Abstract
Post-consolidation immunotherapy with histamine dihydrochloride and interleukin-2 has been shown to improve leukemia-free survival in acute myeloid leukemia in a phase III trial. For this study, treatment efficacy was determined among 145 trial patients with morphological forms of acute myeloid leukemia as defined by the French-American-British classification. Leukemia-free survival was strongly improved in M4/M5 (myelomonocytic/monocytic) leukemia but not in M2 (myeloblastic) leukemia. We also analyzed histamine H2 receptor expression by leukemic cells recovered from 26 newly diagnosed patients. H2 receptors were typically absent from M2 cells but frequently expressed by M4/M5 cells. M4/M5 cells, but not M2 cells, produced reactive oxygen species that triggered apoptosis in adjacent natural killer cells. These events were significantly inhibited by histamine dihydrochloride. Our data demonstrate the presence of functional histamine H2 receptors on human AML cells and suggest that expression of these receptors by leukemic cells may impact on the effectiveness of histamine-based immunotherapy.
Key words: acute myeloid leukemia, remission maintenance, histamine H2 receptors
Introduction
Most adult patients with acute myeloid leukemia (AML) who have achieved complete remission (CR) after induction and consolidation chemotherapy relapse within 12-18 months with poor prospects of post-relapse survival1,2 There is a need for therapies that maintain leukemia free survival (LFS), in particular among patients who are not eligible for allogeneic hematopoietic stem cell transplantation (allo-SCT).
AML prognosis is favorably impacted by the presence of functional NK cells at diagnosis3 and in CR4 These findings have spurred attempts to promote NK cell functions in the post-consolidation phase to prevent relapse, mostly using the NK and T-cell activator IL-2. While the efficacy of IL-2 as a single agent in prolonging LFS has not been proven,5-9 the combination of IL-2 and histamine dihydrochloride (HDC/IL-2) was recently reported to significantly improve LFS in a phase III trial10 Two recent meta-analyses support the view that the HDC component contributes to improvement of LFS11,12
The addition of HDC aims to enhance the effectiveness of IL-213-15 and hence improve immune-mediated elimination of residual leukemia. However, details of the mechanism of action still have to be defined. It was recently reported that leukemic cells recovered from patients with myelomonocytic and monocytic forms of AML (FAB classes M4 and M5) produce immunosuppressive reactive oxygen species (ROS) via a membrane NADPH oxidase and thereby trigger apoptosis in adjacent NK cells and other cytotoxic lymphocytes. AML cells without maturation (FAB-M1) and myeloblastic AML cells with maturation (FAB-M2) did not produce ROS and did not induce NK cell apoptosis.16
For the present study, we performed post hoc analyses of efficacy within the HDC/IL-2 phase III trial to clarify whether the benefit of HDC-based immunotherapy may differ between morphological subtypes of AML. We also determined histamine H2 receptor (H2R) expression on leukemic cells in a separate cohort of newly diagnosed, untreated AML patients. Our results demonstrate the presence of functional H2Rs on human AML cells and suggest that subclasses of AML in which leukemic cells express H2RS respond favorably to treatment with HDC/IL-2.
Design and Methods
Phase III trial patients
In an open-label, randomized phase III study, 320 AML patients were enrolled after induction/consolidation therapies and randomly assigned to an HDC/IL-2 arm (ten 3-week cycles with 3-week and later 6-week rest periods over 18 months) or a control arm (no treatment).10 The present analysis involved patients in first remission (CR1) under 60 years of age and diagnosed with non-M3 FAB classes of AML (n=145). A subgroup analysis included patients who had received high-dose cytarabine or autologous-SCT (allo-SCT) during pre-treatment. All clinical data were analyzed according to ITT and evaluated by the log rank test according to the pre-specified statistical plan.
Bone marrow and peripheral blood from newly diagnosed patients
Peripheral blood or bone marrow samples were obtained from 26 newly diagnosed untreated AML patients (Online Supplementary Table S1) presenting at Sahlgrenska University Hospital or Lund University Hospital, Sweden. Written informed consent was obtained from all participants. The study was approved by the Ethical Committee at the University of Gothenburg.
