Abstract
Nonmedical use of prescription stimulant medication such as methylphenidate (MPH) has increased among college students over the past several years. Common motivations for use include enhancements in cognition and subjective arousal. As it is unclear whether stimulant medication exerts the same effect on healthy individuals as for those with ADHD, it is possible that many reported effects of prescription stimulants by healthy individuals may stem from placebo effects, which may be an important mechanism underlying initiation and maintenance of nonmedical use. This study examined whether placebo effects influence reports of subjective mood and cognitive performance among college students who endorsed several risk factors for prescription stimulant misuse (i.e., low GPA, fraternity/sorority involvement, binge drinking, cannabis use). Ninety-six subjects (60% male) completed a battery of cognitive tests and questionnaires assessing present mood state on two occasions. Forty-seven participants were randomized to an experimental condition and orally ingested what they believed to be MPH, though actually placebo, on one visit and received no medication on the other visit. The control group received no medication on either visit. During the administration visit, experimental participants reported feeling significantly more high and stimulated compared to the non-administration visit and to the control subjects. However, cognitive enhancement differences were not generally seen between visits or groups. This research demonstrates that placebo effects for prescription stimulants do influence subjective mood and may be implicated in nonmedical stimulant use. This knowledge may be useful in challenging prescription stimulant-related expectancies to decrease the prevalence of use among college students.
Keywords: prescription stimulants, placebo effects, expectancy effects, cognition, college students
Introduction
Prescription stimulant medications, such as methylphenidate (MPH; Ritalin, Concerta), and amphetamine-dextroamphetamine combination (Adderall) are primarily used in the treatment of attention-deficit/hyperactivity disorder (ADHD). MPH is the most frequently prescribed ADHD medication (Greenhill et al., 2002). According to the US Drug Enforcement Administration (DEA), MPH has been the fourth most prescribed controlled substance in the United States since 2003, behind hydrocodone, oxycodone, and codeine, with over 58,000 Americans purchasing MPH in 2006 (Department of Justice, 2008). Both the production and prescription of MPH have risen steadily since 1990, as the diagnosis of ADHD has concurrently increased (Goldman, Genel, Bezman, & Slanetz, 1998; Safer, Zito, & Fine, 1996).
Prevalence of nonmedical prescription stimulant use
Research on nonmedical prescription stimulant use (i.e., use of a prescription stimulant without a prescription or overuse of one’s medication) was largely overlooked prior to 2000. However, between 1989 and 1993, emergency department admissions involving MPH increased by as much as 715%, suggesting that nonmedical MPH use may have been increasing over the past two decades (Substance Abuse and Mental Health Association [SAMHSA], 1990, 1996). More recently, several studies have been published on nonmedical prescription stimulant use, the majority of which have focused exclusively on prevalence rates of MPH misuse and associated risk factors. Empirical studies of adolescents and young adults, including large nationwide surveys, provide support for the idea that nonmedical MPH use is occurring at escalating rates. The past decade has seen a surge in prevalence rates of nonmedical stimulant use among both adolescents (Johnston, O’Malley, Backman, & Schulenberg, 2005) and young adults (SAMHSA, 2004), resulting in increased substance-related problems (Stockl et al., 2003). Additionally, the DEA has expressed concern that the diversion of MPH is increasing and that rises in MPH production have resulted in increased illicit availability of the drug (DEA, 2000). A recent paper by Arria and DuPont (2010) highlights the need to address the increasing prevalence of nonmedical use and urges healthcare providers, parents, university officials, and law enforcement to take action to discourage and reduce this behavior.
Though nonmedical use of prescription stimulants has increased for several age groups, the majority of research on nonmedical use has focused on undergraduate college students. Over the past decade, the nonmedical use of several prescription drugs has increased in this population, to the extent that nonmedical use of prescription medication is second only to marijuana as the most common form of illicit drug use among college students (Johnston et al., 2005). A 2001 nationwide survey of more than 10000 students from 4-year universities in the United States found that nearly 7% reported lifetime nonmedical stimulant use and approximately 4% reported past-year use (McCabe, Knight, Teter, & Wechsler, 2005). However, prevalence rates varied as a function of the individual college, with past-year rates ranging from 0% to 25%. Colleges with the highest past-year prevalence rates were typically located in the northeastern United States, which is corroborated by other reports (e.g., Johnston et al., 2005). A more recent review revealed that lifetime rates of nonmedical stimulant use range from 5% to as high as 35%, demonstrating the increasing prevalence of nonmedical use (Wilens et al, 2008).
Prevalence rates for nonmedical use of prescription stimulants additionally appear to be highest amongst individuals who do not have a legitimate prescription for the medication. For example, White, Becker-Blease, and Grace-Bishop (2006) found that 90% of participants reporting nonmedical stimulant use in their sample had never been diagnosed with an attention disorder and did not have a prescription. Common motivations for nonmedical stimulant use are to improve concentration, enhance alertness, and to get high/party (Barrett, Darredeau, Bordy, & Pihl, 2005; Low & Gendaszek, 2002, Teter et al., 2005). Though increasing prevalence rates of nonmedical stimulant use have been documented, no empirical studies have examined prevention strategies in this population.
Effects of prescription stimulant medication in healthy adults
Several researchers have examined the subjective effects elicited by MPH. MPH appears to produce a reliable pattern of subjective effects in both healthy adults and those with histories of substance abuse. Increases in feeling stimulated, alert, and good are frequently reported (Heil et al., 2002; Rush & Baker, 2001; Stoops et al., 2005). In healthy adults, the amphetamine scale of the Addiction Research Center Inventory (ARCI) shows increased scores with MPH compared to placebo (Heil et al., 2002; Rush, Essman, Simpson, & Baker, 2001), and Volkow et al. (2004) reported increased ratings of interest and motivation on a mathematical task following administration of MPH. Cocaine users report similar subjective effects following administration of MPH as with administration of cocaine, such as feeling good, increased motivation, and willingness to take again and pay for the drug (Rush & Baker, 2001).
However, subjective effects of several drugs are affected by expectation to receive the drug, or placebo effects (Kirk, J.M., Doty, P., & De Wit, H., 1998; Yamamoto, R.T., Karlsgodt, K.H., Rott, D., Lukas, S.E., & Elman, 2007). Expectancy theory posits that placebo effects are mediated by explicit expectations; thus, a drug produces an effect because an individual ingesting the drug expects that effect (Stewart-Williams & Podd, 2004). Placebo effects have been elicited by expectation to receive MPH, as Volkow et al. (2003) demonstrated that cocaine users reported substantially greater feelings of being high when they expected and received MPH compared to when they received MPH but did not expect it. Furthermore, route of administration may interact with expectancies to influence reports of subjective mood. Lile and colleagues (in press) found that reports of feeling high and stimulated were only consistently significantly elevated compared to placebo following intranasal doses of d-amphetamine, but not following oral doses.
