Abstract
Asymmetric catalytic aza-Morita-Baylis-Hillman (aza-MBH) reaction of isatin-derived ketimines with MVK has been established by using chiral amino and phosphino catalysts. The reaction resulted in biomedically important 3-substituted 3-amino-2-oxindoles in good yields (>80% for most cases) and excellent enantioselectivity (90–99%ee). Twenty-eight cases assembled with chiral quaternary stereogenic centers have been examined under convenient systems.
3-Substituted-3-amino-2-oxindoles are core structures in a variety of natural products and biologically active compounds,1 such as the potent gastrin/CCK-B receptor antagonist AG-041R,2 the vasopressin VIb receptor antagonist SSR-14941533 and the antimalarial drug candidate NITD609.4 The development of efficient synthetic protocols leading to these products, particularly, those assembled with quaternary chiral carbon centers has been desired and extremely challenging. Recently, a significant advance has been achieved in the development of synthetic methodologies for these targets; These methods include organocatalytic and metal-catalyzed asymmetric α-amination,5 chiral auxiliary-controlled diastereoselective synthesis of chiral 3-substituted 3-aminooxindoles6 and enantioselective additions of isatin-derived ketimines.7 Very recently, Wang and co-workers reported the asymmetric addition of 1,3-dicarbonyl compounds onto N-alkoxycarbonyl ketimines and asymmetric aza-Friedel-Craft reaction of indoles and pyrroles with N-Boc ketimines in the presence of chiral phosphoric acid catalysts;8 the latter led to the formation of 3-aminooxindoles in good yields with excellent enantioselectivities.9
In the meanwhile, the Morita-Baylis-Hillman (MBH) has become a powerful and atom-economic tool for constructing enantiomerically enriched α-hydroxycarbonyl or α-aminocarbonyl compounds. In the past one decade, we and others have extensively studied the Morita-Baylis-Hillman (MBH) reaction and its aza versions;10,11 among that is the MBH reaction of N-protected isatin with electron-deficient alkenes leading to the construction of 3-substituted 3-hydroxyoxindoles in good yields and excellent enantioselectivities.12 Obviously, the more challenging work is to achieve an asymmetric catalytic aza-Morita-Baylis-Hillman approach to chiral 3-aminooxindoles. Herein, we wish to report a highly enantioselective aza-MBH reaction of isatin-derived N-Boc ketimines with methyl vinyl ketone (MVK) for the efficient synthesis of chiral 3-aminooxindoles.
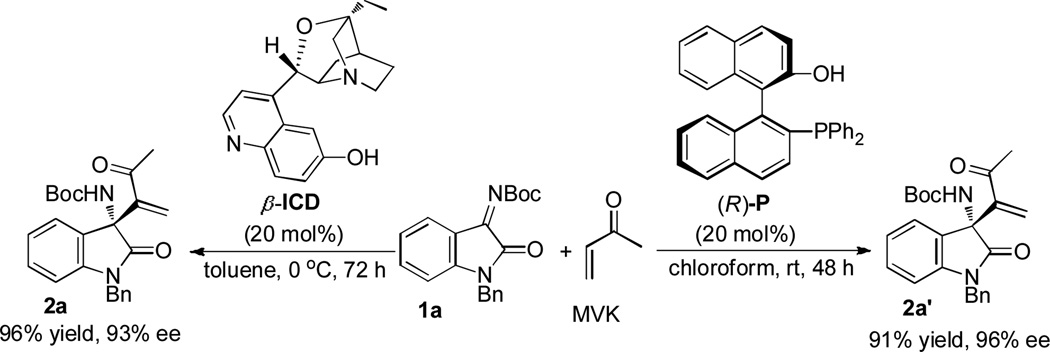
Inspired by the fact that chiral amino13,10a and phosphino14–15,10c,d derivatives are commonly utilized for asymmetric MBH and aza-MBH reactions, we came up with chiral β-isocupreidine (β-ICD) and (R)-2'-diphenylphosphanyl-[1,1']binaphthalenyl-2-ol ((R)-P) as the catalyst candidates for the reaction of N-Boc ketimine 1a and MVK for our initial investigation. After the catalytic conditions were screened extensively (for details, see Tables SI-1 and 2 in the Supporting Information), we found the best condition is as below: 0.10 mmol of 1a was subjected to reaction with 0.20 mmol of MVK in the presence of catalyst β-ICD (20 mol%) in toluene (2 mL). The reaction occurred to completion at 0 °C in 72 hours to give product 2a in 96% yield and 93%ee. We also found that when 20 mol% of (R)-P was employed as the catalyst, the reaction can occurred to completion in chloroform (2 mL) within a shorter period of 48 h to afford 2a' in 91% yield and 96%ee (Scheme 1, numbering 2a and 2a' is for outcomes' differentiation).
