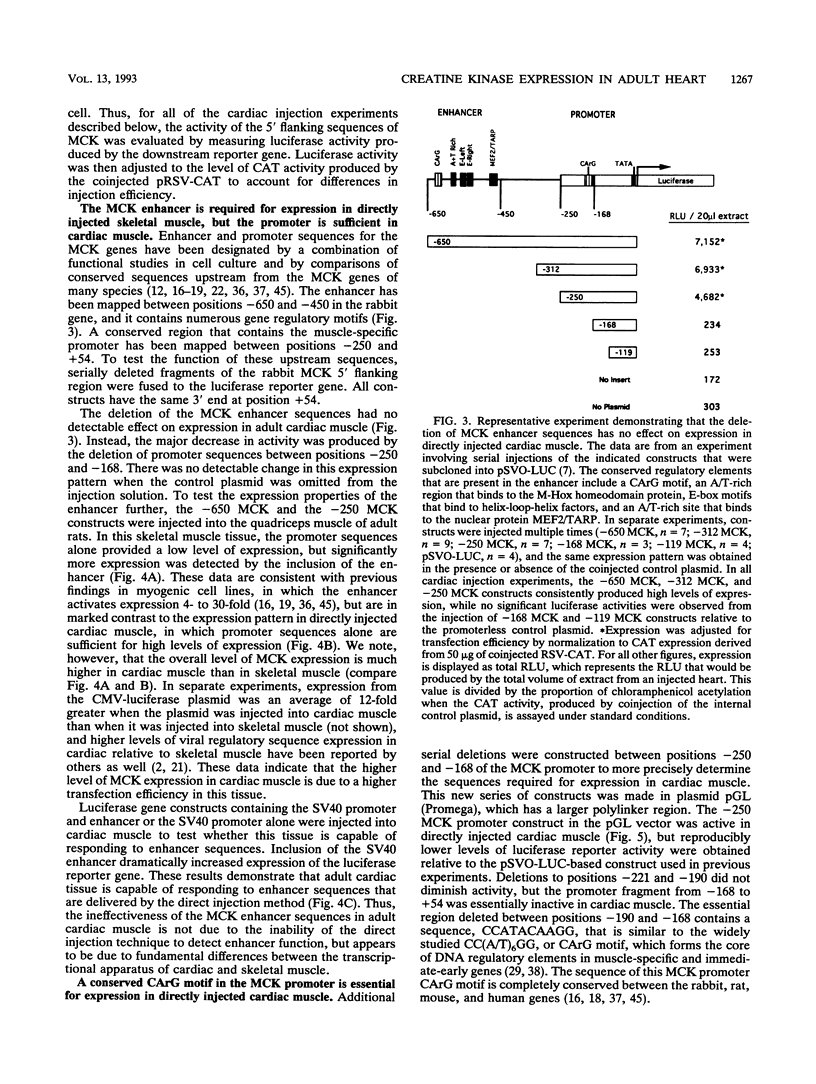
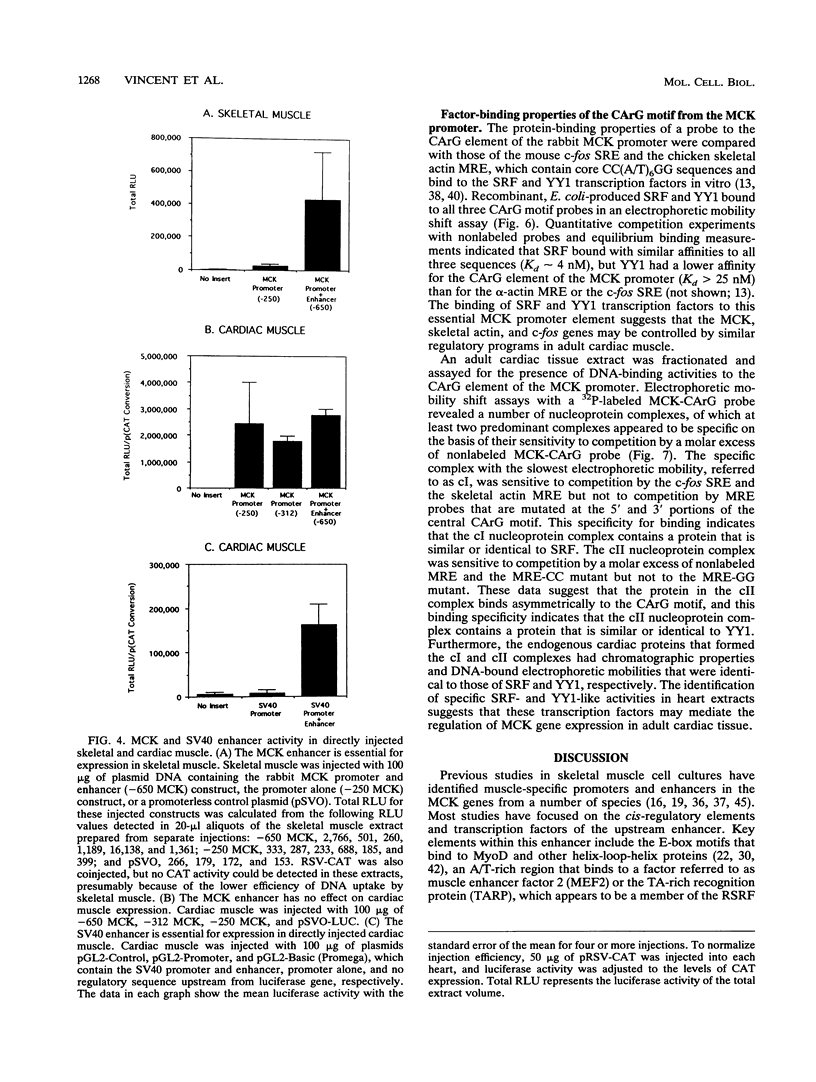
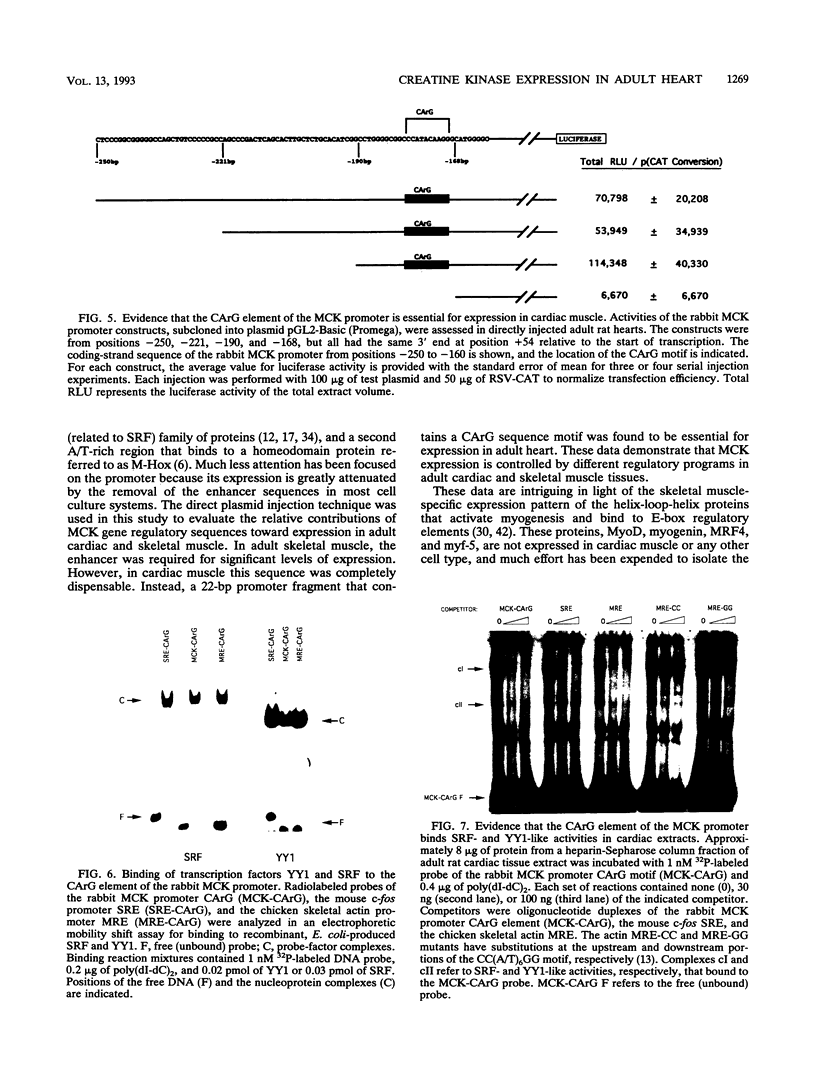
Abstract
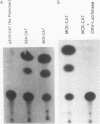
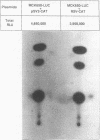
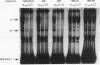
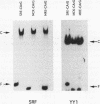
Regulatory sequences of the M isozyme of the creatine kinase (MCK) gene have been extensively mapped in skeletal muscle, but little is known about the sequences that control cardiac-specific expression. The promoter and enhancer sequences required for MCK gene expression were assayed by the direct injection of plasmid DNA constructs into adult rat cardiac and skeletal muscle. A 700-nucleotide fragment containing the enhancer and promoter of the rabbit MCK gene activated the expression of a downstream reporter gene in both muscle tissues. Deletion of the enhancer significantly decreased expression in skeletal muscle but had no detectable effect on expression in cardiac muscle. Further deletions revealed a CArG sequence motif at position -179 within the promoter that was essential for cardiac-specific expression. The CArG element of the MCK promoter bound to the recombinant serum response factor and YY1, transcription factors which control expression from structurally similar elements in the skeletal actin and c-fos promoters. MCK-CArG-binding activities that were similar or identical to serum response factor and YY1 were also detected in extracts from adult cardiac muscle. These data suggest that the MCK gene is controlled by different regulatory programs in adult cardiac and skeletal muscle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Dickson G., Love D. R., Jani A., Walsh F. S., Gurusinghe A., Wolff J. A., Davies K. E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991 Aug 29;352(6338):815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- Acsadi G., Jiao S. S., Jani A., Duke D., Williams P., Chong W., Wolff J. A. Direct gene transfer and expression into rat heart in vivo. New Biol. 1991 Jan;3(1):71–81. [PubMed] [Google Scholar]