There is abundant research documenting the efficacy of MPH in improving cognition in individuals with ADHD (see Spencer et al., 1996). This effect may directly contribute to the nonmedical use of prescription stimulant medication to improve academic performance. However, little research has examined cognitive enhancement by MPH in healthy adults. The extant literature is inconclusive. For example, Stoops and colleagues (2005) determined that MPH dose-dependently increased the ability to correctly answer arithmetic problems. Similarly, MPH decreased reaction time and errors on a continuous performance task (Cooper et al., 2005) and a vigilance task (Camp-Bruno & Herting, 1994) relative to placebo. However, no significant improvement has been seen in immediate and delayed free recall (Camp-Bruno & Herting, 1994). Two studies found no significant improvement in psychomotor performance as measured by the Digit Symbol Substitution Task (Heil et al., 2002; Rush et al., 2001), though a recent study demonstrated improvement under conditions of intranasal and oral d-amphetamine (Lile et al., in press). A recent review posits that stimulant medication may not truly be the efficacious ‘cognitive enhancers’ that many believe them to be, both for adults with and without ADHD (Advokat, 2010). Though studies have confirmed that stimulant medication improves sustained attention, at least among adults diagnosed with ADHD, research also suggests that other indices of cognition such as distractibility, planning, and impulsivity are not enhanced. Additionally, adults without ADHD may actually demonstrate cognitive impairment on several tasks due to symptoms of arousal from stimulant medication.
Moreover, Turner et al. (2005) examined the neurocognitive effects of MPH in participants with a diagnosis of ADHD and those with “attentional difficulties”, but without a DSM diagnosis. The authors found that although individuals with ADHD improved on several indices of cognition (e.g., sustained attention, visual memory), those with “attentional difficulties” did not show the same improvement. Thus, MPH may not improve cognition in adults without ADHD in the same way that it does for adults with an ADHD diagnosis. Given these discrepancies, cognitive improvements by MPH in healthy adults may also be a function of expectancy and placebo effects rather than true pharmacological effects. This possible explanation deserves further attention.
Current study
The purpose of the current study was to examine whether one’s expectation alone to receive a prescription stimulant medication, specifically MPH, would alter mood and cognitive performance. Though much research has demonstrated that placebo effects influence behavior and mood for a variety of substances of abuse (e.g., Kirk, J.M., Doty, P., & De Wit, H., 1998; Yamamoto, R.T., Karlsgodt, K.H., Rott, D., Lukas, S.E., & Elman, 2007), placebo effects for prescription stimulants have not been adequately examined. We hypothesized that when healthy college students expect to receive MPH, they will exhibit superior cognitive performance and will report elevated positive mood ratings (e.g., stimulated, good, high), compared to when they do not expect to receive any drug. As nonmedical users of prescription stimulants often cite using these drugs to enhance concentration and attention, typically in an academic setting, as well as to feel high and euphoric, knowledge of placebo effects for prescription stimulants may be important in developing treatment programs for this behavior. Specifically, challenging individuals’ expectancies about the effects of a drug by demonstrating the influence of placebo effects is effective in reducing alcohol use (Darkes & Goldman, 1993) and may also help to prevent and reduce nonmedical prescription stimulant use.
Method
Participants
Participants were recruited via flyers posted on a Northeastern university campus. Interested participants completed a telephone screen to determine eligibility. Participants were eligible for the study if they were between 18 to 25 years of age and currently enrolled in college, which are risk factors for nonmedical prescription stimulant use (Johnston et al., 2005; Kroutil et al., 2006). Participants also had to report lifetime nonuse of any prescription stimulant medication and be native English speakers. Participants were prescription stimulant-naïve because we wanted to create a credible placebo and did not want participants’ previous experiences with stimulants to affect the results of the current study. Additionally, participants had to report at least 2 of the following risk factors for prescription stimulant misuse: (1) involvement in a fraternity of sorority (McCabe et al., 2005; Shillington et al., 2006), (2) GPA below 3.5 (Teter et al., 2005; McCabe, Teter, & Boyd, 2006), (3) at least one episode of binge drinking in the past two weeks (defined as the consumption of at least five drinks in a row for men and at least four drinks in a row for women; Herman-Stahl, Krebs, Kroutil, & Heller, 2007; McCabe et al., 2005; Shillington et al., 2006), and (4) current cannabis use (defined as past month cannabis use; McCabe et al., 2005). We aimed to recruit participants who were considered at-risk for prescription stimulant misuse to best generalize our results to the population of nonmedical prescription stimulant users and to assess whether they would initiate nonmedical use in the future, which is part of a separate investigation. Though Caucasian and male individuals have been found to be at-risk for prescription stimulant misuse, we did not recruit based on those criteria because we did not want to unfairly bias our sample in terms of sex or race. Individuals with a current or past psychiatric diagnosis, current or past prescription for psychiatric medication, history of psychiatric illness in first-degree relatives, and lifetime use of illicit drugs other than cannabis on more than 50 occasions were excluded. All participants were provided monetary compensation for their involvement. This study was approved by the University at Albany Institutional Review Board and informed consent was obtained from all participants prior to beginning the study.
One hundred and six individuals consented to participate in the study. Nine participants did not complete the study and another participant was withdrawn from the study due to health reasons, resulting in 96 completers. Fifty-seven participants were male (60%) and participants ranged in age from 18 to 23 (M = 19.57, SD = 1.26). Average years of education was 13.49 (SD = 1.07) and participants were primarily Caucasian (71%). Other ethnicities reported were African American (8%), Hispanic (8%), Asian (4%), mixed race (4%), and Native American (1%). All participants were currently enrolled full-time in a 4-year college.
Procedure
Experimental procedure
Eligible participants were invited to participate in our study and were informed that they would visit our laboratory on two occasions in the morning. They were provided with a cover story stating that the purpose of the study was to examine the influence of MPH on mood and cognitive performance. They were informed that they would complete questionnaires and cognitive tests on both laboratory visits and that they may receive a small dose of Ritalin (20mg) on one of the visits; otherwise, they would receive no medication. Participants completed two laboratory visits approximately two weeks apart. Latency to return for visit 2 ranged from 14 – 32 days (M = 16.50, SD = 4.14).
Prior to beginning the study, participants were randomized to either an experimental condition or a control condition. Participants in the experimental condition received what they were told was 20mg of Ritalin on one of the two laboratory visits, counterbalanced. In fact, all experimental participants received a gelatin placebo pill that resembled MPH. The placebo MPH was given to experimental participants at the beginning of the laboratory visit prior to completing any measures of mood or drug effects. Twenty minutes elapsed between orally ingesting the pill and beginning the assessment portion of the study. Participants were informed that this was to allow the medication enough time to take effect, thus creating a more credible placebo. Experimental participants would not receive any medication on the other visit. Control participants were never given medication on either visit. Following the end of the second visit, all experimental participants were debriefed and informed that they received a placebo medication. They completed a manipulation check questionnaire including a 10-point Likert scale to assess the extent to which they believed they had ingested active MPH. Control participants were not debriefed about the true purpose of the study at the end of visit 2 because they were still being assessed during a 6-month follow-up period, which is the subject of an ongoing investigation regarding initiation of prescription stimulant misuse.
