SECTION 1
A 28-year-old man presented with progressive lethargy and confusion over 2 days. He has a longstanding history of IV heroin use. His last use was 2 weeks ago, and he completed inpatient detoxification 2 days before admission. After he returned home from rehabilitation, his roommate noticed that he became increasingly confused. On the day of admission, he was febrile, unable to get out of bed, or speak, although he was able to nod yes and no to simple questions. He also lost urinary control.
His medical history was significant for hepatitis C and cardiomyopathy, both presumed to be associated with IV drug use. His cardiac ejection fraction was 25% to 30% 8 months prior, and improved to 45% to 50% 3 months before admission. He refused to take all medications.
On examination, the patient's temperature was 38.9°C, pulse 82, respiration 28, and blood pressure 206/83 mm Hg. His neck was supple. He was lethargic, confused, and was only able to partially follow simple, 1-step commands. His pupils were equal and fully reactive. His eyes tracked to voice in all directions. He had no facial asymmetry but had moderate drooling. His right upper and lower extremities appeared to be weaker compared with the left. He withdrew to painful stimuli equally in all extremities. There was no evidence of abnormal movements such as dystonia, chorea, tremor, or dysmetria. The right patella reflex was brisk compared with the left; deep tendon reflexes were otherwise normal. He had a right Babinski and left plantar flexion.
Questions for consideration:
What is the differential diagnosis at this time?
What tests would you order?
SECTION 2
Given the patient's clinical picture of fever and global cerebral dysfunction with additional focal neurologic deficit in the setting of recent IV drug use, meningoencephalitis was primary in the differential diagnosis. Source of infections include septic emboli from possible bacterial endocarditis based on the history of IV drug use and cardiomyopathy, as well as direct hematogenous spread from an unclean IV site or contaminated drug. Coccidiomycosis is a common infection in the southwest United States and should be considered. However, the course of this disease is usually more indolent; acute deterioration is uncommon. Ischemic infarcts and toxic metabolic encephalopathy due to drug overdose were also possible, although less likely in the setting of a febrile illness.
The patient's mental status deteriorated rapidly in the emergency department. He was unable to protect his airway, and was intubated to ensure adequate ventilation. Initial laboratory studies were remarkable for a white blood cell count of 21,800/mm3 (80% segmented forms) and a sodium level of 128 mmol/L. A CT scan of the head revealed a 4.2-cm hypodensity in the region of the left basal ganglia with local mass effect and effacement of the ventricular system. A toxicology screen was positive for benzodiazepine and cannabinoids. Results of blood cultures, HIV test, rapid plasma reagin, and coccidiomycosis serum and CSF titers were negative. CSF analysis showed glucose 57 mg/dL, protein 111 mg/dL, white blood cell count 400/mm3 with 67% lymphocytes, and red blood cell count 77,000/mm3 (traumatic). Gram stain, bacterial and fungal cultures, viral PCRs, as well as acid-fast bacilli culture and smear were all negative in the CSF. MRI of the brain (figure 1, A–F) showed restricted diffusion and T2 hyperintensity of the basal ganglia (L > R) with minimal contrast enhancement. The T2 signal abnormality also extended to the left medial temporal area and left cerebral peduncle, which did not demonstrate diffusion restriction. Other diagnostic tests performed during the patient's hospital course include a transesophageal echocardiogram showing normal ejection fraction without any valvular abnormalities, and an EEG showing diffused slowing without epileptiform abnormalities.
Figure 1. Neuroimaging.
