Abstract
Radiotherapy is a major treatment modality used to treat muscle-invasive bladder cancer, with patient outcomes similar to surgery. However, radioresistance is a significant factor in treatment failure. Cell-free extracts of muscle-invasive bladder tumours are defective in non-homologous end-joining (NHEJ), and this phenotype might be exploited clinically by combining radiotherapy with a radiosensitising drug that targets homologous recombination (HR), thereby sparing normal tissues with intact NHEJ. The response of the HR protein RAD51 to radiation is inhibited by the small molecule tyrosine kinase inhibitor (TKI) imatinib. Stable RT112 bladder cancer Ku knockdown (Ku80KD) cells were generated using shRNA technology to mimic the invasive tumour phenotype, and also RAD51 knockdown (RAD51KD) cells to demonstrate imatinib’s pathway selectivity. Ku80KD, RAD51KD, non-silencing vector control and parental RT112 cells were treated with radiation in combination with either imatinib or lapatinib, which inhibits NHEJ, and cell survival assessed by clonogenic assay. Drug doses were chosen at approximately IC40 and IC10 (non-toxic) levels. Imatinib radiosensitised Ku80KD cells to a greater extent than RAD51KD or RT112 cells. In contrast, lapatinib radiosensitised RAD51KD and RT112 cells, but not Ku80KD cells. Taken together, our findings suggest a new application for imatinib in concurrent use with radiotherapy to treat muscle-invasive bladder cancer.
Keywords: Imatinib, homologous recombination, microhomology-mediated end-joining, radiosensitisation, bladder cancer
Introduction
Bladder cancer is the 4th most common cancer in men in the UK (1). In a population-based study, radiotherapy was found to be as effective as cystectomy in the treatment of muscle-invasive disease, and is being increasingly required as the population ages (2). Radiotherapy to the bladder results in acute bladder and bowel toxicities in most patients and, more rarely, causes long-term toxicity in which the most severe cases may require a cystectomy for alleviation of symptoms. Conventional cytotoxic chemotherapy agents have been used to improve the outcome of radiation treatment in muscle-invasive bladder cancer (3-5). However, elderly patients are not always able to tolerate conventional chemotherapy agents when used as radiosensitisers.
For tumours with genomic aberrations/alterations, therapies targeted towards the expressed proteins, such as the tyrosine kinase inhibitors imatinib and lapatinib, are an attractive option as they do not have the myelosuppressive or neurotoxic side effects of chemotherapy, although they do cause diarrhoea, skin rash and very rarely lung fibrosis, and most are available as oral preparations. However, it is important that such costly agents are targeted to those patients most likely to benefit. Imatinib selectively inhibits the tyrosine kinase activity of ABL as well as several receptor tyrosine kinases: the platelet-derived growth factor receptors (PDGFR-α and PDGFR–β), the stem cell factor (SCF) receptor (KIT), the discoidin domain receptors (DDR1 and DDR2) and the colony stimulating factor receptor (CSF-1R) (6, 7) and is used to treat chronic myelogenous leukaemia (CML) and gastrointestinal stromal tumours (GIST). There are currently no clinical trials involving imatinib in bladder cancer. Lapatinib is a tyrosine kinase inhibitor that selectively targets the EGF receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) (8) and is indicated in the treatment of HER2 positive breast cancer. Clinically, lapatinib is primarily being investigated in metastatic bladder cancer. However, there is an ongoing pilot study of neo-adjuvant lapatinib prior to cystectomy (9).
Bladder tumours express tyrosine kinases to varying extents: 50% and 45% over-express EGFR and HER2 respectively (10) whilst PDGFR was reportedly expressed in approximately 80% in a Chinese cohort, published only in Chinese. Although KIT expression has been described in both upper tract tumours and small cell carcinomas of the bladder (11, 12), neither KIT nor ABL expression has been studied in transitional cell carcinomas of the bladder to our knowledge.
Ionising radiation causes DNA damage, including base damage, single-strand breaks (SSBs) and double-strand breaks (DSBs). Unrepaired or mis-repaired DSBs are lethal, resulting in cell death both in tumours and normal tissues (13, 14). Mammalian cells employ two major pathways to repair DSBs, namely homologous recombination (HR) and non-homologous end-joining (NHEJ) (15, 16). HR is an error-free pathway, predominantly used in the G2/S phase of the cell cycle, which requires the sister chromatid to act as a DNA template. A major HR protein is RAD51, which is involved in ATP-dependent DNA strand exchange. RAD51 expression is increased following ionising radiation (IR) in tumour cells and induces the formation of RAD51 nuclear foci at sites of DSB (17). NHEJ is the major DSB repair pathway used in G0 and G1 (18), and involves the DNA binding complex Ku70/Ku80 and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs)(19). In addition to the classical DSB repair pathways, a less efficient Ku-independent pathway has been described in Ku-deficient yeast cells, which involves microhomology mediated end-joining (MMEJ) (20). We have previously demonstrated the MMEJ phenotype in vitro using cell-free extracts from muscle-invasive bladder tumours, and this is associated with reduced Ku-DNA binding and loss of TP53 function (21). Pucci et al also showed reduced Ku-DNA binding in five advanced breast and muscle-invasive bladder tumours (22). This error-prone repair was not detected in normal human urothelial cell extracts (23), which suggests a therapeutic window that could be targeted by novel therapies. Negroni et al (24) inhibited Ku80 expression by RNA interference using short hairpin RNA in RT112 bladder cancer cells and demonstrated increased radiosensitivity and reduced Ku-DNA binding compared to parental RT112 vector-transfected cells.
The outcome of clinical radiotherapy depends, in part, upon the extent of DNA damage and how efficiently the cells can repair this damage. Selective targeting of DNA repair pathways could increase tumour cell kill whilst sparing normal tissues, thus increasing the therapeutic ratio. ABL upregulates RAD51 gene expression, and imatinib reduces RAD51 protein expression and RAD51-chromatin binding (25) and reduces error-free homologous recombination efficiency (17). Imatinib also reduces the increased RAD51 expression induced by IR and reduces the associated RAD51 nuclear focus formation in glioma cell lines (25), and in bladder, pancreatic, prostate and lung carcinoma cell lines, imatinib increases cell kill in combination with IR, due in part to mitotic catastrophe (17), unlike normal fibroblasts, where cell survival is unaffected. In xenograft studies, imatinib increases growth delay following fractionated radiotherapy in glioblastoma, epidermoid and prostate carcinoma models with no apparent increase in toxicity (17, 26).