Assessments
During visit 1, all participants completed a demographic questionnaire and were assessed for lifetime substance use via the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/NP; First, Spitzer, Gibbon, & Williams, 2002). Following either this or the 20-minute medication delay (for experimental subjects, if the first visit was the administration visit), participants then completed the 49-item Addiction Research Center Inventory Short Form (ARCI; Martin et al., 1971) to assess for current drug-related effects (i.e., euphoria (MBG scale), dysphoria (LSD scale), intellectual energy and efficiency (BG scale), amphetamine effects (AMPH scale), and sedation (PCAG scale)) and several visual analogue scales (VAS; Roache & Griffiths, 1989) to assess current mood and physiological state (i.e., “good”, “bad”, “sick”, “high”, “stimulated”, and “motivated”), as well as their rating of how well they expected to perform on the upcoming cognitive tests. The 7 analog scales consisted of 100 mm lines, anchored at each end by “not at all” and “extremely”. Participants were instructed to complete these questionnaires in accordance with how they were feeling immediately at that moment.
Immediately following these questionnaires, participants completed a battery of cognitive tests in the following order: the California Verbal Learning Test – Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), the Rivermead Behavioural Memory Test II – Story Recall (RMBT-II; Wilson, Cockburn, & Baddeley, 2000), the Digit Span and Digit Symbol Substitution Test (DSST) subtests from the Wechsler Adult Intelligence Scale-III (Wechsler, 1997), verbal and quantitative SAT questions (Princeton Review, 2006), and the Conners’ Continuous Performance Test II (CPT II; Connors & MHS Staff, 2000). Instructions were presented at the beginning of each test. These tests were selected to assess performance in a variety of different cognitive domains including contextual and non-contextual short- and long-term memory, working memory, psychomotor processing, attention, impulsivity, and vigilance. The SAT questions were included to provide a measure of ecological validity. Following the cognitive tests, participants again completed the ARCI and the VAS in accordance with their current mood and physiological state. The prior VAS question assessing anticipated performance was changed to assess how well the participant believed he/she performed. Participants completed the same questionnaires and tests on both laboratory visits and were presented with the same instructions. Alternate versions of the CVLT-II, RMBT, SAT questions, and CPT II were used in an effort to reduce the influence of practice effects.
In an attempt to control for confounding variables which may affect mood and performance on a given study visit, participants began both study sessions between the hours of 8–11am. Study visits were completed between 1 – 1.5 hours, with the initial study visit and the administration visit necessitating the most time. Both study visits began at the same time in the morning for each individual. Participants were asked to abstain from any intoxicating substances, food, or beverages except water for at least 8 hours prior to each study visit. Participants’ reports of last use of food, alcohol, caffeine, cigarettes, and illicit substances were obtained to ensure that participants followed these instructions. Additionally, participants reported the hours of sleep obtained during the previous night, and completed the State-Trait Anxiety Inventory (Spielberger, 1983) prior to beginning cognitive testing. Finally, all study sessions and cognitive testing were completed by the first author.
Data Analysis
All data were analyzed via SPSS 17.0 for Windows. To examine whether improved performance and elevated subjective mood ratings were associated with the expectation to receive MPH, we first analyzed the experimental group using repeated measures ANOVAs and MANOVAs. Because many of the constructs of interest have multiple correlated dependent measures, theoretically similar DVs were grouped together and examined using MANOVAs. The main effect for visit (administration or non-administration visit) and the interaction between visit and order (receiving placebo administration on the 1st or 2nd visit) was analyzed to determine whether any order effects exist. Between-subjects analyses were then employed using a mixed-effects design to examine performance and mood across visits (administration or non-administration) and between groups (experimental and control). During randomization, the visit in which administration would have occurred was assigned and counterbalanced for control subjects for the purpose of these analyses, even though they would never expect to receive medication. Though there is no procedural difference in study visits for control participants, we wanted to ensure that differences between visits for the experimental group could only be attributed to expectation to receive MPH. Thus, we chose to present the control subjects’ data for both study visits separately rather than average their scores in order to assess the stability of participants’ responses across visits, which will aid in interpretation of results and assessing the validity of the placebo manipulation. Repeated measures ANCOVAs and MANCOVAs were used for these analyses while controlling for the effects of order. As both groups had equal sex proportions, supplemental analyses were conducted to examine mood and cognition varied by sex.
Results
Forty-seven participants were randomized to the experimental group and 49 participants were in the control group. Groups were not significantly different in terms of sex, age, race, involvement in the Greek system, years of education, GPA, past-month marijuana use, or diagnoses of alcohol abuse, alcohol dependence, marijuana abuse, and marijuana dependence (all ps > .05). Participants in the expectancy condition were more likely to report recent binge drinking (χ2 = 4.00, df = 1, p = .045); however, the expected frequency for 2 cells was less than 2, which may have increased the sensitivity of this test to finding a significant difference. In fact, a logistic regression predicting group membership from binge drinking was non-significant (Wald χ2 = .000, df = 1, p > .05), with an odds ratio of .000, indicating a very small effect. Demographic information for both conditions is presented in Table 1.
Table 1.
Comparisons of Demographic Indices by Condition
| Experimental: Mean (SD) n = 47 | Control: Mean (SD) n = 49 | |
|---|---|---|
| Sex | 28 M/19 F | 29 M/20 F |
| Age | 19.64 (1.29) | 19.51 (1.24) |
| Years of Education | 13.49 (1.08) | 13.49 (1.06) |
| GPA | 3.05 (0.48) | 3.04 (0.56) |
| Ethnicity | 35 C/3 AA/2 H/3 AS/1 NA/3 M | 36 C/5 AA/6 H/1 AS/1 M |
| Greek Involvement | 12.8% | 16.3% |
| Binge Drinking (past 2 weeks) | 100%* | 91.8% |
| Marijuana Use (past month) | 68.1% | 79.6% |
| Alcohol Abuse | 8.5% | 10.2% |
| Alcohol Dependence | 23.4% | 24.5% |
| Marijuana Abuse | 21.3% | 18.4% |
| Marijuana Dependence | 10.6% | 18.4% |
Note. Sex: M = Male, F = Female. Ethnicity: C = Caucasian, AA = African American, H = Hispanic, AS = Asian, NA = Native American, M = Mixed race. Percentage numbers indicate positive responses.
p < .05
Experimental (within-subject) group differences on administration and non-administration visits
To determine whether subjective mood and performance differences occurred on the placebo administration visit where participants expected to take Ritalin compared to the non-administration visit where no medication was expected, we first examined the 47 participants in the experimental group separately. Twenty-four participants received the placebo MPH administration on visit 1 while 23 participants received placebo administration on visit 2. Hours of sleep (administration: M = 6.81, SD = 1.28; non-administration: M = 6.89, SD = 1.26) and anxiety scores (administration: M = 11.94, SD = 7.68; non-administration: M = 12.34, SD = 7.83) were not significantly different across visits (sleep: t (46) = −0.39, p = .695; anxiety: t (46) = −0.40, p = .690) and thus were not used as covariates in our analyses. Participants reported a mean believability score of 5.14 (SD = 2.78) for the extent to which they thought they ingested active MPH, with a range from 0 – 9. Analyses were conducted both for the entire group and again after removing participants whose believability score was lower than 3 (n = 6). The results were not significantly different between analyses, so to be conservative we are presenting data from the whole sample.