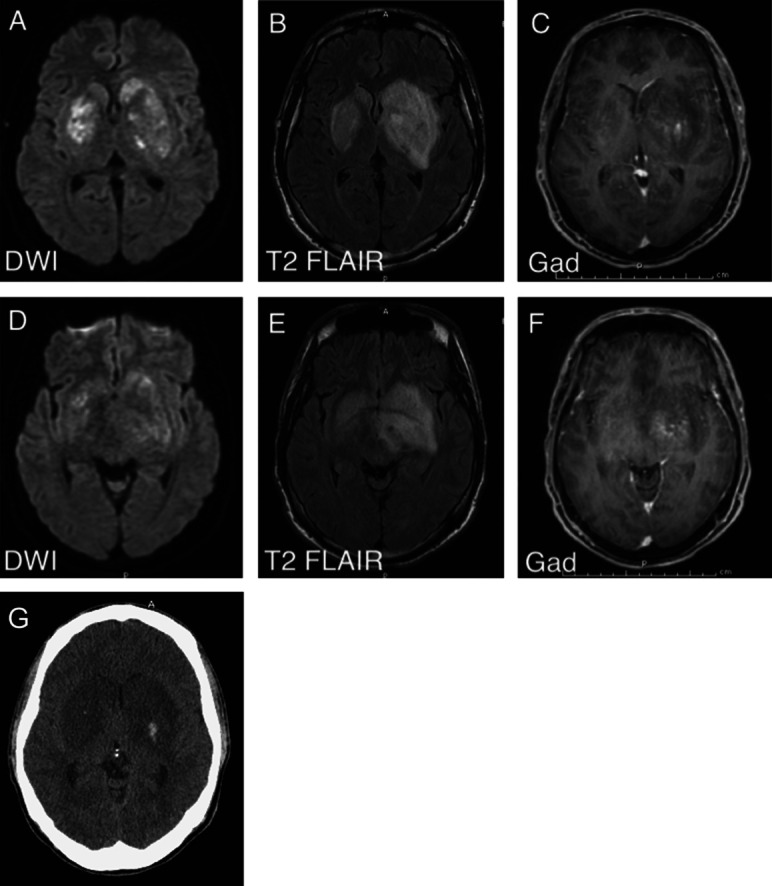
(A) MRI diffusion-weighted imaging (DWI) shows restricted diffusion in the lateral basal ganglia. (B) T2 fluid-attenuated inversion recovery (FLAIR) shows vasogenic and cytotoxic edema in the bilateral basal ganglia. (C) T1 postcontrast image shows minimal enhancement in the left basal ganglia. (D, E, F) Corresponding DWI, T2 FLAIR, and T1 postcontrast sequences at the level of the midbrain. (G) CT scan showing hemorrhagic transformation of the bilateral basal ganglia lesions on hospital admission day 3.
Questions for consideration:
How does the above information change your differential diagnosis?
What is the next step in management of this patient?
SECTION 3
At this time, brain abscess remains high on the list of differential diagnosis based on the CSF results and lesions found on brain MRI. CNS neoplasm was also considered; however, the rapidly progressive course made it less likely. The patient was empirically treated with vancomycin and ceftriaxone in the emergency department. Acyclovir was added for empiric treatment of herpes encephalitis based on the involvement of the left mesial temporal lobe. Antifungal therapy was considered but not started because the serologic testing and CNS fungal cultures were negative. Repeat head CT on hospital day 3 showed focal areas of hemorrhagic transformation (figure 1G). Despite treatments, the patient continued to deteriorate clinically with decerebrate posturing and dilated, minimally reactive pupils. Ventilation support was withdrawn after family consent was obtained. He died on hospital day 5.
At autopsy, the brain was grossly edematous. A 9.1 × 3.2 × 3.1 cm focus of hemorrhage and necrosis was found in the left basal ganglia. Right basal ganglia demonstrated similar pathology, but to a lesser extent (figure 2A). The midbrain, pons, and medulla had many dark petechial hemorrhages. Hematoxylin & eosin–stained sections of the caudate, accumbens, and putamen revealed multinucleate giant cells surrounding broad aseptate fungal hyphae typical of the order Mucorales, highlighted by Grocott methenamine-silver stain (figure 2, B and C).
Figure 2. Pathology.
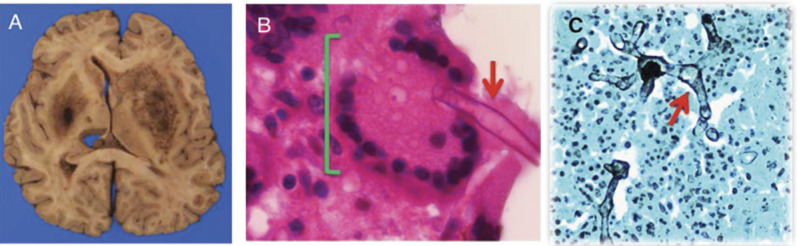
(A) Gross histologic section of brain showing hemorrhage and necrosis in the bilateral basal ganglia. (B) Microscopic histologic section from the left basal ganglia showing multinucleate giant cell (green bracket) and a broad aseptate fungal hyphae (red arrow) consistent with mucormycosis (hematoxylin & eosin, 1,000× magnification). (C) Branched, aseptate hyphae (red arrow) highlighted by Grocott methenamine-silver stain (400× magnification).
DISCUSSION
Cerebral mucormycosis is a fungal disease with an aggressive course and high mortality. It is angioinvasive and causes tissue necrosis.1 The infection typically presents in immunocompromised patients with diabetes mellitus, chronic immunosuppressive therapy, or hematologic/lymphoproliferative malignancies.2 The spread of the infection usually starts in the rhinosinus region and penetrates into the orbit, optic nerve, and brain parenchyma, resulting in lesions in the frontal and temporal lobes.