Cell preparation
Peripheral blood from was diluted 1:1 in PBS and layered on Lymphoprep (Axis-Shield PoC). After centrifugation (850 g, 20 min, RT) PBMC were recovered from the interphase. Lymphocytes and monocytes were further separated using counter-current elutriation as previously described.14 Purified NK cells (purity>95%) were obtained from PBMC using MACS isolation kits (Miltenyi Biotec) according to the manufacturer's instructions. Sorted populations (purity>99%) of FAB-M4/M5 mature leukemic cells (CD14+33+) were obtained by FACS.
Flow cytometry
Phenotype of nucleated cells was determined by flow cytometry using antibodies against CD14, CD15, CD33, CD34, gp91phox and H2R. H2R expression intensity was normalized against a PBMC control and an internal negative control to account for day-to-day variations and balance discrepancies between sample preparations. Myeloid cells from AML patients were defined as cells expressing CD33 and/or CD34. Sorting and phenotyping were carried out using a BD FACSAria or a BD LSRFortessa (BD Biosciences). Antibodies and reagents used in the study are listed in the Online Supplementary Appendix.
Confocal microscopy
Cells were stained with anti-H2R followed by a PE-Cy5.5 conjugated secondary antibody. Blasts and mature cells were separated according to CD33 expression, FACS-sorted onto poly-Llysine coated slides (SigmaAldrich, St Louis, MO, USA) and mounted in Prolong Gold Antifade with DAPI (Invitrogen). Confocal micrographs were acquired using a Zeiss LSM700 confocal microscope (Carl Zeiss) using a 63x/1.40 Oil DIC objective.
ROS production assay
Extracellular ROS production from unseparated bone marrow samples or FACS-sorted FAB-M4/M5 mature leukemic cells was measured using a chemiluminescence assay as described.17 Briefly, cells (106 cells/mL) were suspended in Krebs Ringer glucose and fMLF-triggered (10-7M) ROS production was determined in the presence or absence of histamine (50-100 μM) using a Berthold Biolumat LB 9505 (Berthold Technologies Co.).
NK cell apoptosis
NK cells from healthy donors were co-cultured overnight with FACS-sorted FAB-M4/M5 mature leukemic cells in the presence or absence of histamine (100 μM). Lymphocyte death was determined using the violet amine-reactive Dead Cell Stain Kit (Invitrogen) as described.18
Fluorescence in situ hybridization analysis
LSI probes from Vysis (Abbott Scandinavia AB, Solna, Sweden) were used for fluorescence in situ hybridization (FISH) analysis on interphase cells for the detection of CBFB (Dual Color, Break Apart Rearrangement Probe) according to the manufacturer's instructions.
Statistical analysis
Clinical data were evaluated by the log rank test and results were stratified according to country. For multiple comparisons within a dataset, one-way ANOVAs were performed, followed by Bonferroni's or Dunn's Multiple Comparisons Test. Student's ttest was used for single comparisons if not otherwise stated. All indicated P values are two-sided.