EGFR inhibitors, such as lapatinib, can act as radiosensitisers by targeting the intracellular signalling cascades (including Ras-MAPK and PI3K-AKT), which are triggered by binding of ligand to the trans-membrane EGFR ligand-binding domain (27, 28). These cascades are normally activated by IR in EGFR-overexpressing tumours, resulting in radioresistance (27). Moreover, they also act as radiosensitisers by repressing DNA repair in irradiated cells, although this occurs via the NHEJ pathway, through inhibition of the PI3K-mediated stimulation of DNA PKcs, and by blocking of the nuclear interaction between EGFR and DNA PKcs normally induced by IR (reviewed by Baumann et al (27)).
We hypothesised that in muscle-invasive bladder cancer it would be better to use an agent that targets the HR pathway rather than the NHEJ pathway. This would result in a form of ‘synthetic sickness’ (see (29) for recent review), where tumour cells already deficient in NHEJ would have reduced HR efficiency and repair and thus increased IR-induced lethality. As imatinib is known to target HR via RAD51 (17, 25), it may be one such agent, and the combination of imatinib and radiotherapy should result in an increased therapeutic ratio for muscle-invasive bladder cancer.
We therefore sought to see whether ABL, KIT, PDGFR, HER-2 or EGFR are targets in bladder cancer by determining their role in radiotherapy response. We then moved to an experimental system to test the effectiveness of therapies that might be used in combination with radiotherapy to enhance radiosensitivity. This included targeting the ABL/RAD51 and EGFR pathways.
Materials and Methods
Tissue samples and imunohistochemistry
Ninety-one formalin-fixed paraffin-embedded bladder tumour biopsy samples were obtained from patients treated with radical radiotherapy for transitional cell carcinoma of the bladder at the Leeds Cancer Centre, West Yorkshire, United Kingdom, from 2002 to 2005. Details of the patients and radiotherapy treatments have been described previously (30). Patients gave informed consent for use of their tissues and local ethical approval was obtained from the Leeds (East) Research Ethics Committee (project 04/Q1206/62).
Antibody conditions were optimised by staining sections from control tissues, namely breast tumour for ABL, HER2, EGFR and PDGFR, and skin tumour for KIT. Then 4 μm sections from the bladder tumour specimens were heated, dewaxed, and hydrated in xylene, graded alcohols and water. Antigen retrieval was achieved by boiling in ethylenediaminetetraacetic acid (1 mM at pH 8.0) or citric acid (pH 6.0) for 2 min of pressure cooking before quenching endogenous peroxidase activity with 3% H2O2 for 30 min. Endogenous protein-binding activity was blocked using Avidin-biotin blocking agent (Vector, Peterborough, UK) and normal goat serum (Dako, Cambridgeshire, UK) before incubation with primary antibody: anti-ABL (1:250, NeoMarkers, Fremont, CA), anti-c-HER2 (1:200, Dako, Cambridgeshire, UK), anti-EGFR (1:100, Novocastra, Peterborough, UK), anti-KIT (1:40, Novocastra, Peterborough, UK), and anti-PDGFRβ (1:25, Cell Signalling, Hertfordshire, UK) diluted in diluent (Dako, Cambridgeshire, UK), for 1 hour. Samples were then incubated for 30 minutes with secondary antibody conjugated to horseradish peroxidase using the DAKO ChemMate detection kit (Dako, Cambridgeshire, UK). Immunoreactivity was revealed by incubation of sections with 3-3′-diaminobenzidine for 10 min before washing, taking through graded alcohols, clearing in xylene and counterstaining with haematoxylin (VWR, Leicestershire, UK) before mounting in dibutylphthalate xylene (Leica, Peterborough, UK). Digital images were captured within invasive tumour areas (3-10 images per slide, x400 magnification) using an Olympus BX50 microscope and c-3030 camera.
Assessment was made of the percentage and intensity of tumour cells with membranous staining based on the recommendations for interpretation of the HercepTest™: Gastric Cancer (31). Briefly, for all antibodies studied, complete, basolateral or lateral membrane staining was scored as an intensity of 3+ for strong intensity and 2+ for weak to moderate intensity, where at least 10% of the tumours cells stained positive. Partial membrane staining or only faint/barely perceptible intensity staining in at least 10% of tumour cells was scored as a 1+. Staining intensity was scored independently in a blinded manner by two observers, discordant scores were reviewed, and a consensus was reached.
Cause-specific survival was defined from Day 1 of radiotherapy until death from bladder cancer. Death from another cause was considered a censored observation. Kaplan-Meier curves were plotted for cause-specific survival and the log-rank statistic used to compare survival times across categories of protein expression.
Reagents
Imatinib was a generous gift from Novartis Pharma AG (Switzerland) and was later purchased from Stratatech Scientific Ltd; lapatinib was a generous gift from GlaxoSmithKline plc (UK). For cell culture experiments, imatinib and lapatinib were dissolved in DMSO (Sigma, UK) to a stock concentration of 10 mM and stored in single-use aliquots at −20°C.
Cell culture conditions
The TP53 wild-type RT112 bladder transitional cell carcinoma cell line has been authenticated in MAK’s laboratory by extensive genomic analysis (microsatellite typing, conventional karyotypic analysis, MFISH, array-based copy number analysis). Cells were grown in RPMI 1640 (Sigma, UK) supplemented with 10% v/v foetal bovine serum (Sigma, UK) and 2 mM L-glutamine (Sigma, UK) in a humidified atmosphere containing 5% CO2 at 37°C. Exponentially growing cells were used in all experiments.
Cell irradiation
Cells were harvested from exponential-phase cultures and diluted to 1000 cells/ml. Five millilitre cell suspensions were then irradiated in tubes at a dose-rate of 1.0 Gy/min using an X-ray machine (Irradiator 320, NDT Equipment Services Ltd, Cleveland, UK), or Caesium-137 source at 1.12 Gy/min using a Gamma-Service Medical GmbH GSR D1 irradiator. The cells were then replated into 10 cm dishes at appropriate cell densities.
Chemosensitivity studies
Exponentially growing cells were incubated in 75 cm2 flasks, for 24 hours at appropriate drug concentrations (specified in the figure legends) and the cells then trypsinised and resuspended in medium containing drug as required.