Subjective mood
Four repeated-measures MANOVAs were conducted to examine differences on the 5 drug effect subscales for the ARCI and the 6 VAS items at pre- and post- test. There were significant multivariate main effects of visit for both measures at pre- and post- test (ARCI pre-test: F(5,41) = 6.49, p < .001, ηp2= .442; ARCI post-test: F(5,41) = 3.49, p < .05, ηp2 = .299; VAS pre-test: F(6,40) = 5.88, p < .001, ηp2 = .468; VAS post-test: F(6,40) = 2.93, p < .05, ηp2 = .305), with no significant main effects of order or visit x order interactions (all p’s > .05).
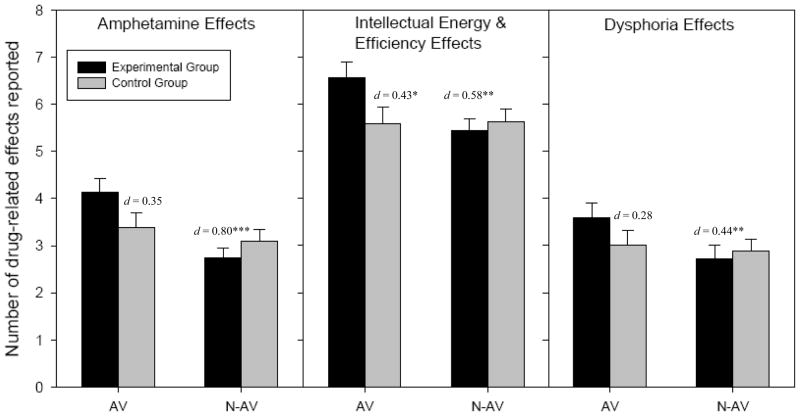
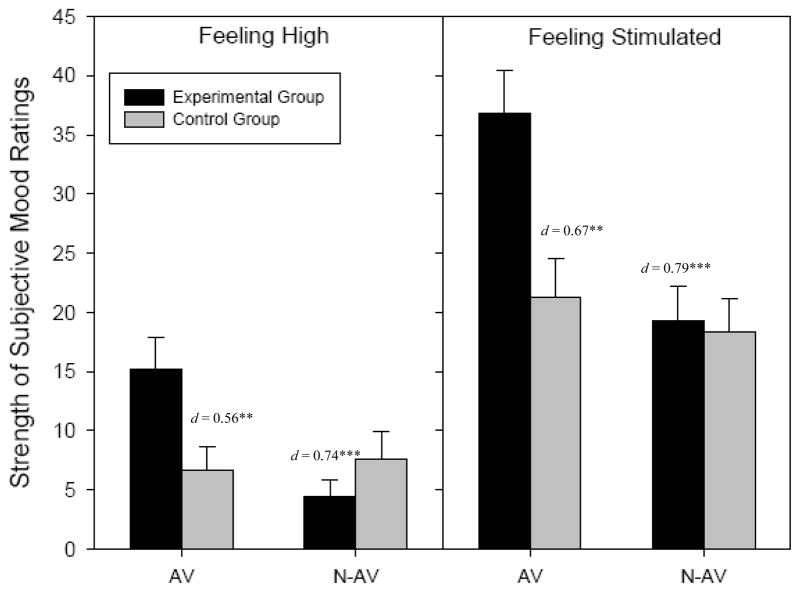
Examination of the specific ARCI subscales showed that during the administration visit, experimental participants reported higher levels of amphetamine effects and dysphoria at pre-and post-test (amphetamine: d = 0.80 and 0.47 respectively; dysphoria: d = 0.44 and 0.49 respectively), while also reporting higher intellectual energy and efficiency effects at pre-test (d = 0.58). During the administration visit, at both pre- and post-test participants also reported feeling significantly more high and more stimulated on the VAS (high: d = 0.74 and 0.70 respectively; stimulated: d = 0.79 and 0.41 respectively). There were no significant main effects of sex at pre- or post-test, nor were there significant sex x visit interactions for any mood indices (all p’s > .05).
Cognitive performance
Five repeated-measures MANOVAs were conducted to examine differences on 3 indices of the CVLT-II (immediate free recall total, short delay free recall score, and long delay free recall score), 2 indices of the RBMT-II Story Recall (immediate score and delayed score), 2 indices of Digit Span (forward and backward), 2 SAT scores (verbal and quantitative), and 3 CPT II subscales (inattention, impulsivity, and vigilance). Two repeated-measures ANOVAs were conducted to examine the performance expectancy VAS item and DSST score.
There was no significant main effect of visit for the rating of how well experimental subjects expected to perform at pre-test (F(1,45) = 1.24, p = .27, ηp2 = .027), though there was a significant visit x order interaction (F(1,45) = 6.38, p < .05, ηp2 = .124), demonstrating that subjects all expected to perform worse at visit 2. At the post-test assessment of how well subjects thought they performed, both the main effect for visit (F(1,45) = 4.14, p < .05, ηp2 = .084) and the visit x order interaction (F(1,45) = 5.91, p < .05, ηp2 = .116) were significant. Subjects thought they performed better on visit 2, though this effect was stronger for subjects who received the placebo administration on visit 1. There was not a significant effect of sex at pre- (F(1,43) = 1.51, p = .23) or post-test (F(1,43) = 2.17, p = .15).
Contrary to our hypothesis, experimental subjects generally did not exhibit superior cognitive performance on the administration visit. On most tests, subjects actually performed slightly worse on the administration visit, though this difference was not significant. No significant main effects for visit or order were observed for the CVLT-II, RMBT-II Story Recall, Digit Span, DSST, or SAT questions (all p’s > .05). There were significant visit x order interactions for the RBMT-II Story Recall (F(2,44) = 14.93, p < .001, ηp2 = .404), Digit Span (F(2,44) = 11.34, p < .001, ηp2 = .340), DSST (F(1,45) = 66.98, p < .001, ηp2 = .598), and SAT questions (F(2,44) = 43.77, p < .001, ηp2 = .666). Participants performed better on the second visit regardless of administration, though this effect tended to be stronger for participants who received placebo administration on visit 1.