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R., Tate J. E., Habener J. F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989 Nov-Dec;7(10):1116–1122. [PubMed] [Google Scholar]
- Buttrick P. M., Kass A., Kitsis R. N., Kaplan M. L., Leinwand L. A. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992 Jan;70(1):193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Eppenberger-Eberhardt M., Flamme I., Kurer V., Eppenberger H. M. Reexpression of alpha-smooth muscle actin isoform in cultured adult rat cardiomyocytes. Dev Biol. 1990 Jun;139(2):269–278. doi: 10.1016/0012-1606(90)90296-u. [DOI] [PubMed] [Google Scholar]
- Eppenberger M. E., Hauser I., Baechi T., Schaub M. C., Brunner U. T., Dechesne C. A., Eppenberger H. M. Immunocytochemical analysis of the regeneration of myofibrils in long-term cultures of adult cardiomyocytes of the rat. Dev Biol. 1988 Nov;130(1):1–15. doi: 10.1016/0012-1606(88)90408-3. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., LePage D., Pons G., Mader S. L., Park K., Atchison M. L., Walsh K. Functional antagonism between YY1 and the serum response factor. Mol Cell Biol. 1992 Sep;12(9):4209–4214. doi: 10.1128/mcb.12.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. A., Spencer M., Sen A., Kumar C., Siddiqui M. A., Chien K. R. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem. 1989 Oct 25;264(30):18142–18148. [PubMed] [Google Scholar]
- Horlick R. A., Benfield P. A. The upstream muscle-specific enhancer of the rat muscle creatine kinase gene is composed of multiple elements. Mol Cell Biol. 1989 Jun;9(6):2396–2413. doi: 10.1128/mcb.9.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlick R. A., Hobson G. M., Patterson J. H., Mitchell M. T., Benfield P. A. Brain and muscle creatine kinase genes contain common TA-rich recognition protein-binding regulatory elements. Mol Cell Biol. 1990 Sep;10(9):4826–4836. doi: 10.1128/mcb.10.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsis R. N., Buttrick P. M., McNally E. M., Kaplan M. L., Leinwand L. A. Hormonal modulation of a gene injected into rat heart in vivo. