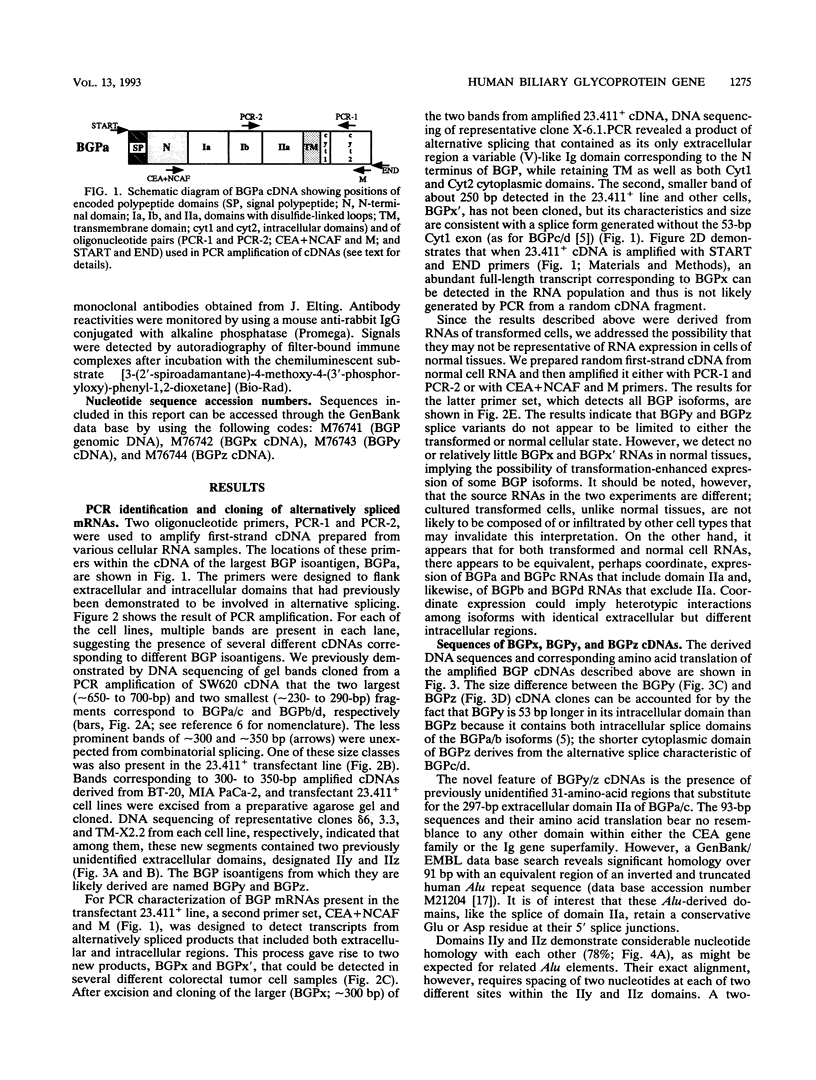
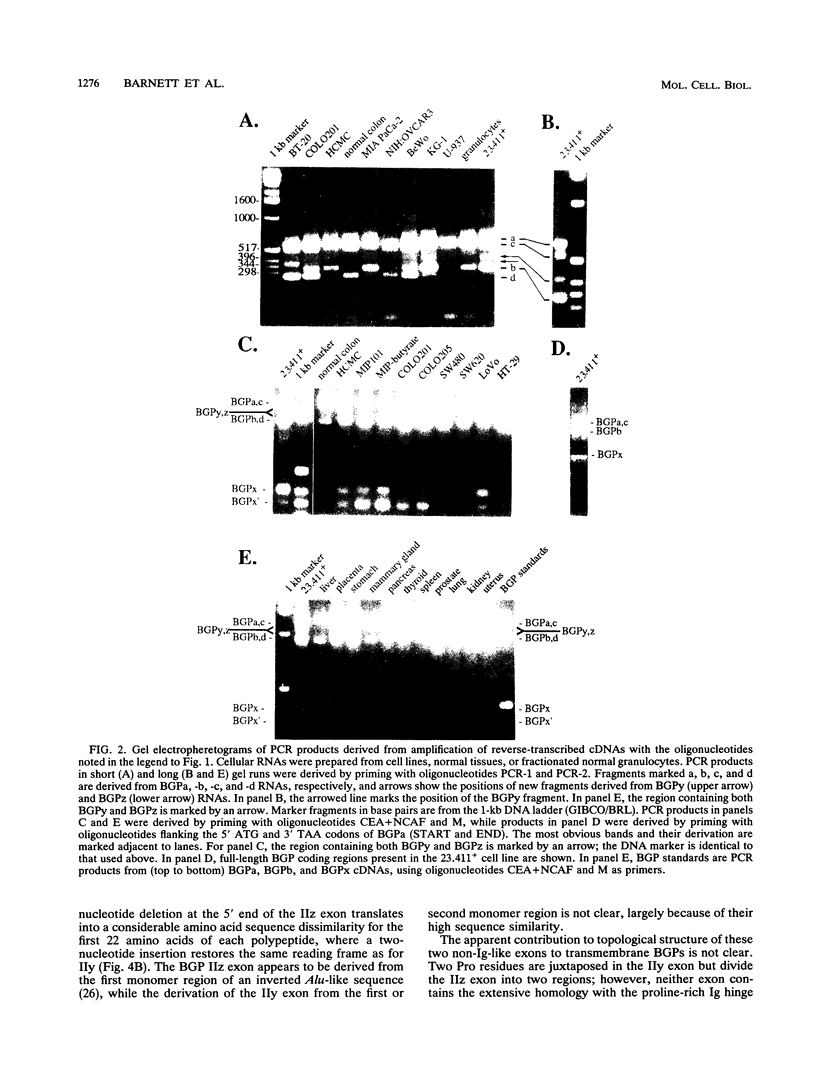
Abstract
Eight different human biliary glycoprotein (BGP) isoantigens, structurally related members of the carcinoembryonic antigen family, CD66/67 family, and immunoglobulin superfamily, are derived by alternative splicing from a single genomic transcription unit. Novel BGP isoforms have been identified by polymerase chain reaction amplification and by DNA sequencing of amplified cDNA segments. In addition to verifying previously documented BGPs, we describe four new forms, two of which have unusual nonimmunoglobulin exons contributed by inverted Alu repeats. Determination of the genomic DNA sequence encompassing most of the known extracellular and intracellular domains demonstrates that the translatable Alu-like sequences are encoded in bona fide exons. The third novel BGP isoform contains none of the extracellular disulfide-linked immunoglobulin-like domains typical of these molecules but retains N-terminal and intracellular domains, suggesting distinct functions for N-terminal versus other disulfide-linked domains. cDNAs coding for each identified isoform have been transfected into COS7 monkey cells, and the resulting polypeptides are heavily N glycosylated but can be deglycosylated to their expected primary sizes. Many of these deglycosylated forms can be correlated with unique patterns of BGP expression in different cell lines, while in granulocytes, some previously undescribed or alternatively modified forms may predominate. The BGP family represents a potentially large but unknown source of functional diversity among cells of epithelial and hematopoietic origin. The availability of a defined set of expressed of BGP cDNAs should permit critical definition of their function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afar D. E., Stanners C. P., Bell J. C. Tyrosine phosphorylation of biliary glycoprotein, a cell adhesion molecule related to carcinoembryonic antigen. Biochim Biophys Acta. 1992 Feb 19;1134(1):46–52. doi: 10.1016/0167-4889(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Aurivillius M., Hansen O. C., Lazrek M. B., Bock E., Obrink B. The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett. 1990 May 21;264(2):267–269. doi: 10.1016/0014-5793(90)80264-j. [DOI] [PubMed] [Google Scholar]
- Barker P., Miller F., Murphy R. Analysis of NGF receptor gene products: generation of artifactual splice variants by PCR. Biotechniques. 1992 Feb;12(2):216–218. [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T., Goebel S. J., Nothdurft M. A., Elting J. J. Carcinoembryonic antigen family: characterization of cDNAs coding for NCA and CEA and suggestion of nonrandom sequence variation in their conserved loop-domains. Genomics. 1988 Jul;3(1):59–66. doi: 10.1016/0888-7543(88)90160-7. [DOI] [PubMed] [Google Scholar]