Scheme 1.
Chiral Amine or Phosphine-catalyzed Asymmetric aza-MBH Reaction.
With the optimized condition in hand, we then turned our attention to the examination of scope and limitations of this reaction using N-protected ketimines 1 with different substituents attached on their benzene rings. We found that whether electron-withdrawing or donating groups were attached on 5-, 6- or 7-position of benzene ring of N-protected ketimines 1, the reaction can smoothly undergo to completion to give product 2 in good to high yields (up to 98%) and excellent enantioselectivities (90% - 94%ee) (Table 1, entries 1-11 and entry 17). As for ketimine 1m with two substituents on its benzene ring, the corresponding aza-MBH product 2m was obtained in 97% yield and 90%ee (Table 1, entry 12). Unfortunately, when the electron-withdrawing or donating substituents were introduced at 4-position of the benzene ring, no desired products were formed (Table 1, entries 13 and 14), which is presumably due to the steric hindrance between the substituents on 4-position of the benzene ring and N-Boc group. N-Boc ketimines 1p and 1q derived from N-methyl and N-allyl protected isatins, respectively, were also found to be suitable for this reaction, affording aza-MBH adducts, 2p and 2q, in excellent yields and enantioselectivity (Table 1, entries 15 and 16). However, when the t-Bu group of Boc moiety was replaced by ethyl group, the yield and ee value decreased remarkably (Table 1, entry 18). In order to resolve the steric problem with 4-substituted substrates in which the t-Bu group of Boc moiety has been replaced by ethyl group, we also synthesized compound 1t and used it in this asymmetric aza-MBH reaction. However, no desired products were formed under the standard conditions (Table 1, entry 19). The absolute chemistry of this reaction is represented by the unambiguous determination of crystals of product 2e via X-ray diffraction to be "R" configuration (see Supporting Information).
Table 1.
β-ICD-catalyzed Asymmetric aza-MBH Reaction
 | ||||
|---|---|---|---|---|
| entrya | R1 | R2 | yield (%)b | ee (%)c |
| 1 | 1b, 5-CH3 | Bn | 2b, 86 | 91 |
| 2 | 1c, 5-F | Bn | 2c, 97 | 94 |
| 3 | 1d, 5-Cl | Bn | 2d, 98 | 93 |
| 4 | 1e, 5-Br | Bn | 2e, 97 | 94 |
| 5 | 1f, 6-CH3 | Bn | 2f, 93 | 92 |
| 6 | 1g, 6-Cl | Bn | 2g, 98 | 94 |
| 7 | 1h, 6-Br | Bn | 2h, 98 | 94 |
| 8 | 1i, 7-F | Bn | 2i, 97 | 92 |
| 9 | 1j, 7-Cl | Bn | 2j, 98 | 92 |
| 10 | 1k, 7-Br | Bn | 2k, 92 | 91 |
| 11 | 1l, 7-CF3 | Bn | 2l, 84 | 91 |
| 12 | 1m, 5-Cl, 7-CH3 | Bn | 2m, 97 | 90 |
| 13 | 1n, 4-CH3 | Bn | trace | ndd |
| 14 | 1o, 4,7-Cl2 | Bn | trace | ndd |
| 15 | 1p, H | Me | 2p, 85 | 90 |
| 16 | 1q, H | Allyl | 2q, 96 | 90 |
| 17 | 1r, 5-CH3O | Bn | 2r, 80 | 90 |
| 18e | 1s, H | Bn | 2s, 32 | 62 |
| 19e | 1t, 4,7-Cl2 | Bn | trace | ndd |
1 (0.1 mmol), MVK (0.2 mmol) and catalyst (0.02 mmol) were stirred in 2 mL of toluene within 72 h at 0 °C.
