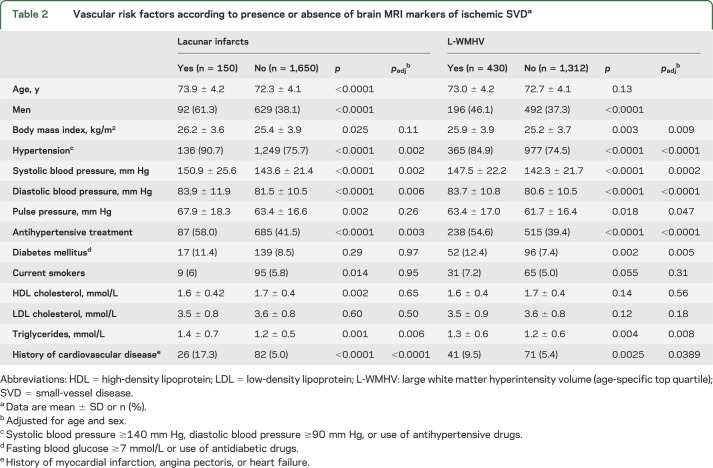
Characteristics of the study population are detailed in table 1. Among the 1,800 individuals studied, 150 (8.3%) had >1 LI and mean WMHV was 5.5 ± 4.9 cm3. Male sex, increasing age, history of cardiovascular disease, hypertension, and triglycerides were significantly associated with L-WMHV and LIs; diabetes and body mass index were significantly associated with L-WMHV (table 2).
Table 1.
Principal characteristics of the study population (n = 1,800)a
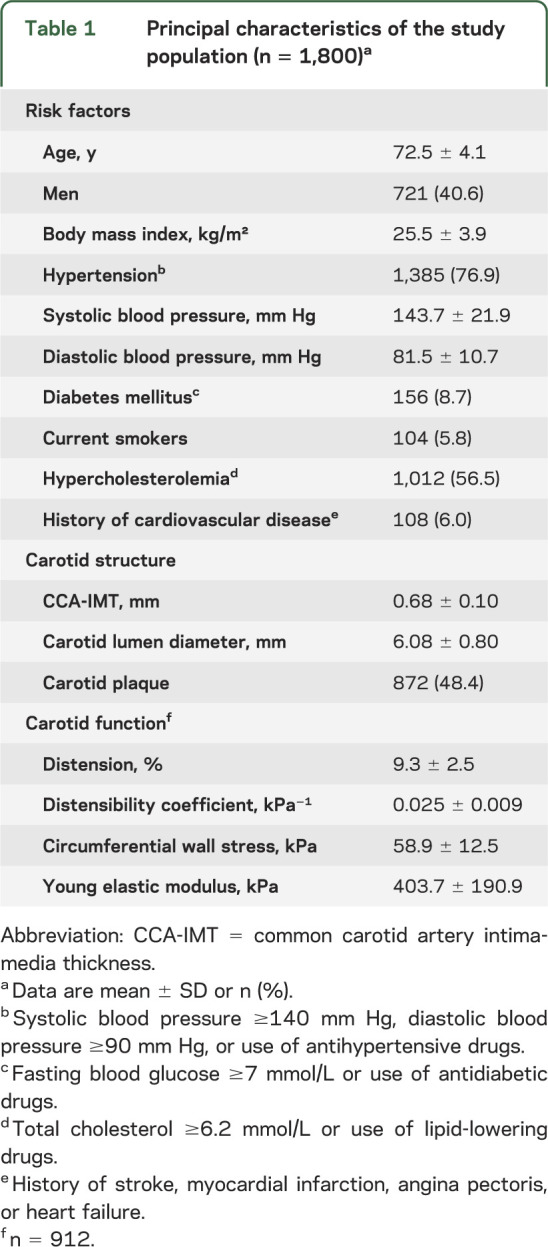
Table 2.
Vascular risk factors according to presence or absence of brain MRI markers of ischemic SVDa
Carotid structure and MRI markers of cerebral ischemic SVD.
