Abstract
Background
Alloreactive T-cells and anti-HLA antibodies mediate transplant injury. Environmental exposures, including vaccinations, may activate the alloimmune repertoire leading to accelerated allograft injury. To test whether vaccination impacts human alloimmunity we analyzed humoral and cellular immune reactivity in subjects undergoing influenza vaccination.
Methods
We serially obtained blood samples from 30 healthy subjects and 8 kidney and 9 lung transplant recipients who received influenza vaccination, and from 20 healthy unvaccinated controls. We measured cellular and humoral anti-influenza responses, anti-HLA antibodies and alloreactive T cell immunity (IFN-γ ELISPOT) at 0, 2, 4 and 12 weeks after vaccination.
Results
Vaccination induced influenza-reactive humoral and cellular responses in control subjects and in transplant recipients. Two of 30 vaccinated volunteers developed new alloantibodies but in none of the transplant patients. Vaccination also specifically and significantly augmented cellular alloimmunity based on reactivity to a panel of stimulators in both healthy subjects and in transplant recipients within 4 weeks of vaccination. The enhanced cellular alloresponse waned toward prevaccine levels by week 12.
Conclusion
Our findings newly demonstrate that influenza vaccination can have a significant impact on the potency of the alloimmune repertoire. Because the strength of the alloresponse influences long term graft function, our results suggest that further investigation of alloimmune monitoring following vaccination is needed.
Keywords: T cell, influenza, vaccination, allosensitization, heterologous immunity
Introduction
T cell- and antibody-mediated allograft injury continues to limit the long-term success of organ transplantation. Preformed anti-HLA alloantibodies and primed donor-reactive T cells are particularly problematic as they resist standard immunosuppression and can rapidly engage effector functions without the need for in vivo reactivation (1-4). B cell and T cell sensitization to alloantigens may be induced upon exposure to alloantigens from previous transplantation, pregnancy or blood transfusions, but also can be found in human transplant candidates who have never been knowingly exposed to HLA antigens, presumably due to cross reactivity to environmental antigens (5, 6).
Emerging evidence from animal models and from select human studies indicates that alloreactive T cells can be activated by antigens derived from infectious pathogens that cross-react with alloantigens, a phenomenon that has been termed heterologous immunity (7-9). In addition to direct cross-reactivity between pathogens and alloantigens, pathogen-induced activation of the innate immune system (e.g. via toll like receptor stimulation) can augment cytokine induction and upregulate costimulatory molecule expression, thereby enhancing expansion and differentiation of the alloimmune repertoire regardless of the initially inciting antigen (10-12).
Over the last decade newer immunosuppressive medications have significantly decreased the rate of acute cellular rejection with the consequences of over-immunosuppression and the risk of infection (13). Viral infections, including influenza infection, occur at higher frequency following effective immunosuppression(14, 15). As a consequence systematic preventive strategies including empirical prophylaxis, routine screening, and vaccination are commonly employed. Current recommendations urge annual influenza vaccination for solid organ transplant recipients, supported by evidence suggesting that influenza vaccination of transplant recipients induces effective humoral and cellular immune response against viral antigens without significant adverse events (16-20).
While the benefits of vaccination are known, exposure to influenza antigens through vaccination could directly activate alloreactive T and B cells (heterologous immunity). Vaccination could also activate innate immunity, indirectly augmenting alloreactive memory T and B cells, which could translate into an elevated risk for subsequent graft injury. Although controversial, the most recent studies suggest that vaccinations do not increase the risk of clinical acute rejection (21-23). However, the impact of vaccination on chronic graft injury remains unknown. This is particularly relevant because sustained elevations of primed alloreactive T cells and or alloantibodies are associated with the development of chronic allograft injury (24, 25). It therefore becomes essential to understand whether and how vaccination, particularly with a commonly used vaccine, influences the alloimmune repertoire, both in transplant candidates and in immunosuppressed transplant recipients.
To test this, we studied the effects of seasonal influenza vaccination on anti-HLA antibodies by flow cytometry, and alloreactive T cells by the ELISPOT-based panel of reactive T cells (PRT assay), in a cohort of healthy volunteers and in lung and kidney transplant recipients.