Clonogenic assays
Following the necessary treatments, cells were plated at appropriate cell numbers in triplicate in 10-cm culture dishes containing 10 ml of fresh medium, with or without drug as required (see relevant figure legends). After 14 days incubation, the cells were stained with 1% methylene blue (Sigma, UK) in 50% ethanol, and colonies with more than 50 cells were counted. The surviving fraction was determined as the total number of colonies formed divided by the total number of cells plated multiplied by the plating efficiency, as determined in untreated cells. Radiation survival curves were plotted after normalisation for the cytotoxicity induced by control or drug alone, in GraphPad Prism, using the linear-quadratic model with the equation SF = exp -(αD + GβD2). Each point on the survival curve represents the mean surviving fraction from at least three independent experiments.
RNA interference using short hairpin RNA
The siRNA expression vector pSilencer 2.1-U6 neomycin (Ambion, Warrington, UK), which contains a human U6 RNA polymerase III promoter able to transcribe short hairpin RNAs, was used in these experiments. Two constructs were made for each of Ku80 and RAD51 (see Supplementary Table 1 and Supplementary Figure 1), whereby complementary oligonucleotides were used to encode hairpin siRNA inserts, designed to target a 21-mer sequence of human Ku80 coding region or 3′UTR mRNA, or RAD51 coding region mRNA, respectively. These were designed using the Ambion Insert Design Tool for pSilencer vectors and purchased from Sigma, and were then annealed and ligated into the linearised pSilencer vector. Circular negative control pSilencer neovector, that expresses a hairpin siRNA with limited homology to any known sequence in the human genome, was used in experiments as a non-specific negative control.
The target sequences in the human Ku80 or RAD51 gene were determined empirically and then analysed by BLAST search, to confirm a lack of homology to other coding sequences, as per manufacturer’s recommendations. The target sequences of clones used for clonogenic assays were Ku80 5′-AAC TCC ATT CCT GGT ATA GAA-3′ (Ku80 coding region target sequence 1) and for RAD51 5′-AAT CAC TAA TCA GGT GGT AGC-3′(RAD51 coding region target sequence 2, Supplementary Table 1). The corresponding targeting oligonucleotide sequences were for Ku80: top strand 5′-GAT CCG CTC CAT TCC TGG TAT AGA ATT CAA GAG ATT CTA TAC CAG GAA TGG AGT TTT TTG GAA A-3′ and bottom strand 5′-AGC TTT TCC AAA AAA CTC CAT TCC TGG TAT AGA ATC TCT TGA ATT CTA TAC CAG GAA TGG AGC G-3′, and for RAD51: top strand 5′-GAT CCG TCA CTA ATC AGG TGG TAG CTT CAA GAG AGC TAC CAC CTG ATT AGT GAT TTT TTG GAA A-3′ and bottom strand: 5′-AGC TTT TCC AAA AAA TCA CTA ATC AGG TGG TAG CTC TCT TGA AGC TAC CAC CTG ATT AGT GAC G-3′.
Stable transfection
RT112 cells were seeded into 75cm2 flasks and the following day transfected at 60% confluence with pSilencer-Ku80, pSilencer-RAD51 or circular negative control pSilencer neovector, using Lipofectamine 2000 (Invitrogen, UK), according to the manufacturer’s instructions. After 24 hours, fresh medium was added containing the selection reagent G418 (600 μg/ml; Gibco, Invitrogen, UK). Selection was continued for 14 days, with the medium refreshed every other day. Single clones were picked and tested for Ku80 and RAD51 expression, respectively, by western blotting and subsequently tested for IR sensitivity using clonogenic assays.
Western blotting analysis
Cells were lysed on ice in RIPA buffer (Sigma, UK) with 1% of protease inhibitor and phosphatase inhibitor (Sigma, UK). The cells were allowed to swell on ice for 20 min; then the lysate was centrifuged for 30s at 12,000 g. The supernatant was carefully removed and stored at −20°C. Total protein concentration in cell lysates was determined by the method of Bradford (Sigma, UK). Thirty to fifty micrograms of protein was resolved on 4-20% polyacrylamide gels and transferred onto nitrocellulose membranes. The resulting membranes were incubated with blocking buffer (Li-cor, Cambridge, UK) and primary antibodies. The antibodies used were rabbit polyclonal ABL (2862, Cell Signalling, UK), rabbit polyclonal Rad51 (ab63801, Abcam, UK), rabbit polyclonal EGFR (sc-03, Santa Cruz Biotechnology, UK), mouse monoclonal anti-Ku80 (Ab-2, Neomarkers, Freemont), mouse monoclonal β-tubulin (clone TUB2.1 TT4026, Sigma) and mouse monoclonal β-actin (ab8226, Abcam, UK).
Fluorochrome-conjugated secondary antibodies (Li-cor, UK) were used and detected by IR scanning densitometry using the Li-cor Odyssey infra-red detection system (Li-cor Biosciences UK Ltd, Cambridge, UK). Quantification was based on normalisation to β-actin. The immunoblotting experiments were performed at least thrice.
Cell cycle analysis
Propidium iodide (PI) staining and flow cytometry were used to determine cell cycle stages. Cells from the batches used for clonogenic assays were washed with PBS, fixed in ice-cold 70% ethanol and stored at 4°C prior to analysis. Cells were spun down and resuspended in propidium iodide solution (50 μg/ml propidium iodide, 0.5 mg/ml RNase, Applied Biosystem, UK) and incubated at room temperature in the dark at least for 30 minutes. DNA content was detected by flow cytometry (Beckman FACScan system). The relative proportions of cells in the G1, S and G2/M phases of the cell cycle were determined using ModFit LT 3.2 software.
Statistical analysis
All statistical analyses were performed using SPSS16.0 software. Clonogenic assays were performed in triplicate at least thrice, with the results expressed as mean plus standard deviation (SD) as appropriate. Western blots were performed at least three times independently. Results were considered statistically significant at a p<0.05, using a two-tailed, unpaired Student’s t-test. Sensitiser enhancement ratios (SER) were calculated at a surviving fraction of 0.1 (10% survival). Cause-specific survival was defined from Day 1 of radiotherapy until death of the patient from bladder cancer. Death from another cause was considered a censored observation. Kaplan-Meier curves were plotted for cause-specific survival and the log-rank statistic used to compare survival times across categories of protein expression.