Results and Discussion
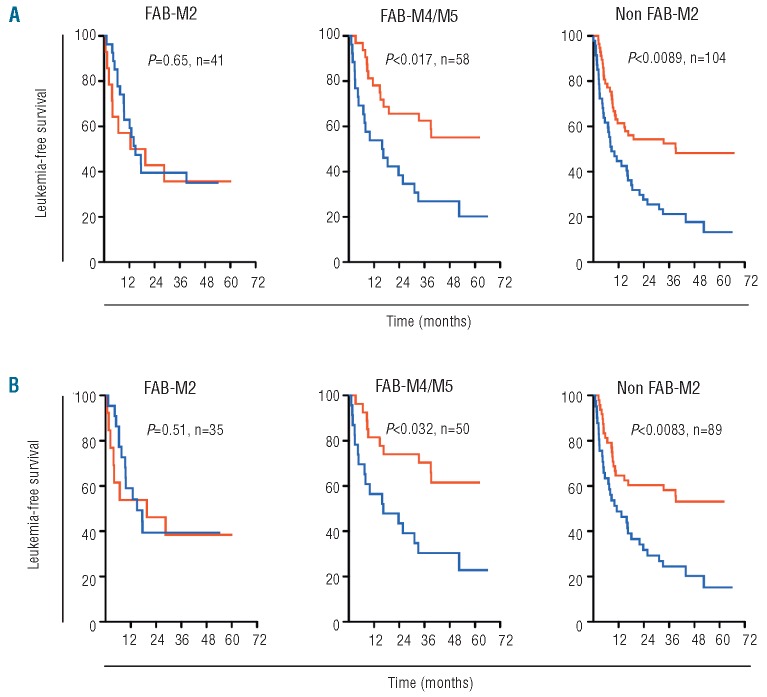
Post hoc analyses of efficacy in morphological subtypes of AML among patients participating in the HDC/IL-2 phase III trial10 showed a non-significant trend towards improvement of LFS for HDC/IL-2-treated patients with FAB-M0/M1 AML versus controls (P=0.16, n=41; Table 1). No benefit of treatment was observed in FAB-M2 AML (P=0.65, n=41; Figure 1A, left panel; Table 1), while HDC/IL-2 significantly improved LFS among patients with FAB-M4/M5 AML (P=0.017, n=58; Figure 1A, middle panel; Table 1). Treatment efficacy was also improved in patients with non-M2 AML with LFS rates at three years of 52.5% (HDC/IL-2) versus 21.7% (controls; P=0.0089, n=104, Figure 1A, right panel; Table 1). Similar results were achieved among patients who had received high-dose cytarabine or allo-ASCT prior to random assignment (Figure 1B in Table 1). We observed no significant improvement of LFS among patients over 60 years of age, regardless of AML FAB class (data not shown). Analyses of overall survival showed trends similar to those observed for LFS (P=0.06 for FAB-M4/M5 and P=0.09 for non-M2), thus supporting the view that LFS is a valid surrogate end point for OS for AML patients in CR.19
Table 1.
Efficacy of HDC/IL-2 immunotherapy in FAB classes of acute myeloid leukemia.
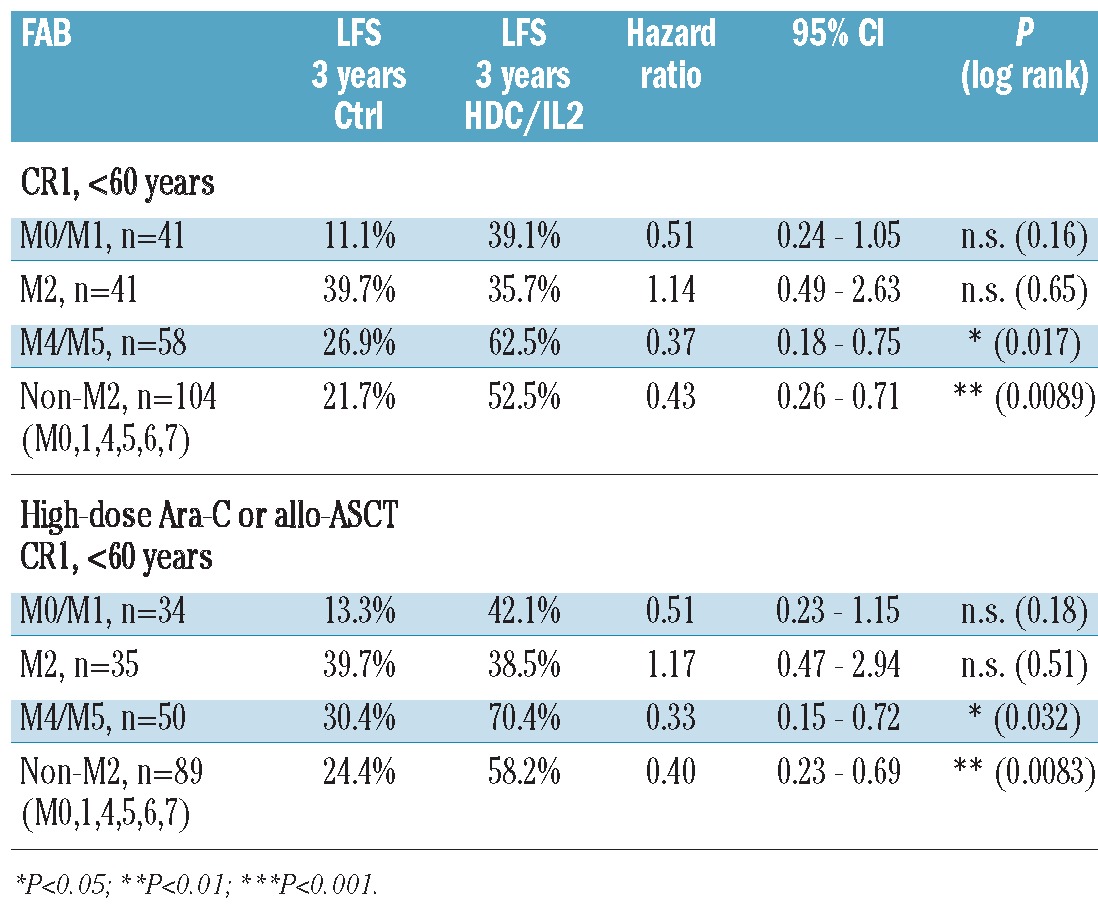
Figure 1.