- Barnett T., Zimmermann W. Workshop report: proposed nomenclature for the carcinoembryonic antigen (CEA) gene family. Tumour Biol. 1990;11(1-2):59–63. doi: 10.1159/000217643. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hinoda Y., Imai K., Nakagawa N., Ibayashi Y., Nakano T., Paxton R. J., Shively J. E., Yachi A. Transcription of biliary glycoprotein I gene in malignant and non-malignant human liver tissues. Int J Cancer. 1990 May 15;45(5):875–878. doi: 10.1002/ijc.2910450516. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Lu J., Chen H., Werner S., Williams L. T. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991 Sep;11(9):4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck M. E., Elting J. J., Hart J. T., Goebel S. J., Rae P. M., Nothdurft M. A., Nedwin J. J., Barnett T. R. Carcinoembryonic antigen family: expression in a mouse L-cell transfectant and characterization of a partial cDNA in bacteriophage lambda gt11. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5350–5354. doi: 10.1073/pnas.84.15.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M., Arakawa F., Matsuo Y., Oikawa S., Nakazato H., Matsuoka Y. Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library. Biochem Biophys Res Commun. 1991 Apr 30;176(2):578–585. doi: 10.1016/s0006-291x(05)80223-2. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Goldthwait D. A., Samols D. Identification of Alu transposition in human lung carcinoma cells. Cell. 1988 Jul 15;54(2):153–159. doi: 10.1016/0092-8674(88)90547-8. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Lundwall A. B., Wetsel R. A., Kristensen T., Whitehead A. S., Woods D. E., Ogden R. C., Colten H. R., Tack B. F. Isolation and sequence analysis of a cDNA clone encoding the fifth complement component. J Biol Chem. 1985 Feb 25;260(4):2108–2112. [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Smith B. R., Barbosa J. A., Crimmins M. A., Herrmann S. H., Burakoff S. J. CTL adhesion and antigen recognition are discrete steps in the human CTL-target cell interaction. J Immunol. 1987 Mar 1;138(5):1325–1330. [PubMed] [Google Scholar]
- Miki T., Bottaro D. P., Fleming T. P., Smith C. L., Burgess W. H., Chan A. M., Aaronson S. A. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklind C., Odin P., Obrink B. Two different cell adhesion molecules--cell-CAM 105 and a calcium-dependent protein--occur on the surface of rat hepatocytes. Exp Cell Res. 1984 Mar;151(1):29–45. doi: 10.1016/0014-4827(84)90353-7. [DOI] [PubMed] [Google Scholar]