Materials and methods
Study population
Patients and healthy volunteers who were offered influenza vaccination during the 2006-2007 and 2007-2008 seasons at the Cleveland Clinic and University Hospitals of Cleveland were considered for participation in this study. After Institutional Review Board approval, enrolled subjects provided informed consent prior to participation. A total of 17 transplant recipients (9 lung transplant recipients and 8 kidney transplant recipients) and 30 non-transplant subjects who opted to receive the vaccination as well as 20 non-transplant controls that refused vaccination enrolled in the study. No transplant recipient refused vaccination. All transplant recipients were on triple drug immunosuppression including prednisone, a calcineurin inhibitor (CNI) and an anti-proliferative agent. CNI levels were recorded during the study for comparison. Volunteers did not have any medical conditions requiring immunosuppressive medications. Blood samples were collected just prior to vaccination, 2 weeks, 4 weeks and at 12 weeks post-vaccination in all subjects. In transplant recipients, graft function was monitored for one year following vaccination. Serum creatinine was used to assess graft function in kidney transplant recipients and forced expiratory volume at 1 second was used in lung transplant recipients.
Vaccine
All participants received a 0.5 mL dose of commercially available trivalent split influenza vaccine (Fluzone, SanofiAventis) by IM injection. The 2006-2007 and the 2007-2008 vaccines used the same antigens and each contained 15 μg of hemagglutinin of the following strains: A/Wisconsin/67/2005 (H3N2-like virus), A/New Caledonia/20/99 (H1N1-like virus) and B/Malaysia/2506/2004-like virus. Other components of the vaccine were also the same.
Alloreactive and anti-influenza T cell detection
Peripheral blood samples were obtained in heparinized tubes at the specified time points. Aliquots of serum were stored at −70°C and used for antibody testing. Unfraccionated peripheral blood mononuclear cells (PBMCs) were prepared and tested in INF-γ ELISPOT assays as previously described (16, 26-29). The responder cells were tested in triplicate against medium alone (negative control), a panel of 5 HLA-typed allostimulator cells (using 100,000 B cells per well), and phytohemagglutinin (PHA, positive control). Results were depicted as the mean number of IFN-γ spots per 200,000 recipient PBLs based on triplicate measurements in a given assay and against any given stimulator. The frequencies of alloreactive IFN-γ ELISPOTs used for analyses were obtained after subtracting those derived from non-stimulated wells (“background”). The frequencies of IFN-γ spots prior to vaccination were considered the baseline response of each subject to any particular stimulator. Post-vaccination frequencies of IFN-γ spots against each of the five stimulators of the panel were assessed in relation to the baseline or pre-vaccination response (fold increase), and thus, each subject served as its own control. The response to the panel is based on the fold increase response at any given time point over the baseline. A response of at least 100% increase over baseline (doubling of frequencies) was considered a positive response. Panel of reactive T cell or PRT assays were constructed based on the number of positive responses to each stimulator in relation to the total of 5 stimulators, that is, if a particular subject had at least doubling of frequencies at a determined follow up time point in 2 of the 5 stimulators, this subject was considered to have a PRT assay of 40%. PRT values were calculated at 2 and 4 weeks with the highest of each response considered as a measure of the peak response. A 12-week PRT value was also calculated as a measure of residual response.
To assess the possibility of a non-specific adjuvant stimuli from the vaccination as a causative of the post-vaccination effect observed we measured 1) IFN-γ secretion by T cells in response to media (background) in post-vaccination time points in relation to baseline and 2) autologous IFN-γ secretion in 4 vaccinated subjects (2 with high post-vaccination alloresponse and 2 with no post-vaccination response) by incubating each responder’s PBMCs with self B cells cultured under the same stimulating conditions as the panel of allostimulators (28). Briefly, B cells were isolated using a negative selection magnetic separation for B cell (CD19+) enrichment (Stem Cell Technologies), stimulated by CD40L transfected fibroblasts and IL-4 until they reached their log phase of growth, and then stored frozen for use in control experiments. B cells were checked for ability to allo-stimulate prior to use in syngeneic controls.
