Reanalysis of the CALGB1 and AZA0012 studies in advanced myelodysplastic syndrome (MDS) suggests that 5-azacytidine (AZA) is effective for acute myeloblastic leukemia (AML) with less than 30% bone marrow blasts. Most AML patients are elderly (>65 years old) and unfit for intensive chemotherapy and allogeneic transplantation; 3 prognosis is extremely poor with median survival of a few months.4 For these patients, appropriate treatments are best supportive care or low-dose cytarabine.3 Compared with best supportive care, low-dose cytarabine more frequently induces complete remissions (18% vs. 1%) and prolongs survival (3.8 vs. 2.5 months).3 AZA targets DNA methyltransferase and reactivates quiescent genes,5 decreases leukemic cell proliferation and induces cell differentiation. In higher risk MDS or in AML with less than 30% bone marrow blasts,6 AZA outperformed conventional care (best supportive care, low-dose cytarabine, and intensive chemotherapy)7 with longer hematologic improvement and transfusion independence (TI).5 Here, we postulated that patients unfit for standard AML chemotherapy might also benefit from AZA, irrespectively of bone marrow blast count.
We systematically reviewed charts of all patients treated with AZA at our institution between 1st January 2007 and 31st December 2011, and identified 52 consecutive AML patients. These patients were not eligible for intensive chemotherapy due to advanced age (n=33) or comorbidities (n=9), or were refractory to initial intensive chemotherapy (n=10). AZA was administered at 100 mg/m2 sc Days 1-5, over 28-day cycles. This schedule is equivalent to standard Days 1-7 MDS treatment8 and allows weekday outpatient treatment.2 Therapy was continued until disease progression, as survival benefit is extended beyond achievement of best response.9 Indication for transfusion was based on symptoms, comorbidities, and laboratory values (generally Hb<80 g/L and PLT<109/L). Transfusion independence was defined as eight weeks or over without red blood cell (RBC-TI) and/or platelet (PLT-TI) transfusion.1,7 The indication to treat patients with baseline RBC-TI was based on appearance of agranulocytosis (n=3), high peripheral blood or marrow blast counts (n=5), and/or AML relapse (n=1). Overall survival (OS) was measured from the first administration of AZA to death. Five surviving patients were censored on 31st December 2011. The median follow up of censored patients was 12.2 months. Statistical analysis was performed using Stata software (Statacorp LP, College Station, Texas, USA).
At baseline, 15% (n=9) of our patients had RBC-TI and 25% (n=13) PLT-TI. Median overall survival of the 52 enrolled patients was 8.6 months and 12-month survival rate was 28% (95 CI: 15-43%). We excluded 14 patients from transfusion and survival analyses since they did not reach the minimal 8-week observation period. Among them, 11 patients received only one cycle of AZA and 3 received 2 cycles. Treatment was interrupted due to premature death or disease progression (n=12), or availability of a donor for allogeneic stem cell transplantation (n=2). Among these 14 patients, at the start of therapy, 3 had RBC-TI and 3 had PLT-TI. Median OS was 1.3 months (range 0.5-23.7).
Characteristics of the 38 remaining patients are presented in Table 1. Patients with baseline or acquired RBC-TI (n=22) were grouped and compared to patients who never reached RBC-TI (n=16). Among patients with transfusion independence, 6 had RBC-TI at baseline while 16 achieved RBC-TI under therapy. Except for age, which was older for patients with RBC-TI, we found no differences in baseline characteristics. Our 38 patients received a median of 6 (range 3-20) cycles of AZA. Overall response rate was 23% (n=9), distributed in 7 complete response with incomplete blood count recovery (CRi) and 2 partial remission (PR).11 The remaining 29 patients (76%) exhibited resistant disease (RD). RD status was equally distributed among transfusion independent and transfusion dependent groups (13 vs. 16).
Table 1.
Baseline characteristics of study groups according to RBC transfusion independence.
RBC-TI at baseline or under AZA therapy (n=22) was associated with prolonged survival. The median OS of transfusion independent patients was 11.1 months versus 5.0 months for transfusion dependent patients: 12-month OS 40% (95% CI: 19-60%) versus 13% (95% CI: 2-32%) (P=0.0006; Figure 1A). Presence of transfusion independence at baseline (n=6) or its achievement under treatment (n=16) had similar survival outcome: median OS 10.7 versus 11.9; 12-month OS 40% (95% CI: 17-63%) versus 45% (95% CI: 5-75%) (P=NS; Figure 1B). Median OS of transfusion dependent patients reported here (5.0 months) is similar to that observed for patients enrolled in the AML14 trial who received best supportive care (2.5 months) or low-dose cytarabine (3.8 months).3 The impact of PLT-TI on survival was similar although not significant: OS 10.7 versus 5.4 months; 12-month OS 32% (95% CI: 14-52%) versus 21% (95% CI: 5-45%) (P=0.053). The median delay to RBC-TI or PLT-TI after therapy initiation was 85 days (range 60-143) and 73 days (35-154), respectively, (approx. 3 AZA cycles). As observed in MDS patients,1 transfusion independence was achieved in all responding patients by the end of the 6th cycle. In patients with baseline RBC-TI, the median duration of transfusion independence was 22 weeks (range 8-42). When obtained in response to AZA, the median duration of transfusion independence reached 30 weeks (range 10-88). In our country, blood product expenses in transfusion dependent patients (median 5 RBC and 6 PLT transfusions per month for our cohort) were comparable to those of continued AZA therapy in transfusion independent patients.