Results
Expression of tyrosine kinases in bladder tumour samples
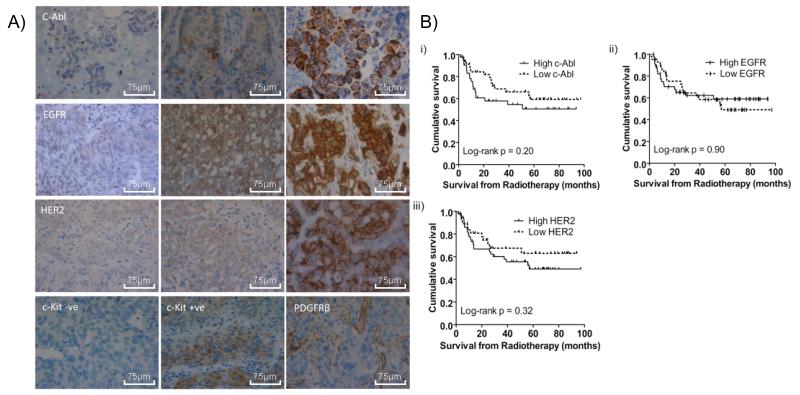
We sought to estimate the percentage of muscle-invasive bladder tumours expressing the TKs of interest (imatinib: ABL, KIT, PDGFR-β; lapatinib: HER2, EGFR), on the basis that this is likely to represent the cohort of patients for which the addition of a TKI to their radiotherapy may be beneficial. We also wished to determine whether these factors were prognostic in these patients, who had not received such treatments in addition to radiotherapy. The Dako scoring system for the HercepTest was applied to our TKs of interest in 91 formalin-fixed paraffin-embedded bladder tumour samples (Figure 1A). A sample was classified as positive if there was membranous staining in at least 10% of cells, which met the threshold criteria for intensity of staining (2+ or 3+). In 75/91 (82%) cases there was positive immunostaining for ABL. In contrast, KIT expression was undetectable in most of the cases, but showed weak staining in eight cases, and PDGFR-β was expressed in endothelial cells and smooth muscle cells, but was undetectable in bladder tumour cell membranes. There were 87/91 cases (96%) positive for EGFR staining and 86/90 (96%) cases HER2 positive.
Figure 1.
Immunohistochemistry studies. a) Representative images, at high-power magnification (x40): ABL 1+, 2+, 3+; EGFR 1+, 2+, 3+; HER2 1+, 2+, 3+; KIT-negative and -positive samples; PDGFRβ example of blood vessel staining but absent tumour cell staining. Scale bars represent 75 μm lengths; b) Kaplan-Meier survival curves from radiotherapy for: i) ABL scoring comparing 3+ (solid line) and 2+ or lower (dashed line); ii) EGFR scoring comparing 3+ (solid line) and 2+ or lower (dashed line); iii) HER2 scoring comparing 3+ (solid line) and 2+ or lower (dashed line).
We also correlated TK expression with patient survival, to look for prognostic significance of high expression. We classified patients into those with low tumour TK expression (equal or less than 2+) and patients with high (3+) expression levels (as there were insufficient tumours scoring 0/1+ for meaningful comparison with 2+/3+). Neither ABL, EGFR nor HER2 were significantly correlated with patient survival (Figure 1B).
Effects of imatinib and lapatinib on RT112 cell proliferation
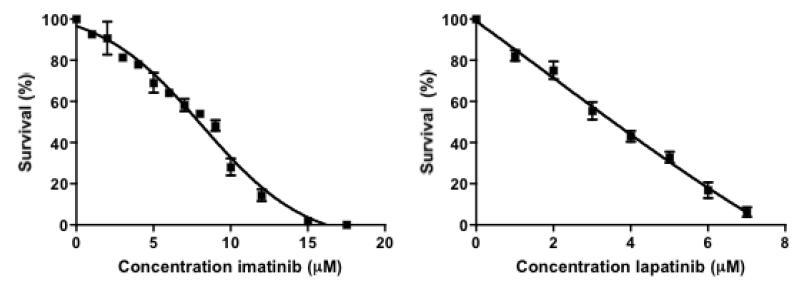
Before combining drug and radiation treatments, it was first necessary to determine the growth-inhibitory activity of the drugs in RT112 cells. Figure 2 shows cell viabilities by clonogenic assay following 14 days treatment with varying concentrations of imatinib or lapatinib. The IC50 value for imatinib in RT112 cells was 8 μM, and for lapatinib 3.5 μM.
Figure 2.
Clonogenic assays following 14 days of treatment with varying concentrations of imatinib (Stratatech) or lapatinib. Bars, mean of at least three independent experiments plus SD.
Inhibition of Ku80 or RAD51 expression in RT112 cells by siRNA
To generate NHEJ-deficient and HR-deficient RT112 cells, we used a small-interfering RNA (siRNA)-based strategy to reduce Ku80 or RAD51 expression in RT112 cells. We tested the effectiveness of two 21-mer siRNAs targeting different sites within the exons or 3′UTR regions of Ku80 and RAD51 (Supplementary Table 1), using pSilencer2.1-U6 neomycin, which drives expression of a short hairpin RNA from the human U6 promoter. The hairpin RNA is then processed into an siRNA, which induces RNAi of the target gene. RT112 cells were stably transfected with each of the vectors and clones picked and tested using western blotting and IR clonogenic assays. An individual clone for each of Ku80 (C13, coding region) and RAD51 (795J) was then selected for drug-IR clonogenic assays. Densitometric analysis of representative western blots revealed a 30% reduction in Ku80 protein expression in C13 cells and 75% reduction in RAD51 expression in 795J cells compared to parental RT112 cells and pSilencer neovector negative control cells (Supplementary Figure 1).