Cerebral mucormycosis is rare among healthy hosts. Including our case described here, only 35 biopsy- or autopsy-proven cases in immunocompetent individuals have been reported in the Western literature since 1970.3–10 Most patients were young with a mean age of 31 years (range 21–53 years). Sixty-three percent of the reported cases were men and 37% were women. Most cases except for 2 were associated with IV drug use. The most common drug of abuse was heroin (29%), followed by cocaine (23%). HIV status was available in approximately half of the reported cases, and did not seem to be a risk factor for developing the disease. Rhizopus and Mucor species were identified in 31% and 6% of the cases, respectively. No species were reported in the remaining 63%.
Interestingly, in contrast to the cortical infection sites in immunocompromised patients, Mucor showed remarkable predilection for the basal ganglia in IV drug users (89%). These lesions were either unilateral or bilateral with minimal contrast enhancement. The basis of such predilection is unclear. One of the proposed mechanisms is that fungal spores from contaminated drugs spread hematogenously and deposit in the heavily vascularized basal ganglia, causing both arterial and venous thrombosis.6 The propensity of Mucor to invade vascular structures together with vessel circumference relative to spore diameter may have a vital role in the location of the infection in these patients.5
In terms of treatment, IV amphotericin B was the only therapy that consistently provided survival benefit.3–10 The disease was uniformly fatal if not treated with amphotericin B. The survival rate increased to 61% with prompt diagnosis and initiation of treatment. Even with early treatment, a significant proportion of survivors had neurologic deficits at the time of their hospital discharge. Among the survivors, no significant differences in outcome were noted in treatment with steroids or surgical drainage.10
When a basal ganglia lesion with minimal contrast enhancement is detected on brain MRI in an IV drug user who presents with acute and rapidly progressive neurologic deficits, mucormycosis should be considered early in the clinical presentation regardless of HIV status. We stress the importance of including mucormycosis in the differential diagnosis because of the extremely high morbidity and mortality rate. Prompt tissue biopsy is necessary in obtaining an accurate diagnosis. Treatment with IV amphotericin B may improve survival rate by more than 60%.
AUTHOR CONTRIBUTIONS
Dr. Lin: resident who initially evaluated and treated the patient in this case, gathered all relevant clinical information, performed literature research, and analyzed all available literature data; manuscript writer. Dr. Thompson: resident who performed autopsy and provided final pathologic diagnosis, histologic images, and pathology descriptions. Dr. Coull: mentor; drafting/revising the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sugar AM. Mucormycosis. Clin Infect Dis 1992;14(suppl 1):S126–S129. [DOI] [PubMed] [Google Scholar]
- 2.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54(suppl 1):S23–S34. [DOI] [PubMed] [Google Scholar]
- 3.Air EL, Vagal AA, Kendler A, McPherson CM. Isolated cerebellar mucormycosis, slowly progressive over 1 year in an immunocompetent patient. Surg Neurol Int 2010;1:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blázquez R, Pinedo A, Cosín J, Miralles P, Lacruz C, Bouza E. Nonsurgical cure of isolated cerebral mucormycosis in an intravenous drug user. Eur J Clin Microbiol Infect Dis 1996;15:598–599. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter M, Polk C, Castellani R, et al. Encephalitis of the basal ganglia in an injection drug user. Clin Infect Dis 2007;4:1522–1524. [DOI] [PubMed] [Google Scholar]
- 6.Gollard RR, Rabb C, Larsen C, Chandrasoma P. Isolated cerebral mucormycosis: case report and therapeutic considerations. Neurosurgery 1994;34:174–177. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RJ, Rothman M, Fiore A, Goldblum SE. Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect Dis 1994;19:1133–1137. [DOI] [PubMed] [Google Scholar]
- 8.Metellus P, Laghmari M, Fuentes S, et al. Successful treatment of a giant isolated cerebral mucormycotic (zygomycotic) abscess using endoscopic debridement: case report and therapeutic considerations. Surg Neurol 2008;69:510–515. [DOI] [PubMed] [Google Scholar]
- 9.Pandian JD, McCarthy JS, Goldschlager T, Robertson T, Henderson RD. Rhizomycosis infection in the basal ganglia. Arch Neurol 2007;64:134–135. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi SU, Freedman JD. Isolated central nervous system mucormycosis. South Med J 1994;87:997–1000. [DOI] [PubMed] [Google Scholar]