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4138–4142. doi: 10.1073/pnas.88.10.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lee K. J., Ross R. S., Rockman H. A., Harris A. N., O'Brien T. X., van Bilsen M., Shubeita H. E., Kandolf R., Brem G., Price J. Myosin light chain-2 luciferase transgenic mice reveal distinct regulatory programs for cardiac and skeletal muscle-specific expression of a single contractile protein gene. J Biol Chem. 1992 Aug 5;267(22):15875–15885. [PubMed] [Google Scholar]
- Lim C. S., Chapman G. D., Gammon R. S., Muhlestein J. B., Bauman R. P., Stack R. S., Swain J. L. Direct in vivo gene transfer into the coronary and peripheral vasculatures of the intact dog. Circulation. 1991 Jun;83(6):2007–2011. doi: 10.1161/01.cir.83.6.2007. [DOI] [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P., Simpson P. C. Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. J Clin Invest. 1989 Mar;83(3):1078–1082. doi: 10.1172/JCI113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Antin P. B., Cooper T. A., Ordahl C. P. Analysis of the upstream regions governing expression of the chicken cardiac troponin T gene in embryonic cardiac and skeletal muscle cells. J Cell Biol. 1988 Aug;107(2):573–585. doi: 10.1083/jcb.107.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer Y., Czosnek H., Zeelon P. E., Yaffe D., Nudel U. Expression of the genes coding for the skeletal muscle and cardiac actions in the heart. Nucleic Acids Res. 1984 Jan 25;12(2):1087–1100. doi: 10.1093/nar/12.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Parmacek M. S., Leiden J. M. Structure and expression of the murine slow/cardiac troponin C gene. J Biol Chem. 1989 Aug 5;264(22):13217–13225. [PubMed] [Google Scholar]