Efficacy of HDC/IL-2 immunotherapy in FAB classes of AML. (A) Kaplan-Meier plots of LFS for patients in CR1 (<60 years old) receiving post consolidation immunotherapy with HDC/IL-2 (red line) or standard-of-care (no treatment, blue line). FAB classification was performed at each participating center at diagnosis, and the FAB classes were grouped as AML with minimal differentiation/AML without maturation (FAB- M0 and FAB-M1), myeloblastic AML with maturation (FAB-M2) and monocytic forms of AML (FAB-M4 and FAB-M5). Non- M2 AML refers to all patients classified as FAB classes M0, M1, M4, M5, M6, or M7. All patients were followed for LFS for at least three years (median follow-up 47 months), and all results were analyzed according to ITT. (B) Corresponding Kaplan-Meier plots for LFS in the subgroup of patients who received more than three days of high-dose cytarabine treatment or ASCT during pre-treatment. Log rank P values are stratified by country according to the trial statistical plan.
Expression of functional H2Rs by myelomonocytic and monocytic acute myeloid leukemia cells
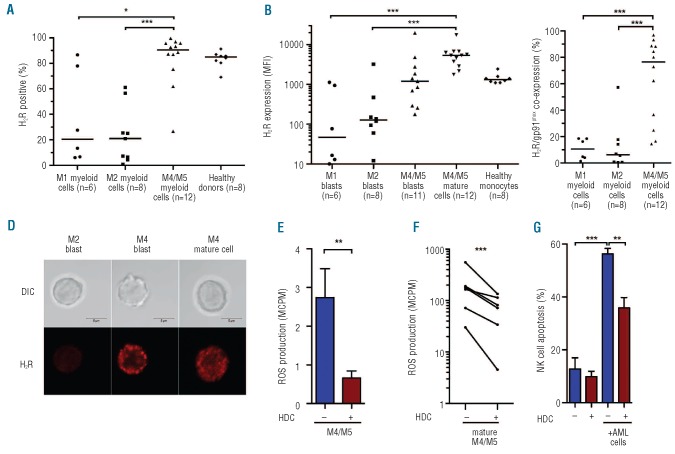
Altogether, the results presented in Figure 1 and Table 1 suggest that the clinical benefit of HDC/IL-2 therapy may be restricted to patients with non-M2 AML and pronounced in patients with monocytic forms of AML. To clarify potential mechanisms of relevance to these findings we determined H2R expression on AML cells recovered from bone marrow or blood recovered from newly diagnosed, untreated patients. H2Rs were more commonly expressed by myeloid cells in FAB-M4/M5 AML than in FAB-M1 (P<0.05) or FAB-M2 AML (P<0.001; Figure 2A).
Figure 2.