Ku80 or RAD51 interference causes a decreased viability of RT112 after X-ray exposure
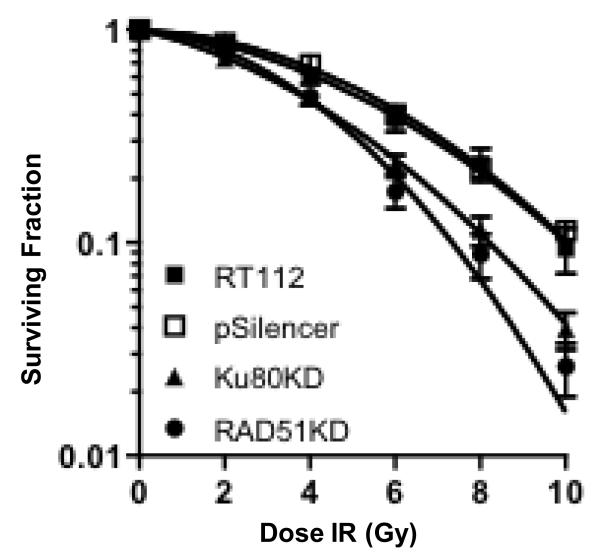
We examined the radiosensitising effect of siRNA-mediated down-regulation of Ku80 and RAD51 expression in RT112 cells using clonogenic assays. We found a statistically significant increase in radiosensitivity for the Ku80KD (p=0.01) and RAD51KD (p=0.02) cells as compared to parental RT112 and RT112-pSilencer vector control cells (Figure 3).
Figure 3.
Clonogenic assays following IR in RT112 parent cells (RT112), and clones selected under G418 from RT112 cells transfected with circular negative control pSilencer neovector (pSilencer), RT112 cells transfected with Ku80-construct (Ku80KD), and RT112 cells transfected with RAD51-construct (RAD51KD). Bars, mean of at least three independent experiments plus SD. Statistical significance was determined by two-tailed, unpaired Student’s t-test (p=0.01).
Imatinib significantly radiosensitised Ku80KD RT112 cells
We then tested our hypothesis that imatinib radiosensitises NHEJ-deficient Ku80 KD cells, while having less effect on the parental RT112 cells with an intact NHEJ pathway, due to targeting the IR-induced increase in RAD51. We performed clonogenic assays on RT112, Ku80KD and RAD51KD cells using relevant drug-IR combinations. Cells were incubated for 24 hours with or without drug, before irradiation and plating followed by 14 days incubation, prior to staining and counting. Drug doses were chosen at approximately IC40 (high) and at IC10 non-toxic (low) levels.
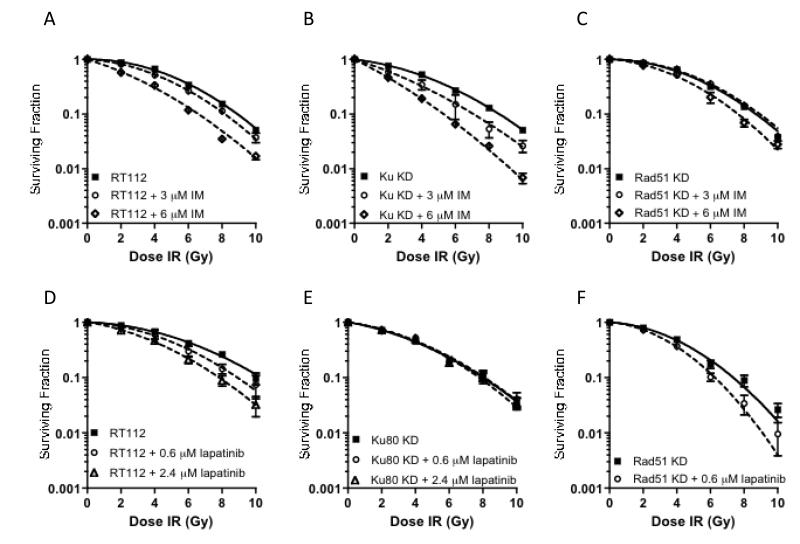
Radiation survival curves were generated for each cell line after normalization for the level of cell killing induced by drug alone. As shown in Figure 4A-C, 3 μM imatinib had a significant radiosensitising effect on Ku80KD cells (SER 1.27, p=0.03), but no significant effect in parental RT112 cells (SER= 1.07, p=0.051) or RAD51 KD cells. At 6 μM concentration, equivalent to approximately IC40, imatinib produced an SER of 1.69 in Ku80 KD cells (p=0.03), but only an SER of 1.34 in the RT112 parental cell line (p=0.03; p= 0.046 compared to Ku80KD 6 μM imatinib survival curve) and 1.11 in the RAD51 KD cells (p=0.03; p=0.04 compared to Ku80KD 6 μM imatinib survival curve).
Figure 4.
Clonogenic survival of cells under IR with or without drug exposure. The radiation survival curves were generated for each cell line after normalization to cell killing by drug alone. Bars, mean survival of at least three independent experiments plus SD. a) RT112, IR/imatinib (3μM, p=0.051; 6 μM, p=0.03); b) Ku80KD, IR/imatinib (3μM, p=0.03; 6 μM, p=0.03); c) RAD51KD, IR/imatinib (3 μM, p=0.08; 6 μM, p=0.03). d) RT112, IR/lapatinib (0.6 μM, p=0.02; 2.4 μM, p=0.01); e) Ku80KD, IR/lapatinib (0.6 μM, p=0.60; 2.4 μM, p=0.86); f) RAD51KD, IR/lapatinib (0.6 μM, p=0.02).
In contrast, lapatinib radiosensitised RT112 cells in a dose-dependent manner (Figure 4D) (SER = 1.13 and 1.30 for 0.6μM, p=0.02, and 2.4 μM, p=0.01), but had no radiosensitising effect on Ku80KD cells at either low (0.6 μM) and high (2.4 μM) concentrations (Figure 4E). However, in contrast to imatinib, lapatinib had a dramatic radiosensitising effect in RAD51KD cells, even at low dose (SER = 1.27, p=0.02), with the high dose resulting in inhibition of RAD51KD cell colony formation, even without IR (Figure 4F).