Isolated yield.
Determined by chiral HPLC.
Not determined.
t-Bu group of Boc moiety in 1a was replaced by ethyl group.
Having examined the β-ICD-catalyzed asymmetric aza-MBH reaction, we next turned our attention to the chiral phosphine-catalyzed reaction, the results are summarized in Table 2. As compared with the β-ICD-catalyzed asymmetric reaction, similar results were obtained, affording aza-MBH adducts 2' in up to 97% yield and 99%ee (Table 2, entries 1-19). The β-ICD catalyst resulted in the same absolute "R" configuration as that of (R)-P-catalyzed aza-MBH process.
Table 2.
(R)-P-catalyzed Asymmetric aza-MBH Reaction
 | ||||
|---|---|---|---|---|
| entrya | R1 | R2 | yield (%)b | ee (%)c |
| 1 | 1b, 5-CH3 | Bn | 2b', 93 | 95 |
| 2 | 1c, 5-F | Bn | 2c', 96 | 93 |
| 3 | 1d, 5-Cl | Bn | 2d', 91 | 99 |
| 4 | 1e, 5-Br | Bn | 2e', 97 | >99 |
| 5 | 1f, 6-CH3 | Bn | 2f', 88 | 99 |
| 6 | 1g, 6-Cl | Bn | 2g', 93 | 96 |
| 7 | 1h, 6-Br | Bn | 2h', 93 | 97 |
| 8 | 1i, 7-F | Bn | 2i', 87 | 95 |
| 9 | 1j, 7-Cl | Bn | 2j', 70 | 99 |
| 10 | 1k, 7-Br | Bn | 2k', 78 | 99 |
| 11 | 1l, 7-CF3 | Bn | 2l', 70 | 94 |
| 12 | 1m, 5-Cl, 7-CH3 | Bn | 2m', 86 | >99 |
| 13 | 1n, 4-CH3 | Bn | trace | ndd |
| 14 | 1o, 4,7-Cl2 | Bn | trace | ndd |
| 15 | 1p, H | Me | 2p', 85 | 95 |
| 16 | 1q, H | Allyl | 2q', 90 | 97 |
| 17 | 1r, 5-CH3O | Bn | 2r, 83 | 90 |
| 18e | 1s, H | Bn | 2s, 86 | 70 |
| 19e | 1t, 4,7-Cl2 | Bn | trace | ndd |
1 (0.1 mmol), MVK (0.2 mmol) and catalyst (0.02 mmol) were stirred in 2 mL of chloroform within 48 h at rt.
Isolated yield.
Determined by chiral HPLC.
Not determined.
t-Bu group of Boc moiety in 1a was replaced by ethyl group.
Furthermore, we also examined the (S)-BINOL-derived catalyst, (S)-P,10d for this reaction. As expected, (S)-P catalyzed the present aza-MBH reaction similarly to (R)-P and resulted in (S)-2a with the opposite enantioselectivity (95%ee) and in 91% yield (Scheme 2).
Scheme 2.
(S)-P-catalyzed Asymmetric aza-MBH Reaction.
For the removal of N-Boc protection group of 3-amino-2-oxindoles, 2p' was used as an example by treating with HCl (conc.) in ethyl acetate. The cleavage product 3 was obtained in 80% yield without observation of a major side-product. After treated with acetic anhydride, N-acyl 3-aminooxindole 4 was generated in 70% yield (Scheme 3). The free amino product 3 would be able to be converted into many others building blocks in future.
Scheme 3.
Representative aza-MBH Product Transformation.