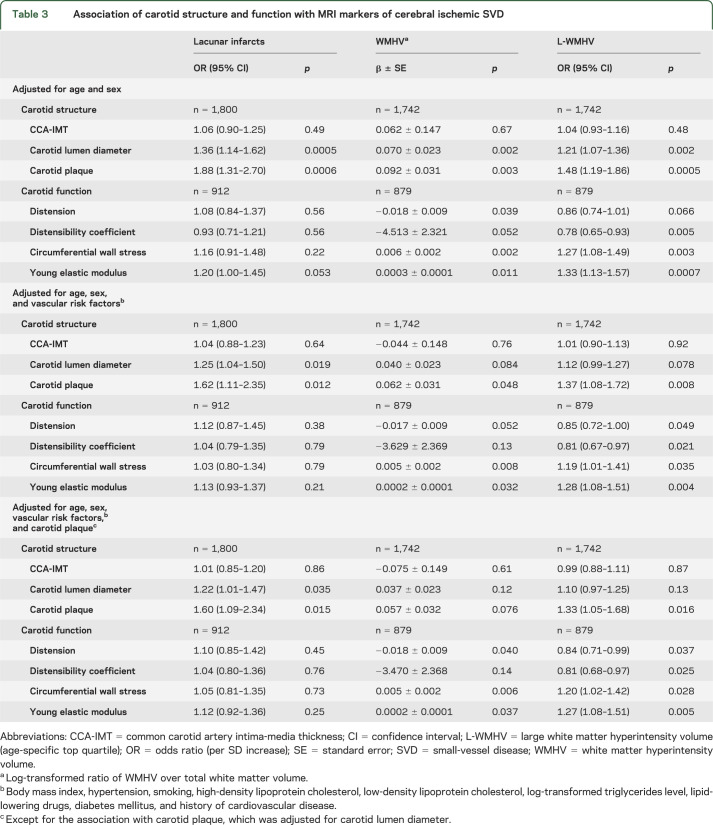
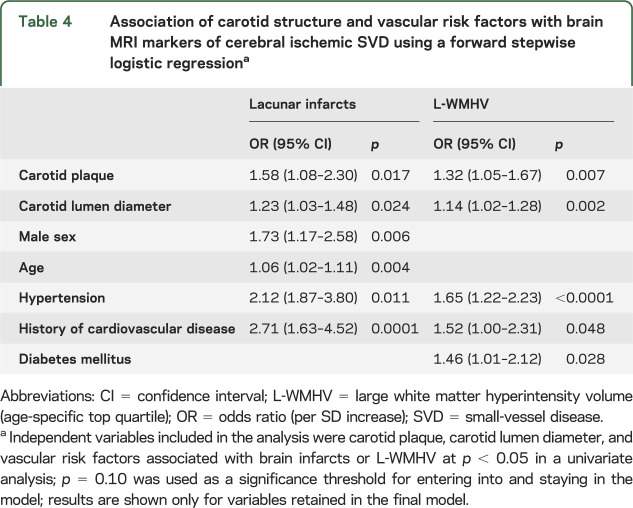
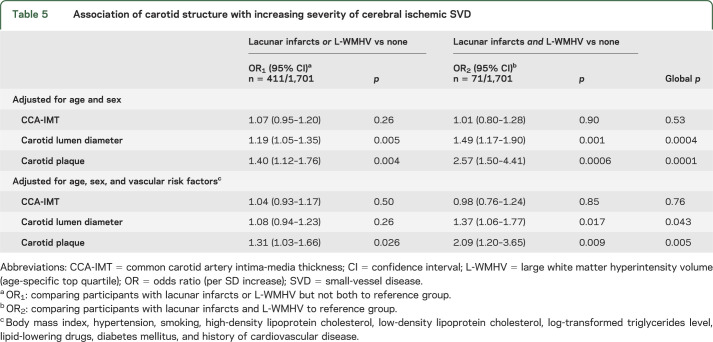
Carotid plaque and increasing carotid lumen diameter were significantly associated with LIs, L-WMHV, and increasing WMHV (table 3). After adjustment for vascular risk factors, these relationships were attenuated, but associations of carotid plaque with LIs, increasing WMHV and L-WMHV, and of carotid lumen diameter with LIs remained significant (table 3). Associations were similar when using brachial pulse pressure and antihypertensive treatment as covariates instead of hypertension, after exclusion of participants with prevalent stroke (data not shown), and in subgroups stratified on sex, body surface area, age, and hypertension (table e-2). Moreover, when we included both carotid plaque and carotid lumen diameter in the same model, associations of each with LIs, and of carotid plaque with L-WMHV, remained significant (table 3). In a forward stepwise logistic regression (table 4) with LIs as an outcome, carotid plaque, carotid lumen diameter, sex, age, hypertension, and history of cardiovascular disease were retained in the model. For L-WMHV, carotid plaque, carotid lumen diameter, hypertension, diabetes, and history of cardiovascular disease were retained (table 4). We observed a graded association of carotid plaque and increasing carotid lumen diameter with increasing SVD severity (table 5). CCA-IMT was not associated with MRI markers of cerebral ischemic SVD. Associations were similar in participants who underwent evaluation of carotid function and those who did not (table e-3).
Table 3.