Anti-viral cellular responses were tested in IFN-γ ELISPOT assays using antigen directly derived from the vaccine at a dilution of 1:10,000. This dilution was found to provide the best read out from titration experiments (data not shown). A positive response was also determined based on the relation of baseline anti-influenza reactive PBLs to follow up time point responses. Fold increase over baseline frequencies were compared among the different cohorts at either 2 or 4 weeks and at 12 weeks post-vaccination.
Alloantibody and anti-viral antibody detection
Serum anti-HLA antibody was determined by flow cytometry using HLA class I and class II antigen-coated latex beads (FlowPRA™ Screening Test, One Lambda, Inc., Canoga Park, CA). The antibody testing was performed according to instructions supplied by the manufacturer. A positive PRA was defined as greater than 10 % reactivity for either class I or class II antigen. A doubling of the PRA percentage at any time point following vaccination from baseline or a 10% increase was considered a positive alloantibody response, and we used single antigen beads to assess for specificity of the alloantibody for positive responses.
Anti influenza antibodies against each of the strains contained in the vaccine were measured by hemagglutination assays at the Glennan Center Laboratory at East Virginia Medical School (Norfolk, VA). Post-vaccination titers at 4 weeks were compared to baseline titers and mean fold increase was calculated to assess vaccination response. Titers were not measured at 2 weeks or 12 weeks post-vaccination. Either a baseline titer to each strain of at least 1:40 or a 4-fold increase in titers over baseline was considered appropriate immunization response to influenza. Seroprotection was defined as having a baseline or follow up anti-influenza titer of at least 1:40 and seroconversion as having at least a 4-fold increase in titers from baseline (30).
Statistical Analysis
All analyses were performed using JMP 7.0 (SAS Institute Inc., Cary, NC). Values are shown as mean ± SD, median and percentiles when data were not normally distributed, and percentages. Categorical variables were compared using the chi-square test or Fisher’s exact test when appropriate. Comparison of mean values was tested using the t-test for independent samples (2-tailed), and median comparison was done using the Wilcoxon/Kruskal-Wallis test. Changes over time compared to baseline were calculated using matched pair t-test and confirmed by Wilcoxon sign rank test in those cases wehre data was non-parametrically distributed. A p value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics of the study cohorts are shown in Table 1. Transplanted patients were older, more likely to be male and more likely to have received prior blood transfusions compared to the healthy subjects.
Table 1.
Population characteristics
| Unvaccinated volunteers n=20 |
Vaccinated volunteers n=30 |
Transplant recipients n=17 |
p value | |
|---|---|---|---|---|
| Female – n (%) | 18 (90.0) | 27 (90.0) | 4 (23.5)* | <0.01 |
| Age – mean (SD) – years | 39 ± 10 | 36 ± 12 | 49 ± 12* | <0.01 |
| Caucasian | 15 (75.0) | 24 (80.0) | 13 (76.5) | 0.90 |
| h/o pregnancies – n (%) | 7 (35.0) | 12 (40.0) | 3 (17.6) | 0.69 |
| h/o blood transfusions – n (%) | 1 (5.0) | 1 (3.3) | 9 (52.9) | <0.01 |
| Months since transplant – median (min – max) |
NA | NA | 25 (9 – 147) | NA |
Note: NA (not applicable)
Influenza vaccination induces viral reactive immunity in normal subjects and transplant recipients
For humoral reactivity to viral antigens (anti-influenza antibodies) we used ELISA and we found seroprotective titers to at least one influenza strain in 13/30 individuals (Figure 1A). Because many antigenic epitopes are shared among viruses from year to year, previous vaccination and or influenza disease is anticipated to induce detectable immunity in a minority of subjects prior to vaccination. Following vaccination, we found seroconversion or seroprotection in 24/30 individuals (p<0.05 vs prevaccination). We similarly noted a significant increase in the percentage of seroconversion in transplant recipients after vaccination (1/17 prevaccination vs. 14/17 postvaccination, p<0.05). In contrast, in the unvaccinated subjects, we found no significant change in the prevalence of seroprotection (8/20 prevaccination vs. 10/20 postvaccination, p=NS).
Figure 1.