Figure 1.
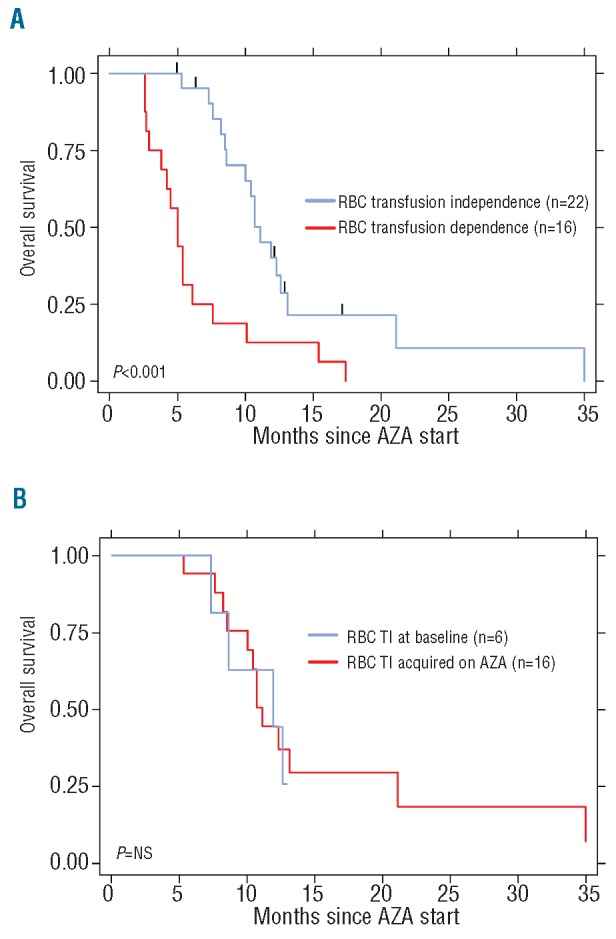
Survival according to RBC-transfusion independency. (A) Overall survival of patients according to transfusion needs. We analyzed 38 patients: 22 RBC-transfusion independent (blue) and 16 RBC-transfusion dependent (red). OS 11.1 versus 5.0 months, P=0.0006. Twelve-month survival rates were 40% (95% CI: 19-60%) and 13% (95% CI: 2-32%), respectively. (B) Overall survival of patients with transfusion independence (TI) according to baseline transfusion needs (n=22). Median OS was 10.7 months for baseline TI (n=6, blue) versus 11.9 months for TI acquired under AZA (n=16, red), 12-month OS 40 (95% CI: 17-63%) versus 45% (95% CI: 5-75%), P=NS.
Univariate analysis of survival showed significant differences in peripheral blood blast count of 20% and over, relapsed/refractory disease, baseline PLT-TI and baseline or acquired RBC-TI. Interestingly, BM blast level was not predictive of survival or response to treatment. In multivariate analysis, baseline or acquired RBC-TI remained the single parameter favorably affecting survival (HR 0.36, 95% CI: 0.16-0.77) (P=0.009).
In conclusion, we report here for the first time that AZA can induce transfusion independence in half of previously transfusion dependent patients and that transfusion independence is a strong prognostic factor in unfit AML patients. Observations made in MDS12 may now be extended to AML. In addition, AZA may improve quality of life by reducing requirements for transfusions and anemia symptoms. Prospective randomized trials are required to validate these observations in larger cohorts.
Supplementary Material
Acknowledgments
Funding: this work was not funded by external sources.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895-903 [DOI] [PubMed] [Google Scholar]
- 2.Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403-11 [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-24 [DOI] [PubMed] [Google Scholar]
- 4.Tilly H, Castaigne S, Bordessoule D, Casassus P, Le Prise PY, Tertian G, et al. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1990;8(2):272-9 [DOI] [PubMed] [Google Scholar]
- 5.Silverman LR. Hypomethylating agents in myelodysplastic syndromes changing the inevitable: the value of azacitidine as maintenance therapy, effects on transfusion and combination with other agents. Leuk Res. 2009;33(Suppl 2):S18-21 [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-9 [DOI] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons RM, Cosgriff T, Modi S, McIntyre H, Beach CL, Backstrom JT. Tolerability and hematologic improvement assessed using three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. ASCO Meeting Abstracts. 2007; 25:7083. [DOI] [PubMed] [Google Scholar]
- 9.Gore SD. New Ways to Use DNA Methyltransferase Inhibitors for the Treatment of Myelodysplastic Syndrome. ASH Education Program Book. 2011;2011:550-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9):3658-66 [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642-9 [DOI] [PubMed] [Google Scholar]
- 12.Seymour JF, Santini V, Fenaux P, Giagounidis AAN, Sanz GF, Finelli C, et al. Achievement of Red Blood Cell (RBC) Transfusion Independence with Azacitidine (AZA) Leads to Improved Survival In Patients with Higher-Risk Myelodysplasias Regardless of Baseline Transfusion Needs. ASH Annual Meeting Abstracts. 2010;116:1856 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