The reaction mechanism for MBH reaction has been extensively investigated by several groups.16 We have studied chiral Lewis base (R)-P-catalyzed asymmetric aza-MBH reaction of N-sulfonated imines with activated olefins.10 The key enolate intermediate, which was stabilized by intramolecular hydrogen bonding, has been observed by 31P and 1H NMR spectroscopy.10d In order to identify the correlation of ee values of the product 2 with those of catalyst (R)-P during the present aza-MBH process, a series of control experiments were performed by employing 1q as substrate and (R)-P with different ee values as catalysts under standard conditions (for details, see Table SI-3 in the Supporting Information). It was confirmed that there is no non-linear effect between the ee value of (R)-P and those of 2q', indicating that the exclusive reaction transition state involves only one molecule of chiral phosphine catalyst played the role in controlling asymmetric induction during the present asymmetric aza-MBH reaction.17
The corresponding aza-MBH reaction using N-phosphonyl and N-phosphinyl imines for GAP (Group-Assisted Purification) synthesis will be explored in due course.18
In conclusion, the asymmetric aza-MBH reaction of isatin-derived N-Boc ketimines with MVK in the presence of chiral amine and phosphine catalysts has been developed for the first time; this reaction provides an efficient enantioselective tool for the synthesis of 3-amino-2-oxindoles bearing quaternary stereogenic centers. The mechanistic study showed that there is no non-linear effect existing in this asymmetric catalytic process. Further efforts will be focused on applications of this reaction for organic and medicinal synthesis.
Supplementary Material
Acknowledgment
We thank the Shanghai Municipal Committee of Science and Technology (11JC1402600), NIH (R21DA031860-01), the Robert Welch Foundation (D-1361), the National Basic Research Program of China (973)-2010CB833302, the Fundamental Research Funds for the Central Universities, and the National Natural Science Foundation of China for financial support (21072206, 20472096, 20872162, 20672127, 21121062, 21102166 and 20732008).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures, characterization data of new compounds, and CCDC 900656.
References
- 1.For reviews, see: Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h. Galliford CV, Scheidt KA. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. Singh GS, Dhooghe M, De Kimpe N. Chem. Rev. 2007;107:2080. doi: 10.1021/cr0680033. Zhou F, Liu Y-L, Zhou J. Adv. Synth. Catal. 2010;352:1381. Shen K, Liu X, Lin L, Feng X-M. Chem. Sci. 2012;3:327. Klein JEMN, Taylor RJK. Eur. J. Org. Chem. 2011:6821. Singh GS, Desta ZY. Chem. Rev. 2012;112:6104. doi: 10.1021/cr300135y.
- 2.Ochi M, Kawasaki K, Kataoka H, Uchio Y. Biochem. Biophys. Res. Commun. 2001;283:1118. doi: 10.1006/bbrc.2001.4911. [DOI] [PubMed] [Google Scholar]
- 3.(a) Decaux G, Soupart A, Vassart G. Lancet. 2008;371:1624. doi: 10.1016/S0140-6736(08)60695-9. [DOI] [PubMed] [Google Scholar]; (b) Shimazaki T, Iijima M, Chaki S. Eur. J. Pharmacol. 2006;543:63. doi: 10.1016/j.ejphar.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. Science. 2010;329:1175. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Cheng L, Liu L, Wang D, Chen Y-J. Org. Lett. 2009;11:3874. doi: 10.1021/ol901405r. [DOI] [PubMed] [Google Scholar]; (b) Qian Z-Q, Zhou F, Du T-P, Wang B-L, Ding M, Zhao X-L, Zhou J. Chem. Commun. 2009:6753. doi: 10.1039/b915257a. [DOI] [PubMed] [Google Scholar]; (c) Bui T, Borregan M, Barbas CF., III Org. Chem. 2009;74:4537. doi: 10.1021/jo902039a. [DOI] [PubMed] [Google Scholar]; (d) Bui T, Hernández-Torres G, Milite C, Barbas CF., III Org. Lett. 2010;12:5696. doi: 10.1021/ol102493q. [DOI] [PubMed] [Google Scholar]; (e) Mouri S, Chen Z, Mitsunuma H, Furutachi M, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2010;132:1255. doi: 10.1021/ja908906n. [DOI] [PubMed] [Google Scholar]; (f) Yang Z, Wang Z, Bai S, Shen K, Chen D, Liu X, Lin L, Feng X-M. Chem. Eur. J. 2010;16:6632. doi: 10.1002/chem.201000126. [DOI] [PubMed] [Google Scholar]; (g) Shen K, Liu X, Wang G, Lin L, Feng X-M. Angew. Chem., Int. Ed. 2011;50:4684. doi: 10.1002/anie.201100758. [DOI] [PubMed] [Google Scholar]