Humoral and cellular responses to Influenza vaccination. 1A: Humoral immune response to Influenza vaccination. Prevaccination represents the percentage of each group with hemagglutination titers >1:40 to any of the three strains of influenza at baseline. Postvaccination represents a titer of >1:40 or a 4-fold increase in hemagglutination response at 4 weeks after vaccination. 1B: Fold increase in anti-influenza IFN-γ spots by 4 weeks after vaccination (p value among three groups of <0.03 by ANOVA and <0.01 by Wilcoxon sign rank test). 1C: Cellular response to influenza vaccination as measured by ELISPOT over the study period for each group (p value non significant between both vaccinated subgroups for any time point).
For cellular reactivity to the vaccine antigens we used IFN-γ ELISPOT (Figure 1B). As we found for humoral immunity, the frequency of influenza-reactive IFN-γ producers increased significantly within 4 weeks post-vaccination in the healthy subjects and the kidney transplant recipients (Figure 1B and 1C) (p<0.01 by matched pair analysis – Wilcoxon/Krustal-Wallis test – in each comparison). Vaccination was less effective at increasing the influenza reactivity in lung transplant recipients than in kidney transplant recipients (1/9 lung transplant recipients showed doubling of the baseline ELISPOT response versus 4/8 kidney transplant recipients), perhaps due to significantly higher tacrolimus levels (mean levels of 10.1 ng/dl and 5.3 ng/dl in lung and kidney recipients, respectively) (p< 0.001). At 12 weeks post vaccination we found a sustained increase in the frequency of influenza-reactive IFN-γ producing PBMCs compared with the prevaccine values in the vaccinated cohort, although on average, the strength of the responses waned compared to the peak values noted at 2-4 weeks.
In contrast to the findings in vaccinated subjects we noted no change in the frequency of influenza-reactive PBMCs in the unvaccinated individuals over 12 weeks (Figures 1B and 1C, p=NS by matched pair analysis). Together, these data indicate that influenza vaccination induces humoral and cellular influenza-reactive immunity in the overwhelming majority of transplant recipients taking maintenance immunosuppressive medications (Table 2).
Table 2.
Humoral and cellular response to influenza vaccination
| Response to Influenza Vaccine |
Unvaccinated n=20 |
Vaccinated n=30 |
Transplant (Lung/Kidney) n=17 (9/8) |
p value |
|---|---|---|---|---|
| Cellular & Humoral | 0 (0%) | 14 (47%) | 6 (3/3) (35%) | <0.01 |
| Humoral only | 10 (50%) | 10 (33%) | 8 (4/4) (47%) | 0.04 |
| Cellular only | 2 (10%) | 4 (13%) | 2 (1/1) (12%) | 0.9 |
| Neither | 8 (40%) | 2 (7%) | 1 (1/0) (6%) | <0.01 |
Influenza vaccination and anti-HLA antibody
At baseline, we observed that the median PRA was <10% for each group. Less than 22% of those subjects about to be vaccinated had either a class I or class II PRA of >10% and less than 3% (none of the transplant patients) were highly sensitized as defined by a PRA of >80%. Following vaccination, 2 of the 30 healthy subjects and none of the transplant patients developed stronger flow PRA values. Two subjects increased their class II PRA greater than 10% that were sustained over the 12 week study period. While in one subject anti-HLA antibodies were not detected by single antigen beads, the other subject demonstrated a significant increase in detectable antibodies to multiple HLA antigens (DR11, DR13, DR16, DR51 and DR103).
Influenza vaccination is associated with an augmented frequency of primed cellular alloimmunity
We compared the strength of the responses to the PRTs assays pre and post vaccination. Figure 2 depicts the percentage of individuals in each group who developed at least a 2 fold increase in alloreactivity to each of the stimulators at 2-4 weeks (panel A) or 12 weeks (panel B). A higher percentage of vaccinated subjects demonstrated increased responses to each of the allostimulators compared to unvaccinated subjects by either 2 or 4 weeks post-vaccination (p<0.01 for stimulators 1-4 and p=0.18 for stimulator 5, Figure 2A). We found that the responses in vaccinated volunteers exceeded the responses in vaccinated transplant subjects at this time point, but it was only statistically significant (p=0.01) for stimulators 2 and 4, while is was not statistically significant for the other three stimulators. The elevated alloresponses were maintained in a subset of individuals for at least 12 weeks after vaccination (Fig 2B).