Cell cycle effects of imatinib and lapatinib
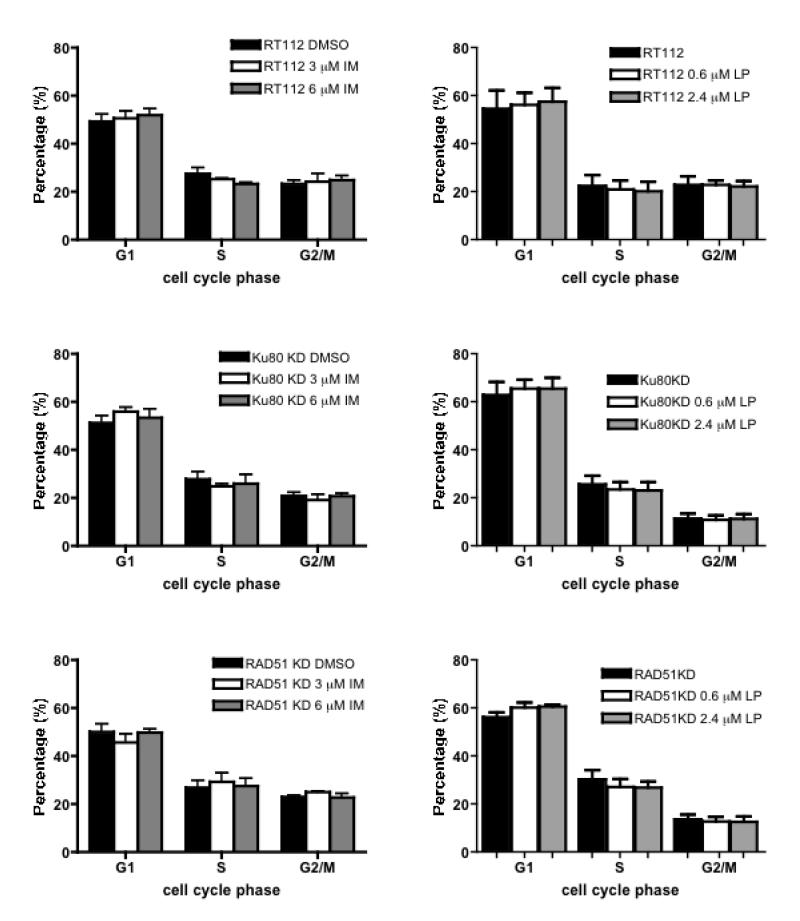
To determine whether the drug-mediated enhancement of radiosensitivity was due to cellular synchronisation into a radiosensitive phase of the cell cycle, propidium iodide staining and flow cytometry were used to determine the cell cycle phase distribution of samples used in each independent clonogenic assay. Neither imatinib nor lapatinib caused obvious cell cycle arrest in treated cells of each cell type, except for a small increase in G1 fraction in RAD51 KD cells treated by lapatinib at both drug concentrations (Figure 5, p=0.02 low dose, p=0.04 high dose).
Figure 5.
Cell cycle phase distribution in cells with or without drug exposure for 24 hours. Column, mean of at least three independent experiments; error bars represent SD. a) RT112, Ku80KD and RAD51KD cells treated with or without imatinib (Stratatech) for 24 hours; b) RT112, Ku80KD and RAD51KD cells treated with or without lapatinib for 24 hours.
Effects of imatinib and lapatinib on RAD51 and EGFR
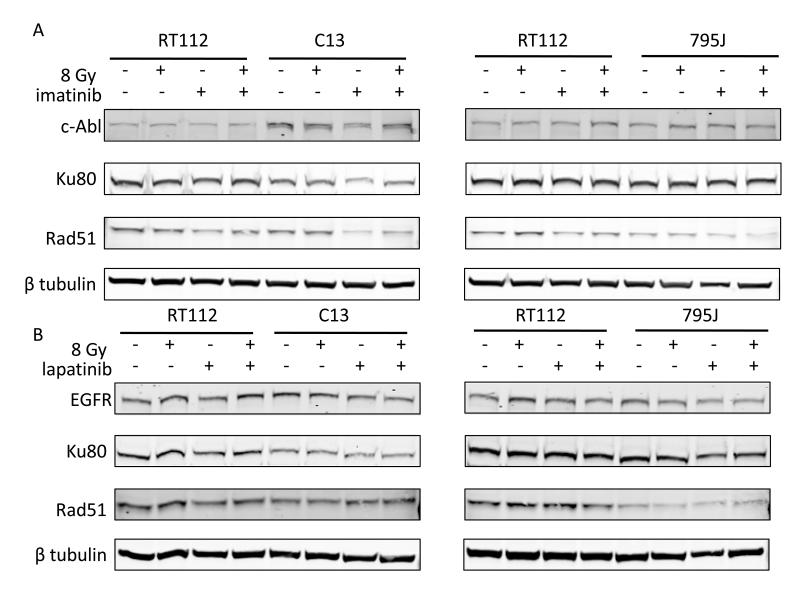
We also measured expression of ABL, EGFR, HER2 and RAD51 using western blotting (Figure 6). Cells were incubated with or without drug for 24 hours and then irradiated to 8 Gy or left untreated. Forty-eight hours later, cells were lysed for western blotting. RT112, Ku80 KD (C13) and RAD51KD (759J) cells all had detectable baseline levels of ABL, EGFR and RAD51, although RAD51 levels were markedly reduced in the RAD51KD cells. HER2 was not detectable in any of the cell lines. We found no major increase in RAD51 levels following 8 Gy ionising radiation. Imatinib treatment was associated with reduced RAD51 levels in RT112 and Ku80KD cells both alone and following 8 Gy IR, but no effect on ABL levels; lapatinib had no effect on RAD51 levels.
Figure 6.
Western blots of ABL, EGFR and RAD51 after a) 12μM (Novartis, IC50 11.6 μM) imatinib treatment and b) 3.5 μM lapatinib treatment. Cells were incubated with or without drug for 24 hours and then irradiated to 8 Gy or left untreated. Cells were lysed 48 hours later. No HER2 was detectable. Full-length blots in Supplementary Figure 2.
Discussion
Radiotherapy is a valid option in the radical treatment of muscle-invasive bladder cancer, with similar survival rates to cystectomy in our recent study (2). Cytotoxic chemotherapy is often combined with radiotherapy to improve survival rates (3-5), but this can be at the expense of late side effects. Moreover, many bladder cancer patients who elect to have radiotherapy are elderly, with poor renal function, and therefore cannot tolerate cisplatin-based chemotherapy regimens. There is therefore an urgent need to identify less toxic agents suitable for these patients.
Our IHC data show that ABL is expressed in over 80% of muscle-invasive bladder tumours, suggesting that imatinib might be useful in such patients. EGFR and HER2 staining was positive in over 95% of bladder tumours. While the Kaplan-Meier survival curves (Figure 1B) demonstrated no prognostic significance, our sample numbers were relatively small, with only 73% power to detect a hazard ratio of 0.4, with 35 cause-specific survival events at p=0.05. Also, we used a scoring system developed for another tumour type and have not validated our findings in an independent patient cohort, so results should be treated with caution. As we did not have access to a patient cohort treated with a TKI and radiotherapy, we cannot comment on the potential predictive value of such markers.