- Parmacek M. S., Vora A. J., Shen T., Barr E., Jung F., Leiden J. M. Identification and characterization of a cardiac-specific transcriptional regulatory element in the slow/cardiac troponin C gene. Mol Cell Biol. 1992 May;12(5):1967–1976. doi: 10.1128/mcb.12.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Santoro I. M., Walsh K. Natural and synthetic DNA elements with the CArG motif differ in expression and protein-binding properties. Mol Cell Biol. 1991 Dec;11(12):6296–6305. doi: 10.1128/mcb.11.12.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry W. M., Vizard D., Weil T., Olson E. N. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988 Jul;8(7):2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask R. V., Strauss A. W., Billadello J. J. Developmental regulation and tissue-specific expression of the human muscle creatine kinase gene. J Biol Chem. 1988 Nov 15;263(32):17142–17149. [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [PubMed] [Google Scholar]
- Vandekerckhove J., Bugaisky G., Buckingham M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem. 1986 Feb 5;261(4):1838–1843. [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Schimmel P. DNA-binding site for two skeletal actin promoter factors is important for expression in muscle cells. Mol Cell Biol. 1988 Apr;8(4):1800–1802. doi: 10.1128/mcb.8.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Dwarki V. J., Verma I., Davis R., Hollenberg S., Snider L., Lassar A., Tapscott S. J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991 Aug;5(8):1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Williams P., Acsadi G., Jiao S., Jani A., Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. Biotechniques. 1991 Oct;11(4):474–485. [PubMed] [Google Scholar]
- Yi T. M., Walsh K., Schimmel P. Rabbit muscle creatine kinase: genomic cloning, sequencing, and analysis of upstream sequences important for expression in myocytes. Nucleic Acids Res. 1991 Jun 11;19(11):3027–3033. doi: 10.1093/nar/19.11.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Garcia A. V., Ross R. S., Evans S. M., Chien K. R. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991 Apr;11(4):2273–2281. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]