Figure 2.

Percent of unvaccinated controls, vaccinated controls and transplant recipients with at least a doubling of the baseline alloresponses to each stimulator at 2-4 weeks (Figure 2A) and 12 weeks (Figure 2B) post-vaccination.
We calculated the overall response to the panel of allostimulators as the proportion of positive responses (doubling from baseline) to the each member of the panel of 5 stimulators. Figure 3 depicts these results at 2 or 4 weeks (peak response) and at 3 months post-vaccination (final response). Vaccinated subjects demonstrated a higher response to the panel independent of the cutoff (number of positive responses) used (p<0.01 for all cutoffs except for PRT-100 where p=0.28). Transplant recipients also demonstrated an increase in alloreactivity after vaccination, although the proportion responding was less than in healthy vaccinated volunteers. Interestingly, while the cellular response appeared to be less evident in lung transplant recipients than in kidney transplant recipients, the PRT response was comparable among the two cohorts (2/9 lung transplant recipients had a PRT response to 40% of the panel versus 2/8 kidney transplant recipients). In conjunction, 4/19 (~24%) transplant recipients had a reactivity to at least 40% of the panel (Figure 3B). Three of the four transplant recipients who reacted to 2 out of 5 stimulators of the PRT panel (PRT-40) were the same who doubled the cellular response to the influenza antigen following vaccination. The remaining transplant recipient (lung) with reactivity to the flu developed a new reactivity to one stimulator (PRT-20) and two of the kidney transplant recipients with reactivity to the influenza antigen did not develop any panel reactivity (PRT negative). Similar patterns of antiviral and PRT panel reactivity were observed in the vaccinated controls (Table 3).
Figure 3.
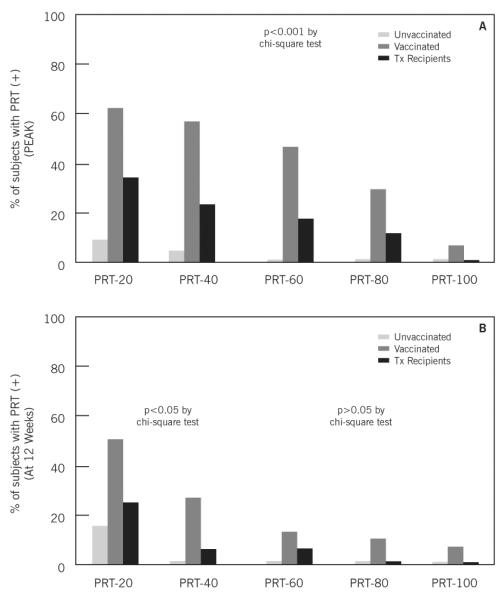
Panel of reactive T cells using different cutoff values for each subgroup at 2-4 weeks post-vaccination (Figure 3A) and 12 weeks post-vaccination (Figure 3B).
Table 3.
Anti-viral and anti-HLA cellular response agreement
| Unvaccinated volunteers n=20 |
Vaccinated volunteers n=30 |
Transplant recipients n=17 |
|
|---|---|---|---|
| Anti-Influenza positive and PRT-40 positive |
1 (5%) | 16 (53.3%) | 3 (17.6%) |
| Anti-Influenza negative and PRT-40 negative |
18 (90%) | 10 (33.3%) | 10 (58.8%) |
| Anti-Influenza positive and PRT-40 negative |
1 (5%) | 3 (10%) | 3 (17.6%) |
| Anti-Influenza negative and PRT-40 positive |
0 (0%) | 1 (3.3%) | 1 (5.8%) |
Analogous to the cellular responses to viral antigens, the alloresponses for the entire vaccinated cohort declined by 12 weeks post-vaccination, Figure 3B (matched pair analysis between mean PRT at 2-4 weeks versus PRT at 12 weeks post-vaccination, p<0.01 by Wilcoxon Signed-Rank). Clinical evidence of graft rejection was not observed in any of the transplant patients within 3 months of vaccination.