On the CPT II, there was a significant main effect of visit for the vigilance measures (F(2,44) = 4.46, p < .05, ηp2 = .169). There was not a significant main effect of order, nor a significant visit x order interaction (all p’s > .05). There was a significant difference between visits for the hit reaction time standard error block change index, such that participants demonstrated better consistent sustained attention during the administration visit (d = −0.59). However, there was not a significant difference for the other measure of vigilance, hit reaction time block change index. There were no significant main effects of visit or order, nor a significant visit x order interaction (all p’s > .05) for the impulsivity and inattention measures on the CPT II.
Sex differences were also examined for cognitive performance. There was a significant multivariate main effect of sex on the RBMT-II Story Recall (F(2,42) = 5.43, p < .01) such that female participants generally recalled more of the story than male participants; however, there was not a significant visit x sex interaction (F(2,42) = 0.92, p = .41). There were no other significant effects of sex on any of the other tests (all p’s > .05).
Between-group comparisons of experimental and control subjects
Analyses of all DVs were conducted again to compare subjective mood and performance between the experimental and control groups on the administration and non-administration visits while controlling for order. Hours of sleep were not significantly different between groups on the administration visit (experimental: M = 6.81, SD = 1.28; control: M = 7.12, SD = 1.24; t (94) = −1.22, p = .23) and the non-administration visit (experimental: M = 6.89, SD = 1.26; control: M = 6.95, SD = 1.75; t (94) = −0.18, p = .86). Anxiety scores also were not significantly different between groups on the administration visit (experimental: M = 11.94, SD = 7.68; control: M = 11.67, SD = 7.16; t (94) = 0.17, p = .86) and the non-administration visit (experimental: M = 12.34, SD = 7.83; control: M = 13.33, SD = 8.07; t (94) = −0.61, p = .55).
Subjective mood
Repeated measures MANCOVAs were conducted for the ARCI and VAS items at pre- and post-test to examine differences across visits between the experimental and control groups while controlling for order. There were significant multivariate visit x group interactions at pre-test for both the ARCI (F(5,89) = 3.26, p < .01, ηp2 = .155) and the VAS (F(6,88) = 2.86, p < .05, ηp2 = .163). These interactions were no longer significant at post-test and there were no significant effects of order at pre- or post-test (all p’s > .05).
Examination of the individual ARCI subscales at pre-test revealed significant visit x group interactions for amphetamine effects and trends toward significance for intellectual energy and efficiency and dysphoric effects, such that experimental subjects reported the strongest effects on the administration visit, and similar or weaker effects than the control group on the non-administration visit (see Figure 1). On the VAS, visit x group interactions were significant for reports of feeling high and stimulated, such that experimental subjects reported feeling the most high and the least high on the administration and non-administration visits, respectively, and the most stimulated on the administration visit, though both groups reported feeling relatively equally stimulated on the non-administration visit (see Figure 2).
Figure 1. Comparisons of ARCI Pre-Test Drug Effects by Group and Visit.
Note: AV = Administration visit; N-AV = Non-Administration visit.
Significance and effect size refers to difference from experimental group on the administration visit. There were no significant differences between visits for the control group so only data from the administration visit is displayed.
*** p < .001 ** p < .01 * p < .05
Figure 2. Comparisons of Pre-Test VAS Subjective Mood by Group and Visit.
Note: AV = Administration visit; N-AV = Non-Administration visit.
Significance and effect size refers to difference from experimental group on the administration visit. There were no significant differences between visits for the control group so only data from the administration visit is displayed.
*** p < .001 ** p < .01
There were no significant sex differences at pre- or post-test on the VAS (all p’s > .05). However, there was a significant multivariate effect of sex on the ARCI at pre-test (F(5,87) = 2.41, p < .05, ηp2 = .122), such that males generally reported significantly higher levels of dysphoria than females. There was not a significant visit x sex interaction, nor was there a significant sex effect at post-test.
Cognitive performance
Repeated measures MANCOVAs were conducted for the CVLT-II, RBMT-II Story Recall, the Digit Span, SAT questions, and the CPT II to examine differences in performance across visits for both groups while controlling for order. Repeated measures ANCOVAs were used to examine the performance expectancy VAS item and DSST score.
There were not significant visit x group interactions for the subjects’ ratings of performance at pre- or post-test (all p’s > .05). There were significant visit x order interactions, such that at pre-test all subjects expected to perform worse on their second visit (F(1,93) = 28.78, p < .001, ηp2 = .236), and at post-test subjects thought they performed better on the second visit, regardless of administration (F(1,93) = 5.02, p < .05, ηp2 = .051). Sex was not a significant variable in either analysis (all p’s > .05).
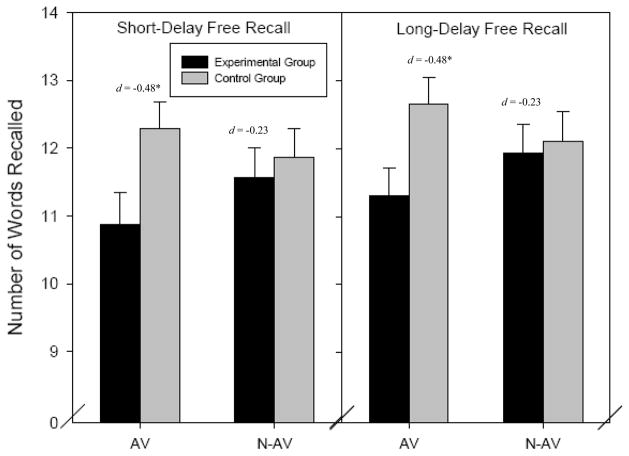
There was a trend toward significance for the visit x group interaction for performance on the CVLT-II (F(3,91) = 2.30, p = .083, ηp2 = .070), with no significant effect of order (F(3,91) = 2.03, p = .12, ηp2 = .063). On both short-delay and long-delay free recall, experimental subjects performed worse than control subjects during the administration visit, though performance was comparable during the non-administration visit (see Figure 3). When sex was entered into the equation, there was a significant main effect (F(3,89) = 3.05, p < .05), such that female participants recalled significantly more words than male participants. However, there was not a significant sex x visit interaction (F(3.89) = 1.44, p = .24).
Figure 3. Comparisons of Number of Words Recalled on the CVLT by Group and Visit.
Note: AV = Administration visit; N-AV = Non-Administration visit. Short-delay free recall refers to word recall ability following an interference test. Long-delay free recall refers to word recall ability following a 20-minute delay.
Significance and effect size refers to difference from experimental group on the administration visit. There were no significant differences between visits for the control group so only data from the administration visit is displayed.