We established that both imatinib and lapatinib are cytotoxic in the RT112 bladder cancer cell-line, which has modest ABL and EGFR expression levels (Figure 2). Choudhury et al (17) found an IC50 value of 20 μM for imatinib in RT112 cells, while ours was 8 μM; McHugh et al found the IC50 for lapatinib in RT112 cells was 1.1 μM MTT assay (32), ours being 3.5 μM on clonogenic assay, perhaps reflecting differences in experimental systems. We then successfully knocked-down both Ku80 and RAD51, using shRNA technology, in TP53 wild-type RT112 cell lines (Figure 3). Negroni et al (24) used the same method to knockdown Ku80 in RT112 cells and demonstrated radiosensitivity and reduced Ku-DNA binding. We previously observed the latter in muscle-invasive bladder tumour extracts. We then the radiosensitising effects of both imatinib and lapatinibin RT112, Ku80 KD and RAD51 KD cells (Figure 4). Imatinib was an effective radiosensitiser in Ku80KD cells but less effective in parental RT112 cells. Although it did not affect ABL expression levels (Figure 6), in both parental RT112 cells and Ku80KD cells, imatinib treatment was associated with reduced RAD51 expression levels both with and without ionising radiation. As imatinib had no radiosensitising effect at low dose and only limited effects at high dose in RAD51KD cells, this appears to support our hypothesis that imatinib works through the HR pathway, but not the NHEJ pathway. In contrast, lapatinib had no radiosensitising effect on Ku80KD cells but a marked effect in RAD51KD cells and also sensitised parental RT112 cells, consistent with lapatinib acting via NHEJ rather than HR. Recently, Myllynen et al (33) showed that both HR and NHEJ are involved in regulation of DSB repair by EGFR, using an I-SceI-based reporter system, with reduction of HR by the TKI erlotinib at 0.5 μM, and less so by the monoclonal antibody cetuximab at 30 nM concentration. However, our experiments suggest that lapatinib does not act on HR at 0.6 μM or 2.4 μM. Other than a small effect on G1 arrest for lapatinib at both concentrations in RAD51KD cells, neither drug affected cell cycle progression at 24 hours. Lapatinib has been found to induce G1 arrest in bladder cancer (RT112, 45% to 65% G1 cells after 72 hours of 1.1 μM lapatinib) (34) and gastric cancer cell lines (58% G1 to 70% after 24 hours of 1 μM lapatinib) (35). Treatment with imatinib (5-6 μM) for 48-72 hours caused a slight increase in the number of cells in G1 phase in head-and-neck squamous carcinoma cell lines (47% G1 to 58%) (36), ovarian cancer cell lines (80% G1 to 94%) (37), but not in small cell lung carcinoma cell lines (38).
Our data support the role of DNA repair in both imatinib and lapatinib’s radiosensitising effects. They suggest that imatinib may be a useful radiosensitiser in muscle-invasive bladder cancer, as our tumours so far have all shown the MMEJ phenotype, with defective Ku-DNA binding and defective TP53 function, while superficial tumours had intact NHEJ (21). We are currently testing further tumour sample extracts. If some muscle-invasive tumours show intact NHEJ, imatinib would not be appropriate as a radiosensitiser in these patients, and there would be a need to develop a pre-radiotherapy predictive end-joining assay for use in muscle-invasive bladder cancer patients. An advantage of imatinib as a radiosensitiser in this context, unlike lapatinib, is that we would expect normal tissue sparing and an increase in therapeutic ratio.
Precis: The tyrosine kinase inhibitor Gleevec may have additional uses to radiosensitize tumors that are defective in non-homologous end joining (NHEJ), with the potential to greatly expand clinical applications of this agent.
Supplementary Material
Acknowledgements
We thank Ms Filomena Estevez for her expert help with immunohistochemistry staining and to Dr Sameer Chilka for outlining the muscle-invasive tumour areas, also Dr Paul Manley of Novartis and Dr Daniel Ridley of Glaxo Smith Kline for critical reading of the manuscript.
Grant support: Yorkshire Cancer Research project grant L336 and Cancer Research UK.
Financial support: BQ was funded by Yorkshire Cancer Research Project Grant L336
BG was funded by the Slovene Human Resources Scholarship Fund
MTWT was funded by Yorkshire Cancer Research Project Grant L350
MAK’s work was funded by Cancer Research UK Programme grant C6228/A7675
RGB was funded by grants from the Canadian Foundation for Innovation and Canadian
Cancer Society and a Canadian Cancer Research Society Career Scientist Award
MK and AEK were funded by Cancer Research UK Programme Grant C5255/A12678
Footnotes
Conflicts of interest: None to declare
References
- 1.Cancer Research UK [Internet] Bladder Cancer Statistics; London: [updated 2012 Sept 7; cited 2013 Jan 3]. c2012. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/ [Google Scholar]
- 2.Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–63. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29:733–8. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- 4.Heney NM, Kaufman DS, Shipley WU. Surgery: selective bladder-preserving therapy for muscle-invasive cancer. Nat Rev Clin Oncol. 2009;6:193–4. doi: 10.1038/nrclinonc.2009.21. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol. 2010;28:4912–8. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- 6.Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, et al. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18:6977–86. doi: 10.1016/j.bmc.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Yerushalmi R, Nordenberg J, Beery E, Uziel O, Lahav M, Luria D, et al. Combined antiproliferative activity of imatinib mesylate (STI-571) with radiation or cisplatin in vitro. Experimental Oncology. 2007;29:126–31. [PubMed] [Google Scholar]
- 8.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–9. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Clinical trials.gov [Internet] A phase 0 of Neoadjuvant Lapatinib in Infiltrative Bladder Carcinoma Before Cystectomu (LAPAINBLAD); Bethesda: [updated 2012 Dec 30; accessed 2013 Jan 3]. c2010-12. Available from http://clinicaltrials.gov/ [Google Scholar]
- 10.McHugh LA, Griffiths TR, Kriajevska M, Symonds RP, Mellon JK. Tyrosine kinase inhibitors of the epidermal growth factor receptor as adjuncts to systemic chemotherapy for muscle-invasive bladder cancer. Urology. 2004;63:619–24. doi: 10.1016/j.urology.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, Koch MO, et al. c-kit Expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implications. Mod Pathol. 2005;18:320–3. doi: 10.1038/modpathol.3800318. [DOI] [PubMed] [Google Scholar]
