Abstract
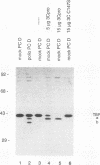
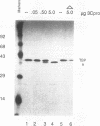
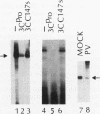
Host cell RNA polymerase II (Pol II)-mediated transcription is inhibited by poliovirus infection. This inhibition is correlated to a specific decrease in the activity of a chromatographic fraction which contains the transcription factor TFIID. To investigate the mechanism by which poliovirus infection results in a decrease of TFIID activity, we have analyzed a component of TFIID, the TATA-binding protein (TBP). Using Western immunoblot analysis, we show that TBP is cleaved in poliovirus-infected cells at the same time postinfection as when Pol II transcription is inhibited. Further, we show that one of the cleaved forms of TBP can be reproduced in vitro by incubating TBP with cloned, purified poliovirus encoded protease 3C. Protease 3C is a poliovirus-encoded protease that specifically cleaves glutamine-glycine bonds in the viral polyprotein. The cleavage of TBP by protease 3C occurs directly. Finally, incubation of an uninfected cell-derived TBP-containing fraction (TFIID) with protease 3C results in significant inhibition of Pol II-mediated transcription in vitro. These results demonstrate that a cellular transcription factor can be directly cleaved both in vitro and in vivo by a viral protease and suggest a role of the poliovirus proteinase 3C in host cell Pol II-mediated transcription shutoff.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M. The effect of Mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. Proc Natl Acad Sci U S A. 1962 Aug;48:1383–1390. doi: 10.1073/pnas.48.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Clark M. E., Dasgupta A. A transcriptionally active form of TFIIIC is modified in poliovirus-infected HeLa cells. Mol Cell Biol. 1990 Oct;10(10):5106–5113. doi: 10.1128/mcb.10.10.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. E., Hämmerle T., Wimmer E., Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991 Oct;10(10):2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Tanese N., Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992 Mar 6;68(5):965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Crawford N., Fire A., Samuels M., Sharp P. A., Baltimore D. Inhibition of transcription factor activity by poliovirus. Cell. 1981 Dec;27(3 Pt 2):555–561. doi: 10.1016/0092-8674(81)90397-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta A. Purification of host factor required for in vitro transcription of poliovirus RNA. Virology. 1983 Jul 15;128(1):245–251. doi: 10.1016/0042-6822(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tomás C. The presence of viral-induced proteins in nuclei from poliovirus-infected HeLa cells. Virology. 1982 Jan 30;116(2):629–634. doi: 10.1016/0042-6822(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Lopez C. A., Wu T., Weinstein I. B. A factor present in fetal calf serum enhances oncogene-induced transformation of rodent fibroblasts. Mol Cell Biol. 1987 Oct;7(10):3380–3385. doi: 10.1128/mcb.7.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerle T., Hellen C. U., Wimmer E. Site-directed mutagenesis of the putative catalytic triad of poliovirus 3C proteinase. J Biol Chem. 1991 Mar 25;266(9):5412–5416. [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Kliewer S., Dasgupta A. An RNA polymerase II transcription factor inactivated in poliovirus-infected cells copurifies with transcription factor TFIID. Mol Cell Biol. 1988 Aug;8(8):3175–3182. doi: 10.1128/mcb.8.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S., Muchardt C., Gaynor R., Dasgupta A. Loss of a phosphorylated form of transcription factor CREB/ATF in poliovirus-infected cells. J Virol. 1990 Sep;64(9):4507–4515. doi: 10.1128/jvi.64.9.4507-4515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Picornavirus protein processing--enzymes, substrates, and genetic regulation. Curr Top Microbiol Immunol. 1990;161:49–87. [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991 Dec;5(12B):2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- Margottin F., Dujardin G., Gérard M., Egly J. M., Huet J., Sentenac A. Participation of the TATA factor in transcription of the yeast U6 gene by RNA polymerase C. Science. 1991 Jan 25;251(4992):424–426. doi: 10.1126/science.1989075. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Harris K. S., Pallai P. V., Wimmer E. Poliovirus proteinase 3C: large-scale expression, purification, and specific cleavage activity on natural and synthetic substrates in vitro. J Virol. 1988 Dec;62(12):4586–4593. doi: 10.1128/jvi.62.12.4586-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Diverse transcriptional functions of the multisubunit eukaryotic TFIID complex. J Biol Chem. 1992 Jan 15;267(2):679–682. [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Rubinstein S. J., Dasgupta A. Inhibition of rRNA synthesis by poliovirus: specific inactivation of transcription factors. J Virol. 1989 Nov;63(11):4689–4696. doi: 10.1128/jvi.63.11.4689-4696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S. J., Hammerle T., Wimmer E., Dasgupta A. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J Virol. 1992 May;66(5):3062–3068. doi: 10.1128/jvi.66.5.3062-3068.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. TATA-binding protein is a classless factor. Cell. 1992 Mar 6;68(5):819–821. doi: 10.1016/0092-8674(92)90023-6. [DOI] [PubMed] [Google Scholar]
- Urzainqui A., Carrasco L. Degradation of cellular proteins during poliovirus infection: studies by two-dimensional gel electrophoresis. J Virol. 1989 Nov;63(11):4729–4735. doi: 10.1128/jvi.63.11.4729-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G., Sawadogo M. Physical analysis of transcription preinitiation complex assembly on a class II gene promoter. Science. 1988 Sep 9;241(4871):1335–1338. doi: 10.1126/science.3413495. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Lieberman P. M., Boyer T. G., Berk A. J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992 Oct;6(10):1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]