Control studies revealed no change in spontaneous IFNγ secretion (media wells) for any of the samples within each group post-vaccination. To further assess for non-specific stimulation by B cell secreted factors, we performed ELISPOT assays using syngeneic, self B cells as stimulators. We tested PBLs from two vaccinated subjects without pre- or post vaccine alloreactivity and two vaccinated subjects with detectable alloimmune responses that were augmented post vaccination. Regardless of the change in alloresponses following vaccination, the responses to self B cells did not change significantly post vaccine in any subject, indicating that the vaccination altered alloimmunity but not self reactivity (data not shown).
Discussion
We present novel data indicating that vaccination against influenza results in temporal changes in cellular alloimmune responses. This information is important as it suggests that 1) vaccination can affect alloimmunity, and 2) anti-viral and anti-HLA immune monitoring could be applicable to transplanted patients. Alloreactivity in general is detrimental to transplant outcomes, with alloreactive effector/memory T cells being central mediators of the immune-mediated injury to the graft.(1, 3, 4) When present, these cells have been shown to impair graft tolerance and to accelerate rejection in animal models.(4, 7) In humans, pre- and post-transplant circulating alloreactive T cells have also been associated with graft rejection.(2, 6, 24, 27, 28) These cells are commonly found in subjects with previous exposures to alloantigens including pregnancy, blood transfusion or transplantation; however, cellular alloimmunity can also be found in subjects without any prior history of such events,(2, 6, 29) suggesting that other responsible mechanisms of allosensitization exist. Hypothesized factors that could cause cross-reactivity with alloantigens are viral infections or vaccinations; however, there are no studies in humans evaluating this theory.
In this study we show that vaccinated subjects – both healthy volunteers and transplant recipients – exhibited increased cellular responses to commonly expressed human alloantigens after vaccination, while the unvaccinated cohort did not. The effects of influenza vaccination on alloreactivity achieved its maximum effect two to four weeks following vaccination, with attenuation in the alloimmune response by three months post-vaccination in most of the studied subjects. The response in vaccinated healthy subjects exceeded that response in transplant recipients, likely due to the non exposure to immunosuppressive drugs in the healthy cohort. Additionally, lung transplant recipients, who had significantly higher tacrolimus levels during the study period, showed decreased responses to the influenza antigen compared to kidney transplant recipients.
In animal models, several mechanisms may explain the development of HLA reactive T cells in the absence of direct exposures to alloantigens. In heterologous immunity alloreactive T cells can be generated by incomplete allelic exclusion leading to allo-specific T cells. A second mechanism is when T cell receptors can directly cross-react to viral antigens due to molecular mimicry.(8) It is also plausible that several non-allo specific stimuli like vaccinations temporarily enhance pre-existent alloreactivity through a bystander effect. Since response to one stimulator predicted response to other allo-stimulators, our data suggest that the observed increase in T cell alloreactivity may be due to a non-specific reactivation of a variety of memory T cell clones, and not specifically those able to cross react with viral antigen. On the other hand, the absence of self-responses and increased alloreactivity in those transplanted subjects who also showed evidence of anti-influenza cellular reactivity suggest that cross-reactivity cannot be disregarded. However, an important point is that irrespective of the underlying mechanism leading to enhanced alloreactivity, identification and closer follow up of transplant subjects during the peri-vaccination period with the use of immune monitoring tools that detect anti-vaccine and anti-HLA cellular reactivity may be justifiable.
Clinically, the concern for the practicing healthcare professional relates to whether vaccination can trigger clinical rejection. Anecdotal reports of influenza vaccination-induced graft rejection were common during the pre-cyclosporine era, (31-34) but direct associations between vaccinations and acute graft rejection have not been recently described.(17, 22, 35, 36) Increased alloreactivity did not translate directly into episodes of acute rejection in our cohort; however, influenza vaccination was associated with increased cellular alloreactivity, an important event that is known to participate in the pathogenesis of chronic rejection.(37, 38) The response attenuated within three months of vaccination but repeated exposure to vaccination could allow for the accumulation of alloreactivity increasing the risk for subsequent chronic immune-mediated graft injury. Further evaluation of the persistence or recurrence of enhanced alloreactivity with repeated exposures is needed. Our data further suggests that the effects of influenza vaccination on alloreactivity are variable and unpredictable, but the use of non-invasive immune assays permitted us to detect and follow the alloimmune response during its peak and subsequent attenuation of the response.