- 6.(a) Emura T, Esaki T, Tachibana K, Shimizu M. J Org. Chem. 2006;71:8559. doi: 10.1021/jo061541v. [DOI] [PubMed] [Google Scholar]; (b) Lesma G, Landoni N, Pilati T, Sacchetti A, Silvani A. J Org. Chem. 2009;74:4537. doi: 10.1021/jo900623c. [DOI] [PubMed] [Google Scholar]; (c) Jung HH, Buesking AW, Ellman JA. Org. Lett. 2011;13:3912. doi: 10.1021/ol201438k. [DOI] [PubMed] [Google Scholar]; (d) Yan W-J, Wang D, Feng J-C, Li P, Wang R. J Org. Chem. 2012;77:3311. doi: 10.1021/jo300110a. [DOI] [PubMed] [Google Scholar]
- 7.(a) Liu Y-L, Zhou F, Cao J-J, Ji C-B, Ding M, Zhou J. Org. Biomol. Chem. 2010;8:3847. doi: 10.1039/c0ob00174k. [DOI] [PubMed] [Google Scholar]; (b) Guo Q-X, Liu Y-W, Li X-C, Zhong L-Z, Peng Y-G. J. Org. Chem. 2012;77:3589. doi: 10.1021/jo202585w. [DOI] [PubMed] [Google Scholar]; (c) Hara N, Nakamura S, Sano M, Tamura R, Funahashi Y, Shibata N. Chem. Eur. J. 2012;18:9276. doi: 10.1002/chem.201200367. [DOI] [PubMed] [Google Scholar]; (d) Lv H, Tiwari B, Mo J-M, Xing C, Chi YGRB. Org. Lett. 2012;14:5412. doi: 10.1021/ol302475g. [DOI] [PubMed] [Google Scholar]; (e) Zhang B, Feng P, Sun L-H, Cui Y-X, Ye S, Jiao N. Chem. Eur. J. 2012;18:9198. doi: 10.1002/chem.201201375. [DOI] [PubMed] [Google Scholar]
- 8.Yan W-J, Wang D, Feng J-C, Li P, Zhao D-P, Wang R. Org. Lett. 2012;14:2512. doi: 10.1021/ol3007953. [DOI] [PubMed] [Google Scholar]
- 9.Feng J-C, Yan W-J, Wang D, Li P, Sun Q-T, Wang R. Chem. Commun. 2012;48:8003. doi: 10.1039/c2cc33200k. [DOI] [PubMed] [Google Scholar]
- 10.(a) Shi M, Xu Y-M. Angew. Chem. Int. Ed. 2002;41:4507. doi: 10.1002/1521-3773(20021202)41:23<4507::AID-ANIE4507>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (b) Shi M, Xu Y-M, Shi Y-L. Chem. Eur. J. 2005;11:1794. doi: 10.1002/chem.200400872. [DOI] [PubMed] [Google Scholar]; (c) Shi M, Chen L-H. Chem. Commun. 2003:1310. doi: 10.1039/b301863f. [DOI] [PubMed] [Google Scholar]; (d) Shi M, Chen L-H, Li C-Q. J. Am. Chem. Soc. 2005;127:3790. doi: 10.1021/ja0447255. [DOI] [PubMed] [Google Scholar]; (e) Shi Y-L, Shi M. Adv. Synth. Catal. 2007;349:2129. [Google Scholar]; (f) Qi M-J, Ai T, Shi M, Li G. Tetrahedron. 2008;64:1181. [Google Scholar]; (g) Guan X-Y, Jiang Y-Q, Shi M. Eur. J. Org. Chem. 2008:2150. [Google Scholar]; (h) Guan X-Y, Wei Y, Shi M. Eur. J. Org. Chem. 2010:4098. [Google Scholar]; (i) Pei W, Wei H-X, Li G. Chem. Comm. 2002:2412. doi: 10.1039/b206736f. [DOI] [PubMed] [Google Scholar]; (j) Pei W, Wei H-X, Li G. Chem. Comm. 2002;40:1856. doi: 10.1039/b204210j. [DOI] [PubMed] [Google Scholar]
- 11.For reviews, see: Drewes SE, Roo GHP. Tetrahedron. 1988;44:4653. Basavaiah D, Rao PD, Hyma RS. Tetrahedron. 1996;52:8001. Langer P. Angew. Chem. Int. Ed. 2000;39:3049. doi: 10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5. Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811. doi: 10.1021/cr010043d. Masson G, Housseman C, Zhu J-P. Angew. Chem. Int. Ed. 2007;46:4614. doi: 10.1002/anie.200604366. Basavaiah D, Rao KV, Reddy RJ. Chem. Soc. Rev. 2007;36:1581. doi: 10.1039/b613741p. Shi Y-L, Shi M. Eur. J. Org. Chem. 2007:2905. Singh V, Batra S. Tetrahedron. 2008;64:4511. Declerck V, Martinez J, Lamaty F. Chem. Rev. 2009;109:1. doi: 10.1021/cr068057c. Wei Y, Shi M. Acc. Chem. Res. 2010;43:1005. doi: 10.1021/ar900271g.