Association of carotid structure and function with MRI markers of cerebral ischemic SVD
Table 4.
Association of carotid structure and vascular risk factors with brain MRI markers of cerebral ischemic SVD using a forward stepwise logistic regressiona
Table 5.
Association of carotid structure with increasing severity of cerebral ischemic SVD
Carotid function and MRI markers of cerebral ischemic SVD.
Increasing Young elastic modulus and circumferential wall stress, as well as decreasing distension and distensibility coefficient, were associated with increasing WMHV and prevalence of L-WMHV (table 3). After adjustment for vascular risk factors, associations remained significant for Young elastic modulus, circumferential wall stress, and distensibility coefficient. These associations also persisted when additionally adjusting for carotid plaque (table 3) and when using brachial pulse pressure and antihypertensive treatment as a covariate instead of hypertension (data not shown). Subgroup analyses stratified by age, sex, body surface area, and hypertension yielded consistent results (table e-2). LIs were not associated with carotid function parameters after adjustment for vascular risk factors (table 3).
DISCUSSION
In a large sample of elderly community participants, carotid plaque was associated with a higher prevalence of LIs and increasing WMHV. Independently of carotid plaque, larger carotid lumen diameter predicted an increased prevalence of LIs, whereas functional markers of carotid stiffness were associated with increasing WMH burden. These associations were attenuated but maintained after adjustment for vascular risk factors. Cerebral ischemic SVD was not associated with CCA-IMT.
The association of LIs with carotid plaque is in agreement with the literature.18,19 Previous publications also described an association among carotid stenosis, number or characteristics of carotid plaque, and WMHV.7,9,20 In the present dataset, we observed an association of carotid plaque with WMHV and L-WMHV, even after accounting for vascular risk factors. Mechanisms underlying the association of carotid plaque with cerebral ischemic SVD are unclear. Factors that may interfere with cerebral blood flow such as concomitant microatheroma at the origin of perforator arteries may have a role.21 Cerebral emboli originating from ruptured or ulcerated carotid plaque could also be involved, although they may not be a major contributor in a population-based setting.9 Carotid plaque may also reflect the overall effect of uncontrolled vascular risk factors, or lifetime exposure to these, better than each vascular risk factor taken individually.
Interestingly, carotid lumen diameter, another much less-studied marker of carotid structure, was also associated with increased prevalence of LIs, and to a lesser extent WMHV. Arterial dilatation may act as a compensatory mechanism to counteract an increase in stiffness and thickness of the arterial wall and maintain arterial compliance in the normal values.22–24 The fact that association of increasing carotid lumen diameter with LIs was maintained after adjusting for carotid plaque, and that we did not observe any association of CCA-IMT with cerebral ischemic SVD, suggest that this relationship was not driven only by carotid atherosclerosis and thickening. The cellular and molecular mechanisms of diameter enlargement are speculative, but may involve an excessive extracellular matrix turnover, a lack of vascular smooth muscle cell proliferation, or apoptosis,25 which could occur not only in the carotid artery, but also concomitantly in small cerebral arteries.
The absence of association of CCA-IMT with MRI markers of cerebral ischemic SVD is in contrast with results from other population-based studies,8,9 although some associations were observed primarily with internal carotid artery IMT.8 In the 3C-Dijon Study, CCA-IMT was measured at a site free of plaque,26 whereas plaques were included in the measurement of carotid IMT in other studies.8,9 In these studies, IMT probably reflects more advanced atherosclerosis, whereas increased IMT at a site free of plaque can also correspond to nonatherosclerotic thickening, such as an adaptive response to altered shear stress or circumferential wall stress.27
Several markers of carotid stiffness were associated with increasing WMH burden, independently of vascular risk factors and carotid plaque. The relationship of pulse wave velocity, a marker of aortic stiffness, with MRI markers of ischemic brain injury was recently assessed, in relatively small samples.19,28–32 After accounting for vascular risk factors, pulse wave velocity was consistently associated with WMH burden,30–32 whereas associations with MRI-defined BIs were controversial.19,28,30–32 Our results lend further support to an association of large-artery stiffness with WMH burden, and extend the findings to markers of carotid stiffness, additionally demonstrating that this association is at least in part independent of carotid atherosclerosis. Markers of carotid stiffness were not associated with LIs in our sample, suggesting that carotid stiffness may have an important role in chronic hypoperfusion but not complete infarction in the territory of small arteries.33
The mechanisms underlying the association between carotid stiffness and WMH burden are speculative. Large-artery stiffening is an important feature of arterial aging, associated with increased risk of cardiovascular events,34 but also with reduced glomerular filtration rate and cognitive impairment.35–37 In contrast with other organs, kidney and brain are continually and passively perfused at high-volume flow throughout systole and diastole.38 This exposes small renal and cerebral vessels to highly pulsatile pressure and flow, eventually leading to hypertrophic remodeling and rarefaction of small arteries, causing chronic ischemia.39 Increase in vasomotor tone of small arteries and capillary rarefaction could in turn promote large-artery stiffening by increasing the amplitude of the reflected wave, leading to a vicious circle. Other mechanisms could include structural damage at the molecular and cellular level, occurring simultaneously in the wall of large and small arteries, such as increased extracellular matrix turnover, impaired vascular smooth muscle cell proliferation, and apoptosis,25 thus promoting ischemia, in the territory of small cerebral arteries. Interestingly, increasing circumferential wall stress was also associated with WMHV after adjustment for pulse pressure, suggesting a defect of arterial wall thickening, unable to normalize wall stress.