* p < .05
There were no significant visit x group interactions for the RBMT-II Story Recall, Digit Span, DSST, or SAT questions (all p’s > .05). There were significant visit x order interactions for these tests (RBMT-II Story Recall: (F(2,92) = 32.81, p < .001, ηp2 = .420); Digit Span: (F(2,92) = 10.61, p < .001, ηp2 = .187); DSST: (F(1,93) = 99.79, p < .001, ηp2 = .518); SAT questions: (F(2,92) = 55.26, p < .001, ηp2 = .546)), such that participants performed better on their second visit, regardless of administration. There were no significant effects of sex on any of these tests (all p’s > .05).
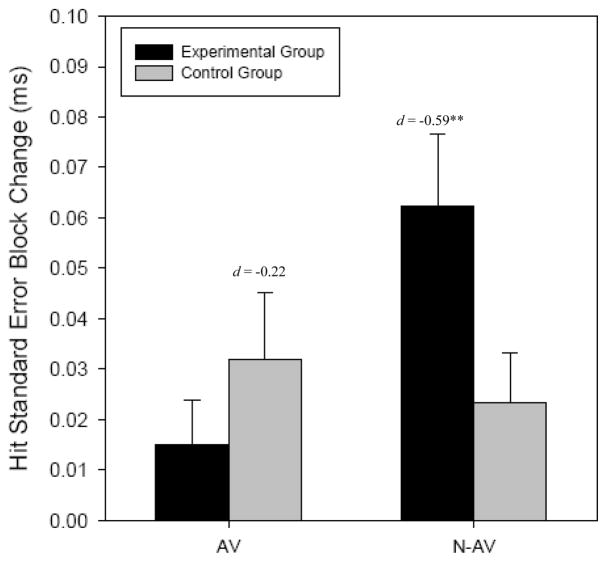
There was a trend toward significance for the multivariate visit x group interaction for the CPT II vigilance measures (F(2,92) = 2.90, p = .060, ηp2 = .059), with no significant effect of order (F(2,92) = 0.37, p = .69, ηp2 = .008). This interaction was significant only for the hit reaction time standard error block change index, revealing that experimental subjects demonstrated both the best sustained attention during the administration visit and the worst sustained attention during the non-medication visit (see Figure 4). There were no significant multivariate visit x group interactions for the CPT II impulsivity or inattention measures (all p’s > .05), though there was a significant effect of order for the impulsivity measures (F(3,91) = 5.26, p < .01, ηp2 = .150). There were no significant effects of sex on these tests (all p’s > .05).
Figure 4. Comparison of a Measure of Vigilance on the CPT II by Group and Visit.
Note: AV = Administration visit; N-AV = Non-Administration visit. Hit standard error block change measures reaction time consistency across the test. It refers to the slope of change in reaction time standard errors across six time blocks. Better vigilance is indicated by fewer milliseconds.
Significance and effect size refers to difference from experimental group on the administration visit. There were no significant differences between visits for the control group so only data from the administration visit is displayed.
** p < .01
Discussion
The present study is the first to examine whether subjective mood and cognitive performance in healthy prescription-stimulant naïve college students can be influenced solely by one’s expectation to receive prescription stimulant medication. We hypothesized that when participants expected to receive MPH, they would report enhancements in positive mood states and in cognitive performance compared to when they did not expect to receive medication. Our hypothesis was largely supported with regard to enhancements in subjective mood. At both pre-test (i.e., 20 minutes following the placebo administration) and post-test, significant increases in subjective mood and drug effects were reported by the experimental group during the placebo-administration visit compared to the non-administration visit and to the control group. Experimental subjects tended to report the strongest effects during the administration visit and the weakest effects during the non-administration visit, while the control subjects’ mood remained relatively stable between visits. Significant differences were seen for amphetamine effects, intellectual energy and efficiency effects, dysphoria, feeling high, and feeling stimulated, regardless of the order of the visits. We did not anticipate that participants would report significant increases on the dysphoria subscale of the ARCI, though previous studies of subjective mood following cocaine administration have also shown elevations in dysphoria (e.g., Penetar et al., 2006). Though we initially conceived of feeling high and stimulated as positive, it is possible that participants perceived these feelings as negative. The effect sizes for these differences between visits for the experimental group were in the medium to large range. Effect size differences between groups were also generally larger during the administration visit than the non-administration visit, providing support for the direct influence of expecting to receive MPH on altering subjective mood ratings.
Unfortunately, our hypothesis of improved cognitive function when expecting to receive MPH was not supported. Experimental participants surprisingly did not expect to perform better or believe they performed better on the administration visit compared to the non-administration visit, or to the control subjects. Instead, performance expectancy was determined by session number, as all participants expected to perform worse, yet believed they performed better on the second visit, regardless of administration. As participants knew they would be completing similar versions of the tests on both visits, it is unclear why this was the case. It is possible that the participants found the tests too difficult and thus lowered their expectations for the second visit, only to ultimately believe they performed better due to realized practice effects. These factors may have exerted stronger influence on performance expectation than beliefs regarding the enhancing effects of MPH, as the participants in this study had no prior direct experience with prescription stimulants.
On nearly all cognitive tests, participants’ cognitive performance did not significantly differ between the administration and non-administration visit; in fact, participants tended to perform worse on the administration visit, though these differences were not significant and were small in effect. Performance also did not largely differ from that of the control subjects. Again, order played a significant role, such that all participants tended to perform better on the second visit, regardless of administration. Though alternate versions of several tests were used, it is likely that practice effects were responsible for this increase in performance on the second visit.
However, significant differences were found on the CVLT-II and CPT II. On the CVLT-II, experimental participants actually recalled significantly fewer words during short-delay free recall and long-delay free recall than the control participants during the administration visit, with medium effect sizes for differences between groups. As the CVLT-II was the first test participants completed during each testing session, it is possible that experimental participants may not have put in much effort on this test during the administration visit because they believed MPH would help them perform well, only to realize that they were performing poorly and consequently increased their effort for subsequent tests. Alternating the order of cognitive tests in future studies would help clarify this effect. Though only a trend emerged for significant multivariate differences between groups, and in the opposite direction of our predicted hypothesis, we thought it was important to present this effect as it is interesting that participants may be exerting decreased effort when using prescription stimulant medication, leading to poorer recall ability. This effect may be particularly salient with regard to short- and long-term memory and/or ability to pay optimal attention to appropriately encode new information, as experimental subjects recalled an average of nearly 3 fewer words than control subjects during the CVLT delay trials on the administration visit. This finding that should be further examined, as it may have important implications for identifying those at risk for misuse (e.g., individuals with little motivation) or potential consequences of misuse (e.g., generalized lack of effort to other areas of life).