Distribution and function of H2Rs on morphological subtypes of AML. H2R expression was analyzed by FACS on PBMC or bone marrow from newly diagnosed AML patients (n=26). (A) Percentage of myeloid cells (defined as CD33+ and/or CD34+) expressing H2Rs in cells from patients with AML of indicated FAB classes. (B) Median fluorescence intensity of H2R expression on immature blasts (CD33+ and/or CD34+, CD14- and CD15- myeloid cells) or mature CD14+ leukemic cells. Horizontal lines represent median values. (C) Fraction of CD33+ and/or CD34+ myeloid cells co-expressing H2R and gp91phox in patients with different morphological subtypes of AML. (D) H2R expression visualized by confocal microscopy on immature FAB-M2 cells, immature FAB-M4 cells, and mature FAB-M4 leukemic cells. Bars represent 5 μm. (E) Bars show extracellular ROS production, assayed in the presence or absence of histamine (100 microM) in bone marrow cells from patients with FAB-M4/M5 AML, evaluated using one-sample t-test. (F) Extracellular ROS production by FACS-sorted mature (CD14+) FAB-M4/M5 cells and its inhibition by histamine (P<0.001, one sample t-test). (G) NK cell apoptosis after overnight culture of NK cells from healthy donors with mature CD33+CD14+ FAB-M4/M5 AML cells. Data are from the first NK:AML cell ratio to reach 40% apoptosis (range 8:1 to 2:1; n=5). *P<0.05, **P<0.01, ***P<0.001.
The malignant clone in FAB-M4 AML comprises a mature population alongside the immature CD34+ blasts whereas the malignant clone in monocytic AML (FAB-M5) is more homogenous with a predominance of CD14+ leukemic cells.20,21 The expression intensity of H2R was higher in mature (CD33+CD14+) FAB-M4/M5 cells than in FAB-M1 or FAB-M2 myeloblasts (P<0.001 and P<0.001, respectively; Figure 2B). CD14- CD15- blasts from FAB-M4 and FAB-M5 AML tended to express higher levels of H2R than myeloblasts from FAB-M1 and FAB-M2 AML (Figure 2B). Co-expression of H2Rs and gp91phox by myeloid cells (CD33+ and/or CD34+) was more frequently observed in FAB-M4 and FAB-M5 AML than in FAB-M1 (P<0.001) or FAB-M2 AML (P<0.001) (Figure 2C). Figure 2D visualizes H2R expression by FAB-M2 blasts and by blasts or mature FAB-M4 cells by confocal microscopy.
Next, we assessed the functionality of H2Rs as reflected by effects of histamine on the production and release of ROS from AML cells. The fMLF-induced extracellular ROS production by unsorted bone marrow-derived FAB- M4/M5 cells was reduced by HDC (P<0.01; Figure 2E). M1 or M2 AML cells produced low or undetectable amounts of ROS (data not shown). The ROS production by FACS-sorted mature (CD33+CD14+) monocytic (FAB M4/M5) populations was reduced by HDC (P<0.001; Figure 2F).
Histamine rescues NK cells from acute myeloid leukemia cell-induced apoptosis
Co-culture experiments showed that FACS-sorted, CD33+CD14+, FAB-M4 and FAB-M5 cells triggered extensive cell death in NK cells from healthy blood donors. The AML cell-induced apoptosis in NK cells was prevented by the NADPH oxidase inhibitor diphenylene iodonium and by catalase, a scavenger of hydrogen peroxide, thus implying that cell death was triggered by NADPH oxidase-derived ROS as reported elsewhere.16 The induction of NK cell apoptosis by CD33+CD14+ FAB-M4/M5 cells was reduced by HDC (P<0.01; Figure 2G). All sorted FAB-M4 samples showed chromosomal or sub-chromosomal aber rations, assessed by FISH and mutational PCR analysis, and more than 95% of the sorted CD14+ malignant cells used in these experiments expressed H2R (data not shown).
In conclusion, the in vitro studies presented herein imply that HDC acts on H2R expressed by FAB-M4 and FAB-M5 AML cells to reduce ROS formation, thus preventing inactivation of NK cells. The post hoc analyses of the phase III trial with HDC/IL-2 showed a pronounced effect of HDC/IL-2 on LFS in H2R+ and NADPH oxidase/gp91phox+ subtypes of AML, i.e. FAB-M4 and FAB-M5. Prospective studies are needed to clarify whether the benefit of HDC-based immunotherapy in AML may involve a direct action of HDC on H2R+ leukemic cells.