Strengths of our study include the large sample size in a population-based setting, the quantitative measurement of brain MRI markers, and simultaneous assessment of carotid structure and function. Although other studies have examined the relationship of large-artery atherosclerosis and stiffness with MRI markers of vascular brain injury, to our knowledge, none have simultaneously assessed their impact on MRI markers of ischemic SVD. Moreover, most studies have used markers of aortic stiffness. Carotid lumen diameter and carotid stiffness markers can be measured noninvasively, simultaneously with carotid plaque, and our data suggest that they could predict cerebral ischemic SVD above and beyond the presence of carotid plaque. Although the sample with measures of carotid function was smaller than the sample with measures of carotid structure, eligibility of participants for the substudy on carotid function depended only on the period of recruitment, thus a selection bias is unlikely. We used brachial blood pressure to calculate markers of carotid stiffness; however, because central and peripheral blood pressures converge with aging, this is unlikely to have influenced our findings substantially.25
The present findings, if confirmed, have 2 major implications. First, they suggest a strong relationship between large-artery aging and cerebral ischemic SVD, markers of carotid structure and function being associated with different components of SVD. This information could be used to optimize prevention strategies for cerebral SVD and their consequences on the risk of stroke and dementia. For instance, it might be of interest to explore whether antihypertensive treatments reducing primarily systolic blood pressure through a “de-stiffening” strategy are more effective than other antihypertensive protocols in preventing progression of WMH burden.40 Second, our findings imply that markers of carotid structure and function could be implemented as a screening tool to triage patients at high risk of cerebral ischemic SVD, requiring further, more costly investigations, including brain MRI.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of the 3C-Dijon Study for their important contributions.
GLOSSARY
- BI
brain infarct
- CCA
common carotid artery
- CI
confidence interval
- IMT
intima-media thickness
- LI
lacunar infarct
- L-WMHV
large white matter hyperintensity volume
- OR
odds ratio
- SVD
small-vessel disease
- WMH
white matter hyperintensity
- WMHV
white matter hyperintensity volume
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Marion Brisset: drafting the manuscript, statistical analysis, analysis and interpretation. Dr. Pierre Boutouyrie: critical revision of the manuscript for important intellectual content, study design, acquisition of data. Dr. Fernando Pico: critical revision of the manuscript for important intellectual content. Dr. Yicheng Zhu: critical revision of the manuscript for important intellectual content, acquisition of data. Dr. Mahmoud Zureik: critical revision of the manuscript for important intellectual content. Mrs. Sabrina Schilling: critical revision of the manuscript for important intellectual content, analysis and interpretation. Dr. Carole Dufouil and Dr. Bernard Mazoyer: critical revision of the manuscript for important intellectual content, acquisition of data. Dr. Stéphane Laurent: critical revision of the manuscript for important intellectual content, study design, acquisition of data. Dr. Christophe Tzourio: critical revision of the manuscript for important intellectual content, study design, acquisition of data, study supervision or coordination, obtaining funding. Dr. Stéphanie Debette: drafting/revising the manuscript for content, statistical analysis, study supervision or coordination.