- Rojas M., Fuks A., Stanners C. P. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990 Nov;1(11):527–533. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni M. J., Barthels D., Vopper G., Boned A., Goridis C., Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989 Feb;8(2):385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Sharma S., Mehta S., Morgan J., Maizel A. Molecular cloning and expression of a human B-cell growth factor gene in Escherichia coli. Science. 1987 Mar 20;235(4795):1489–1492. doi: 10.1126/science.3547651. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Beatty J. D. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2(4):355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Small S. J., Haines S. L., Akeson R. A. Polypeptide variation in an N-CAM extracellular immunoglobulin-like fold is developmentally regulated through alternative splicing. Neuron. 1988 Dec;1(10):1007–1017. doi: 10.1016/0896-6273(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Snow P. M., Van de Rijn M., Terhorst C. Association between the human thymic differentiation antigens T6 and T8. Eur J Immunol. 1985 May;15(5):529–532. doi: 10.1002/eji.1830150520. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Rojas M., Zhou H., Fuks A., Beauchemin N. The CEA family: a system in transitional evolution? Int J Biol Markers. 1992 Jul-Sep;7(3):137–142. doi: 10.1177/172460089200700303. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Sizer K. C., Vollmer A. C., Hunkapiller T., Parnes J. R. The T cell differentiation antigen Leu-2/T8 is homologous to immunoglobulin and T cell receptor variable regions. Cell. 1985 Mar;40(3):591–597. doi: 10.1016/0092-8674(85)90207-7. [DOI] [PubMed] [Google Scholar]
- Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976 May 15;17(5):588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Hammarström S., Zeromski J. Immunofluorescence studies on the occurrence and localization of the CEA-related biliary glycoprotein I (BGP I) in normal human gastrointestinal tissues. Clin Exp Immunol. 1979 Jun;36(3):436–441. [PMC free article] [PubMed] [Google Scholar]
- Watt S. M., Sala-Newby G., Hoang T., Gilmore D. J., Grunert F., Nagel G., Murdoch S. J., Tchilian E., Lennox E. S., Waldmann H. CD66 identifies a neutrophil-specific epitope within the hematopoietic system that is expressed by members of the carcinoembryonic antigen family of adhesion molecules. Blood. 1991 Jul 1;78(1):63–74. [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Lai M. M. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992 Oct;66(10):6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]