However, experimental participants actually exhibited improved performance on a vigilance index of the CPT II during the administration visit compared to the non-administration visit, though their performance was not significantly different from that of the control subjects. Examination of Figure 4 reveals that this within-subject difference was due to experimental participants performing substantially worse on the non-administration visit. Specifically, experimental participants’ reaction time was remarkably consistent throughout the CPT during the administration visit, yet much more variable when they did not believe they ingested MPH. Participants may have believed that they had better ability to focus and persevere, particularly for a sustained amount of time, when they expected to receive MPH. This is similar to circumstances in which participants may engage in nonmedical stimulant use to study or cram for extended hours. On the other hand, when experimental participants did not expect to receive MPH, their attention appeared disrupted, resulting in inconsistent reaction times through the CPT. This is an intriguing finding in that perhaps experimental participants were more influenced by the expectation to not receive MPH than by the expectation to receive it, particularly with regard to their ability to sustain optimal attention. It is difficult to speculate on the explanations for this finding as the CPT II was the sole cognitive test to produce this effect, and only for one measure of vigilance. It would be prudent to examine measures of vigilance more closely in future studies as college students may have stronger expectations for the effects of MPH on enhanced vigilance than for other indices of cognitive functioning, such as memory and reaction time.
Though only one of our hypotheses was supported, this research has important implications and spurs several ideas for further research in this area. Notably, that subjective feelings of being high and stimulated were produced solely by expecting to receive MPH is important to consider when examining initiation and maintenance of nonmedical prescription stimulant use. As motives for nonmedical use include desire to feel high and to party (Barrett et al., 2005), it is likely that individuals who use a prescription stimulant for this purpose will consequently feel high due to these demonstrated placebo effects, which will likely maintain nonmedical use. This expectation is an important one to challenge and disprove in order to reduce the incidence and prevalence of nonmedical use. It appears particularly important to challenge this belief among stimulant-naïve individuals, as this placebo effect was rather large and may be chiefly influential for maintaining early nonmedical use. Future research should examine whether placebo effects for mood enhancements are also evident among experienced prescription stimulant users.
Given that frequent motives for nonmedical prescription stimulant use are to enhance concentration and alertness (Teter et al., 2005), it is surprising that cognitive enhancements were not affected by expectation to receive MPH. Though it is possible that participants did not believe they were ingesting an active drug, believability ratings were generally high and significant enhancements were seen in subjective mood, likely confirming a credible placebo. Instead, it is possible that other factors exerted greater influence on cognitive performance than MPH expectancy, such as motivation. Perhaps significant differences between visits would have emerged if participants had a greater incentive to perform well, as is typically the case when individuals use prescription stimulants to perform well in school. It is also possible that the participants generally found the testing session to be too difficult, which may have decreased motivation, self-efficacy, and effort. Future studies examining cognitive performance during placebo administration in individuals experienced with prescription stimulants may yield different results, as their familiarity with the situation may enhance their expectations and thus their performance.
Several limitations of this research warrant cautious interpretation. Though our sample is demographically representative of nonmedical prescription stimulant users, this study only assessed at-risk college students who did not have experience with prescription stimulants. As such, the results may not generalize to high school students or same-aged individuals who are not currently enrolled in college. As previously mentioned, they also may not generalize to those experienced with prescription stimulants. Additionally, this study relied on self-report to determine history of substance use and it is possible that participants may not have been truthful when asked about their experience with prescription stimulants. However, advertisements for this study did not specifically mention prescription stimulant medication in an effort to conceal the purpose of this study and reduce the chance of biased responding during initial screening. We also relied on self-report to determine whether participants had abstained from other substances which may have influenced performance, such as caffeine or alcohol. Future studies should employ biological confirmation of abstention.
There is the possibility that participants’ reported mood and cognition may have been influenced by factors other than the expectation to receive MPH. On participants’ first study visit, they completed the consent process and initial information gathering (i.e., demographics and substance use history and diagnosis), which was not repeated on the second visit. Counterbalancing of administration visits and comparisons to control subjects were employed to control for this discrepancy in procedure across visits. Additionally, experimental participants only ingested a capsule during the administration visit. Capsules were not ingested during the non-administration visit and were never ingested by control subjects. This was done to ensure that participants did not think there was any possibility they may be receiving MPH during the non-administration visit to better examine differences between an instance in which they expected MPH and an instance in which they did not. However, future studies should take care to ensure strict equivalence across study visits. Finally, there is the possibility of experimenter bias, as the experimenter’s knowledge of the placebo may have inadvertently influenced the results. However, this is unlikely given that significant differences were only seen for subjective mood and not for cognitive performance. Future studies should employ a double-blind experimental design to eliminate any potential experimenter bias.
Future research should also examine whether different types of prescription stimulant medication may elicit stronger or weaker placebo effects. We chose to examine MPH, as the nonmedical prescription stimulant use literature is primarily focused on that medication. However, unpublished results from our laboratory reveal that northeastern college students are more likely to report nonmedical use of Adderall. It is possible that participants may have more fully developed expectations for Adderall use, which may have resulted in different findings had we chosen to use Adderall rather than MPH in our study. Additionally, we only assessed expectations for 20mg of MPH. It is possible that expectations, and thus behavior, may have been altered with the expectation to receive lower or higher doses, and should be a focus of study in future research.
Though this study is not without limitations, this is the first effort to better understand the influence of placebo effects related to nonmedical prescription stimulant use. Future research in this area is imperative and should include use of a balanced-placebo design to determine whether active MPH does in fact enhance subjective mood and cognitive performance above and beyond that of placebo effects. An important question is whether cognitive functioning in non-ADHD individuals is truly enhanced by prescription stimulants, and if so, in which domains of cognition. If enhancements do in fact exist, researchers and practitioners should focus on disseminating information regarding the safety risks of nonmedical stimulant use. As taking prescription medication without a prescription can pose health risks, individuals experiencing problems related to cognitive or academic performance should be encouraged to consult health professionals to fully understand and assess underlying problems, rather than engage in nonmedical stimulant use. This is particularly important given the recent research suggesting that individuals demonstrating attentional difficulties may be predominantly at-risk for engaging in nonmedical stimulant use (Arria et al., in press; Rabiner, Anastopoulos, Costello, Hoyle, & Swartzwelder, 2010). Nevertheless, information obtained from this study and future research on this topic will be extremely useful in challenging the expectancies of those at risk in order to prevent nonmedical use. Expectancy challenges, which are efficacious in decreasing alcohol use (Darkes & Goldman, 1993), may also be useful in preventing or decreasing nonmedical prescription stimulant use, and is a topic of current study.
Acknowledgments
This study was funded by National Research Service Award DA024921 to Alison Looby from the National Institute on Drug Abuse.