STUDY FUNDING
The 3C-Study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Victor Segalen–Bordeaux II University, and the SanofiSynthélabo Company. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C-Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Fondation Plan Alzheimer, Conseils Régionaux of Bourgogne, Fondation de France, Ministry of Research-INSERM Program “Cohortes et collections de données biologiques,” Mutuelle Générale de l’Education Nationale, Institut de la Longévité, Conseil Général de la Côte d’or. Dr. Brisset is a recipient of a grant from the Fondation pour la Recherche Médicale. Dr. Debette is a recipient of a “Chaire d’Excellence Junior” grant from the Agence Nationale de la Recherche (ANR). Dr. Tzourio has received fees from the Fondation Plan Alzheimer, the Fondation de Recherche sur l'Hypertension Artérielle, and the ABBOTT Company for participating in scientific committees. He has also received investigator-initiated research funding from the French National Research Agency (ANR) and the Fondation Plan Alzheimer for the 3C-Study.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Longstreth WT, Jr. Brain vascular disease overt and covert. Stroke 2005;36:2062–2063. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619. [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Bis JC, Fornage M, et al. Genome-wide association studies of MRI-defined brain infarcts: meta-analysis from the CHARGE Consortium. Stroke 2010;41:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol 2011;69:928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996;27:1641–1646. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha I, Takahashi T, Nomura E, et al. Association between central systolic blood pressure, white matter lesions in cerebral MRI and carotid atherosclerosis. Hypertens Res 2009;32:869–874. [DOI] [PubMed] [Google Scholar]
- 8.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke 2009;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolio TA, Burke GL, O'Leary DH, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol 1999;19:356–365. [DOI] [PubMed] [Google Scholar]
- 10.3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003;22:316–325. [DOI] [PubMed] [Google Scholar]
- 11.Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon Study. Biol Psychiatry 2008;63:663–669. [DOI] [PubMed] [Google Scholar]
- 12.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
- 13.Maillard P, Delcroix N, Crivello F, et al. An automated procedure for the assessment of white matter hyperintensities by multispectral (T1, T2, PD) MRI and an evaluation of its between-centre reproducibility based on two large community databases. Neuroradiology 2008;50:31–42. [DOI] [PubMed] [Google Scholar]
- 14.Debette S, Courbon D, Leone N, et al. Tea consumption is inversely associated with carotid plaques in women. Arterioscler Thromb Vasc Biol 2008;28:353–359. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 16.Calvet D, Boutouyrie P, Touze E, Laloux B, Mas JL, Laurent S. Increased stiffness of the carotid wall material in patients with spontaneous cervical artery dissection. Stroke 2004;35:2078–2082. [DOI] [PubMed] [Google Scholar]
- 17.Van Bortel LM, Spek JJ. Influence of aging on arterial compliance. J Hum Hypertens 1998;12:583–586. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K, Matsumoto M, Shono T, Toyokawa S, Moriki A. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J Stroke Cerebrovasc Dis 2007;16:14–20. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens Res 2007;30:767–773. [DOI] [PubMed] [Google Scholar]
- 20.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol 1979;36:65–73. [DOI] [PubMed] [Google Scholar]
- 22.Benetos A, Laurent S, Hoeks AP, Boutouyrie PH, Safar ME. Arterial alterations with aging and high blood pressure: a noninvasive study of carotid and femoral arteries. Arterioscler Thromb 1993;13:90–97. [DOI] [PubMed] [Google Scholar]
- 23.Wikstrand J, Wiklund O. Frontiers in cardiovascular science: quantitative measurements of atherosclerotic manifestations in humans. Arterioscler Thromb 1992;12:114–119. [DOI] [PubMed] [Google Scholar]
- 24.Safar ME, Blacher J, Mourad JJ, London GM. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke 2000;31:782–790. [DOI] [PubMed] [Google Scholar]
- 25.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 26.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006): an update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75–80. [DOI] [PubMed] [Google Scholar]
- 27.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness: adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997;28:2442–2447. [DOI] [PubMed] [Google Scholar]
- 28.Ochi N, Kohara K, Tabara Y, et al. Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am J Hypertens 2010;23:889–894. [DOI] [PubMed] [Google Scholar]
- 29.Kuo HK, Chen CY, Liu HM, et al. Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int J Cardiol 2010;143:184–191. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility—Reykjavik Study. Brain 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 2008;52:1120–1126. [DOI] [PubMed] [Google Scholar]
- 32.van Elderen SG, Brandts A, Westenberg JJ, et al. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol 2010;20:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol 2011;122:171–185. [DOI] [PubMed] [Google Scholar]
- 34.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005;25:932–943. [DOI] [PubMed] [Google Scholar]
- 35.Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007;49:1202–1206. [DOI] [PubMed] [Google Scholar]
- 36.Briet M, Collin C, Karras A, et al. Arterial remodeling associates with CKD progression. J Am Soc Nephrol 2011;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanon O, Haulon S, Lenoir H, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke 2005;36:2193–2197. [DOI] [PubMed] [Google Scholar]
- 38.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 39.Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension 2009;54:388–392. [DOI] [PubMed] [Google Scholar]
- 40.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease: is it possible to break the vicious circle? Atherosclerosis 2011;218:263–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.