- 12.(a) Guan X-Y, Wei Y, Shi M. Chem. Eur. J. 2010;16:13617. doi: 10.1002/chem.201002240. [DOI] [PubMed] [Google Scholar]; (b) Liu Y-L, Wang B-L, Chen L, Zhang Y-X, Wang C, Zhou J. J Am. Chem. Soc. 2010;132:15176. doi: 10.1021/ja107858z. [DOI] [PubMed] [Google Scholar]; (c) Zhong F-R, Chen G-Y, Lu Y-X. Org. Lett. 2011;13:82. doi: 10.1021/ol102597s. [DOI] [PubMed] [Google Scholar]
- 13.For selected examples, see: Iwabuchi Y, Nakatani M, Yokoyama N, Hatakeyama S. J Am. Chem. Soc. 1999;121:10219. Matsui K, Takizawa S, Sasai H. J Am. Chem. Soc. 2005;127:3680. doi: 10.1021/ja0500254. Raheem IT, Jacobsen EN. Adv. Synth. Catal. 2005;347:1701. Abermil N, Masson. G, Zhu J. J Am. Chem. Soc. 2008;130:12596. doi: 10.1021/ja805122j.
- 14.For selected examples, see: Liu Y-H, Chen L-H, Shi M. Adv. Synth. Catal. 2006;348:973. Zhong F-R, Wang Y-Q, Han X-Y, Huang K-W, Lu Y-X. Org. Lett. 2011;13:1310. doi: 10.1021/ol103145g.
- 15.Nakano A, Ushiyama M, Iwabuchi Y, Hatakeyama S. Adv. Synth. Catal. 2005;347:1790. [Google Scholar]
- 16.For recent mechanistic studies on MBH reaction: Santos LS, Pavam CH, Almeida WP, Coelho F, Eberlin MN. Angew. Chem. Int. Ed. 2004;43:4330. doi: 10.1002/anie.200460059. Price KE, Broadwater SJ, Jung HM, McQuade DT. Org. Lett. 2005;7:147. doi: 10.1021/ol047739o. Aggarwal VK, Fulford SY, Lloyd-Jones GC. Angew. Chem. Int. Ed. 2005;44:1706. doi: 10.1002/anie.200462462. Robiette R, Aggarwal VK, Harvey JN. J. Am. Chem. Soc. 2007;129:15513. doi: 10.1021/ja0717865.
- 17.Lin S, Jacobsen EN. Nature Chem. 2012;4:817. doi: 10.1038/nchem.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Kattuboina A, Li G. Tetrahedron Lett. 2008;49:1573. [Google Scholar]; (b) Kaur P, Shakya G, Sun H, Pan Y, Li G. Org. Biomol. Chem. 2010;8:1091. doi: 10.1039/b923914f. [DOI] [PubMed] [Google Scholar]; (c) Pindi S, Kaur P, Shakya G, Li G. Chem. Biol. Drug Design. 2011;77:20. doi: 10.1111/j.1747-0285.2010.01047.x. [DOI] [PubMed] [Google Scholar]; (d) Kattamuri PV, Ai T, Pindi S, Sun Y, Gu P, Shi M, Li G. J Org. Chem. 2011;76:2792. doi: 10.1021/jo200070d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.