References
- Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Arria AM, DuPont RL. Nonmedical prescription stimulant use among college students: Why we need to do something and what we need to do. Journal of Addictive Diseases. 2010;29:417–426. doi: 10.1080/10550887.2010.509273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Garnier-Dykstra LM, Caldeira KM, Vincent KB, O’Grady KE, Wish ED. Persistent nonmedical use of prescription stimulants among college students: Possible association with ADHD symptoms. Journal of Attention Disorders. doi: 10.1177/1087054710367621. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Brody LE, Pihl RO. Characteristics of methylphenidate misuse in a university student sample. Canadian Journal of Psychiatry. 2005;50:457–461. doi: 10.1177/070674370505000805. [DOI] [PubMed] [Google Scholar]
- Camp-Bruno JA, Herting RL. Cognitive effects of milacemide and methylphenidate in healthy young adults. Psychopharmacology. 1994;115:46–52. doi: 10.1007/BF02244750. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff MHS, editors. Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. North Tonwanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- Cooper NJ, Keage H, Hermens D, Williams LM, Dobrota D, Clark CR. The dose-dependent effect of methylphenidate on performance, cognition, and psychophysiology. Journal of Integrative Neuroscience. 2005;4:123–144. doi: 10.1142/S0219635205000744. [DOI] [PubMed] [Google Scholar]
- Darkes J, Goldman MS. Expectancy challenge and drinking reduction: Experimental evidence for a mediational process. Journal of Consulting and Clinical Psychology. 1993;61:344–353. doi: 10.1037//0022-006X.61.2.344. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Department of Justice: Drug Enforcement Administration. ARCOS 2 – Report 7, United States Summary for Retail Drug Purchases by Grams Weight. 2008 Retrieved from http://www.deadiversion.usdoj.gov/arcos/retail_drug_summary/2006/06_rpt7.pdf.
- Drug Enforcement Administration. DEA Congressional Testimony by Terrance Woodworth before the Committee on Education and the Workforce: Subcommmittee on Early Childhood, Youth and Families. 2000 Retrieved from http://www.usdoj.gov/dea/pubs/cngrtest/ct051600.htm.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. Journal of the American Medical Association. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Stock S. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200210000-00003. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug and Alcohol Dependence. 2002;67:149–156. doi: 10.1016/S0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Herman-Stahl MA, Krebs CP, Kroutil LA, Heller DC. Risk and protective factors for methamphetamine use and nonmedical use of prescription stimulants among young adults aged 18 to 25. Addictive Behaviors. 2007;32:1003–1015. doi: 10.1016/j.addbeh.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No 05–5727. Bethesda, MD: National Institute on Drug Abuse; 2005. Monitoring the Future National Survey Results on Drug Use, 1975–2003: Volume I, Secondary School Students. Retrieved from http://monitoringthefuture.org/pubs/monographs/vol1_2004.pdf. [Google Scholar]
- Kirk JM, Doty P, De Wit H. Effects of expectancies on subjective responses to oral delta 9-tetrahydrocannabinol. Pharmacology, Biochemistry, and Behavior. 1998;59:287–293. doi: 10.1016/S0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug and Alcohol Dependence. 2006;84:135–143. doi: 10.1016/j.drugalcdep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Lile JA, Babalonis S, Emurian C, Martin CA, Wermeling DP, Kelly TH. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. Journal of Clinical Pharmacology. doi: 10.1177/0091270010375956. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KG, Gendaszek AE. Illicit use of psychostimulants among college students: a preliminary study. Psychology, Health & Medicine. 2002;7:283–287. doi: 10.1080/13548500220139386. [DOI] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasiniski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clinical Pharmacology and Therapy. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a nationwide survey. Addiction. 2005;99:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. Journal of Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Looby AR, Su Z, Lundahl LH, Eros-Sarnyai M, McNeil JF, et al. Benztropine pretreatment does not affect responses to acute cocaine administration in human volunteers. Human Psychopharamcology: Clinical and Experimental. 2006;21:549–559. doi: 10.1002/hup.810. [DOI] [PubMed] [Google Scholar]
- Princeton Review. 11 Practice Tests for the SAT and PSAT. New York: Princeton Review; 2006. [Google Scholar]
- Rabiner DL, Anastopoulos AD, Costello EJ, Hoyle RH, Swartzwelder HS. Predictors of nonmedical ADHD medication use by college students. Journal of Attention Disorders. 2010;13:640–648. doi: 10.1177/1087054709334505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Abuse liability of anxiolytics and sedative/hypnotics: methods of assessing likelihood of abuse. NIDA Research Monograph. 1989;92:123–146. Retrieved from http://archives.drugabuse.gov/pdf/monographs/92.pdf#page=140. [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Experimental and Clinical Psychopharmacology. 2001;9:59–73. doi: 10.1037//1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. Journal of Clinical Psychopharmacology. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–1088. [PubMed] [Google Scholar]
- Shillington AM, Reed MB, Lange JE, Clapp JD, Henry S. College undergraduate ritalin abusers in southwestern California: Protective and risk factors. Journal of Drug Issues. 2006;36:999–1014. [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: Dissolving the expectancy versus conditioning debate. Psychological Bulletin. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Stockl KM, Hughes TE, Jarrar MA, Secnik K, Perwien AR. Physician perceptions of the use of medications for attention deficit hyperactivity disorder. Journal of Managed Care Pharmacy. 2003;9:416–423. doi: 10.18553/jmcp.2003.9.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;117:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. DHHS Publication No ADM 90–717. Department of Health and Human Services, Public Health Service; Washington, DC: 1990. Statistical series, annual data, 1989. Retrieved from http://www.drugabusestatistics.samhsa.gov/ [Google Scholar]
- Substance Abuse and Mental Health Services Administration. DHHS Publication No SMA 96–3080. Department of Health and Human Services, Public Health Service; Washington, DC: 1996. Statistical series: annual emergency department data, 1993. Retrieved from http://www.drugabusestatistics.samhsa.gov/ [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-25, DHHS Publication No SMA 04–3964. Office of Applied Studies; Rockville, MD: 2004. Results from the 2003 National Survey on Drug Use and Health: National Findings. Retrieved from http://www.drugabusestatistics.samhsa.gov/ [Google Scholar]
- Teter CJ, McCabe SE, Cranford JA, Boyd CJ, Guthrie SK. Prevalence and motives for illicit use of prescription stimulants in an undergraduate student sample. Journal of American College Health. 2005;53:253–262. doi: 10.3200/JACH.53.6.253-262. [DOI] [PubMed] [Google Scholar]
- Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology. 2005;178:286–295. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, Swanson JM. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. American Journal of Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. The Journal of Neuroscience. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- White BP, Becker-Blease KA, Grace-Bishop K. Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. Journal of American College Health. 2006;54:261–267. doi: 10.3200/JACH.54.5.261-268. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: A systematic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. 2. London: Harcourt Assessment; 2003. [Google Scholar]
- Yamamoto RT, Karlsgodt KH, Rott D, Lukas SE, Elman I. Effects of perceived cocaine availability on subjective and objective responses to the drug. Substance Abuse Treatment, Prevention, and Policy. 2007;2:30. doi: 10.1186/1747-597X-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]