- 12.Zigeuner R, Ratschek M, Langner C. Kit (CD117) immunoreactivity is rare in renal cell and upper urinary tract transitional cell carcinomas. BJU Int. 2005;95:315–8. doi: 10.1111/j.1464-410X.2005.05290.x. [DOI] [PubMed] [Google Scholar]
- 13.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nature Genetics. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 14.O’Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nature Reviews Genetics. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 15.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair. 2007;6:923–35. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Molecular and Cellular Biology. 2003;23:5706–15. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury A, Zhao H, Jalali F, Rashid SA, Ran J, Supiot S, et al. Targeting homologous recombination using imatinib results in enhanced tumor cell chemosensitivity and radiosensitivity. Molecular Cancer Therapeutics. 2009;8:203–13. doi: 10.1158/1535-7163.MCT-08-0959. [DOI] [PubMed] [Google Scholar]
- 18.Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, Utsumi H, et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. Embo Journal. 1998;17:5497–508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyagawa K. Clinical relevance of the homologous recombination machinery in cancer therapy. Cancer Science. 2008;99:187–94. doi: 10.1111/j.1349-7006.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma JL, Kim EM, Haber JE, Lee SE. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Molecular and Cellular Biology. 2003;23:8820–8. doi: 10.1128/MCB.23.23.8820-8828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentley J, L’Hote C, Platt F, Hurst CD, Lowery J, Taylor C, et al. Papillary and muscle-invasive bladder tumors with distinct genomic stability profiles have different DNA repair fidelity and KU DNA-binding activities. Genes Chromosomes Cancer. 2009;48:310–21. doi: 10.1002/gcc.20641. [DOI] [PubMed] [Google Scholar]
- 22.Pucci S, Mazzarelli P, Rabitti C, Giai M, Gallucci M, Flammia G, et al. Tumor specific modulation of KU70/80 DNA binding activity in breast and bladder human tumor biopsies. Oncogene. 2001;20:739–47. doi: 10.1038/sj.onc.1204148. [DOI] [PubMed] [Google Scholar]
- 23.Bentley J, Diggle CP, Harnden P, Knowles MA, Kiltie AE. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Research. 2004;32:5249–59. doi: 10.1093/nar/gkh842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negroni A, Stronati L, Grollino MG, Barattini P, Gumiero D, Danesi DT. Radioresistance in a tumour cell line correlates with radiation inducible Ku 70/80 end-binding activity. Int J Radiat Biol. 2008;84:265–76. doi: 10.1080/09553000801953318. [DOI] [PubMed] [Google Scholar]
- 25.Russell JS, Brady K, Burgan WE, Cerra MA, Oswald KA, Camphausen K, et al. Gleevec-mediated inhibition of Rad51 expression and enhancement of tumor cell radiosensitivity. Cancer Res. 2003;63:7377–83. [PubMed] [Google Scholar]
- 26.Oertel S, Krempien R, Lindel K, Zabel A, Milker-Zabel S, Bischof M, et al. Human glioblastoma and carcinoma xenograft tumors treated by combined radiation and imatinib (Gleevec (R)) Strahlentherapie Und Onkologie. 2006;182:400–7. doi: 10.1007/s00066-006-1445-8. [DOI] [PubMed] [Google Scholar]
- 27.Baumann M, Krause M, Dikomey E, Dittmann K, Dorr W, Kasten-Pisula U, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol. 2007;83:238–48. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Sambade MJ, Camp JT, Kimple RJ, Sartor CI, Shields JM. Mechanism of lapatinib-mediated radiosensitization of breast cancer cells is primarily by inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade and radiosensitization of lapatinib-resistant cells restored by direct inhibition of MEK. Radiother Oncol. 2009;93:639–44. doi: 10.1016/j.radonc.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoms J, Bristow RG. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol. 2010;20:217–22. doi: 10.1016/j.semradonc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Choudhury A, Nelson LD, Teo MTW, Chilka S, Bhattarai S, Johnston CF, et al. MRE11 Expression Is Predictive of Cause-Specific Survival following Radical Radiotherapy for Muscle-Invasive Bladder Cancer. Cancer Research. 2010;70:7017–26. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dako A/S [Internet] HercepTest Interpretation Manual – Gastric Cancer; Glostrup: [updated 2010 May 5; cited 2013 Jan 3]. c2002. Available from: http://www.dako.com/uk/29018_05may10_herceptest_interpretation_manual_gastric_cancer. pdf. [Google Scholar]
- 32.McHugh LA, Kriajevska M, Mellon JK, Griffiths TR. Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens--evidence of schedule-dependent synergy. Urology. 2007;69:390–4. doi: 10.1016/j.urology.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Myllynen L, Rieckmann T, Dahm-Daphi J, Kasten-Pisula U, Petersen C, Dikomey E, et al. In tumor cells regulation of DNA double strand break repair through EGF receptor involves both NHEJ and HR and is independent of p53 and K-Ras status. Radiother Oncol. 2011;101:147–51. doi: 10.1016/j.radonc.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 34.McHugh LA, Sayan AE, Mejlvang J, Griffiths TR, Sun Y, Manson MM, et al. Lapatinib, a dual inhibitor of ErbB-1/-2 receptors, enhances effects of combination chemotherapy in bladder cancer cells. Int J Oncol. 2009;34:1155–63. doi: 10.3892/ijo_00000244. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, Kim HP, Im SA, Kang S, Hur HS, Yoon YK, et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 2008;272:296–306. doi: 10.1016/j.canlet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Wang-Rodriguez J, Lopez JP, Altuna X, Chu TS, Weisman RA, Ongkeko WM. STI-571 (Gleevec) potentiates the effect of cisplatin in inhibiting the proliferation of head and neck squamous cell carcinoma in vitro. Laryngoscope. 2006;116:1409–16. doi: 10.1097/01.mlg.0000225895.40732.52. [DOI] [PubMed] [Google Scholar]
- 37.Matei D, Chang DD, Jeng MH. Imatinib mesylate (Gleevec) inhibits ovarian cancer cell growth through a mechanism dependent on platelet-derived growth factor receptor alpha and Akt inactivation. Clin Cancer Res. 2004;10:681–90. doi: 10.1158/1078-0432.ccr-0754-03. [DOI] [PubMed] [Google Scholar]
- 38.Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, et al. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene. 2000;19:3521–8. doi: 10.1038/sj.onc.1203698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.