Supplementary Material
Acknowledgments
the authors would like to thank Ulla Gingsjö and Jenny Hendel for expert technical assistance, the EpiCept Corporation, New York, for providing clinical results from the HDC/IL-2 phase III trial, and Harald Anderson, Lund University, Lund, for performing statistical analyses.
Funding: this work was supported by the Swedish Cancer Society (Cancerfonden), the Swedish Research Council, Torsten och Ragnar Söderberg's Foundation, Inga-Britt and Arne Lundberg Research Foundation, Gunvor and Ivan Svensson Foundation, the Assar Gabrielsson Foundation, The Swedish Society of Medicine, ALF funds at the Sahlgrenska University Hospital, and the Sahlgrenska Academy at the University of Gothenburg.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Appelbaum FR, Rowe JM, Radich J, Dick JE. Acute Myeloid Leukemia. ASH Education Program Book. 2001; (1):62-86 [DOI] [PubMed] [Google Scholar]
- 2.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487-94 [DOI] [PubMed] [Google Scholar]
- 3.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient Expression of NCR in NK Cells from Acute Myeloid Leukemia: Evolution During Leukemia Treatment and Impact of Leukemia Cells in NCRdull Phenotype Induction. Blood. 2007;109(1):323-30 [DOI] [PubMed] [Google Scholar]
- 4.Lowdell MW, Koh MB. Immunotherapy of AML: future directions. J Clin Pathol. 2000;53(1):49-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrozek K, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26(30):4934-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaise D, Attal M, Reiffers J, Michallet M, Bellanger C, Pico JL, et al. Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw. 2000;11(1):91-8 [PubMed] [Google Scholar]
- 7.Kolitz JE, Hars V, DeAngelo DJ, Allen SL, Shea TC, Vij R, et al. Phase III Trial of immunotherapy with recombinant interleukin-2 (rIL-2) versus observation in patients < 60 years with acute myeloid leukemia (AML) in first remission (CR1): preliminary results from Cancer and Leukemia Group B (CALGB) 19808. ASH Annual Meeting Abstracts. 2007;110(11):157 [Google Scholar]
- 8.Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG- 2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group. Blood. 2008;111(3):1044-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808-14 [DOI] [PubMed] [Google Scholar]
- 10.Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88-96 [DOI] [PubMed] [Google Scholar]
- 11.Berry SM, Broglio KR, Berry DA. Addressing the incremental benefit of histamine dihydrochloride when added to interleukin-2 in treating acute myeloid leukemia: a Bayesian meta-analysis. Cancer Invest. 2011;29(4):293-9 [DOI] [PubMed] [Google Scholar]
- 12.Buyse M, Squifflet P, Lange BJ, Alonzo TA, Larson RA, Kolitz JE, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117(26):7007-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96(5):1961-8 [PubMed] [Google Scholar]
- 14.Hellstrand K, Asea A, Dahlgren C, Hermodsson S. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol. 1994;153(11):4940-7 [PubMed] [Google Scholar]
- 15.Martner A, Thoren FB, Aurelius J, Soderholm J, Brune M, Hellstrand K. Immunotherapy with histamine dihydrochloride for the prevention of relapse in acute myeloid leukemia. Expert Rev Hematol. 2010;3(4):381-91 [DOI] [PubMed] [Google Scholar]
- 16.Aurelius J, Thoren FB, Akhiani A, Brune M, Palmqvist L, Hansson M, et al. Monocytic AML cells inactivate anti-leukemic lymphocytes: role of NADPH oxidase/gp91phox expression and the PARP-1/PAR pathway of apoptosis. Blood. 2012;doi: 10.1182/blood-2011-11-391722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232(1-2):3-14 [DOI] [PubMed] [Google Scholar]
- 18.Christenson K, Thoren FB, Bylund J. Analyzing cell death events in cultured leukocytes. Methods in Molecular Biology. 2012;844:65-86 [DOI] [PubMed] [Google Scholar]
- 19.Buyse M, Squifflet P, Lucchesi KJ, Brune ML, Castaigne S, Rowe JM. Assessment of the consistency and robustness of results from a multicenter trial of remission maintenance therapy for acute myeloid leukemia. Trials. 2011;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-51 [DOI] [PubMed] [Google Scholar]
- 21.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.