Common practice at many centers delays vaccination at least three months following transplantation, when the state of heavy immunosuppression early post-transplant has declined to allow an effective anti-viral immune response. It could additionally be hypothesized that because the risk of acute graft rejection is highest early after transplantation, stimulation of the immune system by vaccination may precipitate early graft rejection. These assumptions could be extended to the pre-transplant period when transplantation is anticipated within one to three months to avoid the peak of cellular alloreactivity post-vaccination. Nevertheless, antiviral and anti-HLA cellular and humoral immune monitoring may aid in determining vaccination efficacy and state of alloimmunity in the peritransplant period (immediately prior or after transplantation) or in any circumstances in which immunosuppression is drastically modified, like in minimization regimens or treatment of rejection episodes.
Our study is limited by the lack of dedicated mechanistic studies to help understand the pathophysiologic processes behind the reported observations; however, this study provides the first human evidence that viral vaccinations may play a role in alloimmunity. Moreover, whether temporarily enhanced alloreactivity in humans by any mechanism triggered by viruses indeed have any impact on HLA-immunity mediated graft injury needs to be better elucidated. Our current work is also limited by the evaluation of a single vaccination and therefore whether alloreactivity can also be affected by other organisms or vaccinations is unknown, nor did we specifically study HLA specificities of the cellular alloreactivity. However, studies in animal models have demonstrated that heterologous immunity may occur with a variety of pathogens.(7, 9, 39, 40) Clinical implications of other infectious diseases on the alloimmune response in transplant recipients remains uncertain and requires further investigation. Further, the study did not evaluate repeated episodes of vaccination, limiting the understanding of repeated stimulation on alloreactivity. Finally, sample size was small to elucidate whether this increase in alloreactivity relates to clinical graft rejection and follow up longer than 3 months post-vaccination until the alloresponse leveled off was not pursued.
In conclusion, influenza vaccination elicits an appropriate anti-viral humoral and cellular response in most transplant recipients; however a significant proportion of vaccinated subjects concomitantly demonstrated an increase in alloreactivity against a broad panel of allogeneic stimulators. “In the future, anti-viral and anti-HLA immune monitoring may be potentially clinically useful for surveillance of circulating alloreactive T cells in solid organ transplant candidates and recipients following influenza vaccination and may aid in personalization of care.”
Acknowledgements
Lara Danziger-Isakov holds a NIH K23 RR 022956 and Emilio Poggio holds a NIH/NIAID K23 5K23AI068824. Assay development was supported by the Clinical Trials in Organ Transplantation (NIH U01 AI63594) awarded to Peter S. Heeger.
Footnotes
There is no conflict of interest of any authors in relation to the presented data
References
- 1.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3(5):525. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 2.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267. [PubMed] [Google Scholar]
- 3.Valujskikh A. Memory T cells in allograft rejection. Adv Exp Med Biol. 2007;601:247. doi: 10.1007/978-0-387-72005-0_26. [DOI] [PubMed] [Google Scholar]
- 4.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol. 2007;18(8):2252. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 5.Augustine JJ, Poggio ED, Clemente M, et al. Hemodialysis vintage, black ethnicity, and pretransplantation antidonor cellular immunity in kidney transplant recipients. J Am Soc Nephrol. 2007;18(5):1602. doi: 10.1681/ASN.2006101105. [DOI] [PubMed] [Google Scholar]
- 6.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-Transplant IFN-gamma ELISPOTs Are Associated with Post-Transplant Renal Function in African American Renal Transplant Recipients. Am J Transplant. 2005;5(8):1971. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr Opin Immunol. 2004;16(5):558. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Alegre ML, Leemans J, Le Moine A, et al. The multiple facets of toll-like receptors in transplantation biology. Transplantation. 2008;86(1):1. doi: 10.1097/TP.0b013e31817c11e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Wang T, Zhou P, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6(10):2282. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 12.Porrett PM, Yuan X, LaRosa DF, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181(3):1692. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 15.Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2(3):287. doi: 10.1034/j.1600-6143.2002.20315.x. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2004;4(Suppl 10):160. doi: 10.1111/j.1600-6135.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 17.Avery RK, Michaels M. Update on immunizations in solid organ transplant recipients: what clinicians need to know. Am J Transplant. 2008;8(1):9. doi: 10.1111/j.1600-6143.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Mauer JR. Cell-mediated immune response to influenza vaccination in lung transplant recipients. J Heart Lung Transplant. 2004;23(10):1175. doi: 10.1016/j.healun.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Schilz RJ, Maurer JR. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18(6):971. doi: 10.1183/09031936.01.00215201. [DOI] [PubMed] [Google Scholar]
- 20.Magnani G, Falchetti E, Pollini G, et al. Safety and efficacy of two types of influenza vaccination in heart transplant recipients: a prospective randomised controlled study. J Heart Lung Transplant. 2005;24(5):588. doi: 10.1016/j.healun.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Lawal A, Basler C, Branch A, Gutierrez J, Schwartz M, Schiano TD. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transplant. 2004;4(11):1805. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 22.Scharpe J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8(2):332. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 23.White-Williams C, Brown R, Kirklin J, et al. Improving clinical practice: should we give influenza vaccinations to heart transplant patients? J Heart Lung Transplant. 2006;25(3):320. doi: 10.1016/j.healun.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Poggio ED, Clemente M, Riley J, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15(7):1952. doi: 10.1097/01.asn.0000129980.83334.79. [DOI] [PubMed] [Google Scholar]
- 25.Poggio ED, Roddy M, Riley J, et al. Analysis of immune markers in human cardiac allograft recipients and association with coronary artery vasculopathy. J Heart Lung Transplant. 2005;24(10):1606. doi: 10.1016/j.healun.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 26.Gebauer BS, Hricik DE, Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2(9):857. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 27.Hricik DE, Rodriguez V, Riley J, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3(7):878. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 28.Poggio ED, Augustine JJ, Clemente M, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 29.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17(2):564. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 30.Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103(1-2):125. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Briggs JD, Timbury MC, Paton AM, Bell PR. Viral infection and renal transplant rejection. Br Med J. 1972;4(5839):520. doi: 10.1136/bmj.4.5839.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briggs WA, Rozek RJ, Migdal SD, et al. Influenza vaccination in kidney transplant recipients: cellular and humoral immune responses. Ann Intern Med. 1980;92(4):471. doi: 10.7326/0003-4819-92-4-471. [DOI] [PubMed] [Google Scholar]
- 33.Lopez C, Simmons RL, Mauer SM, Najarian JS, Good RA, Gentry S. Association of renal allograft rejection with virus infections. Am J Med. 1974;56(3):280. doi: 10.1016/0002-9343(74)90609-3. [DOI] [PubMed] [Google Scholar]
- 34.Gabriel R, Selwyn S, Brown D, et al. Virus infections and acute renal transplant rejection. Nephron. 1976;16(4):282. doi: 10.1159/000180612. [DOI] [PubMed] [Google Scholar]
- 35.Grekas D, Alivanis P, Kiriazopoulou V, et al. Influenza vaccination on renal transplant patients is safe and serologically effective. Int J Clin Pharmacol Ther Toxicol. 1993;31(11):553. [PubMed] [Google Scholar]
- 36.Kobashigawa JA, Warner-Stevenson L, Johnson BL, et al. Influenza vaccine does not cause rejection after cardiac transplantation. Transplant Proc. 1993;25(4):2738. [PubMed] [Google Scholar]
- 37.Avery RK. Cardiac-allograft vasculopathy. N Engl J Med. 2003;349(9):829. doi: 10.1056/NEJMp038124. [DOI] [PubMed] [Google Scholar]
- 38.Avery RK. Viral triggers of cardiac-allograft dysfunction. N Engl J Med. 2001;344(20):1545. doi: 10.1056/NEJM200105173442010. [DOI] [PubMed] [Google Scholar]
- 39.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169(7):3686. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 40.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2(11):1067. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]


