Abstract
Background
The protection against pneumococcal infections provided by currently available pneumococcal polysaccharide conjugate vaccines are restricted to the limited number of the serotypes included in the vaccine. In the present study, we evaluated the distribution of the pneumococcal capsular type and surface protein A (PspA) family of pneumococcal isolates from upper respiratory tract infections in Japan.
Methods
A total of 251 S. pneumoniae isolates from patients seeking treatment for upper respiratory tract infections were characterized for PspA family, antibiotic resistance and capsular type.
Results
Among the 251 pneumococci studied, the majority (49.4%) was identified as belonging to PspA family 2, while most of the remaining isolates (44.6%) belonged to family 1. There were no significant differences between the distributions of PspA1 versus PspA2 isolates based on the age or gender of the patient, source of the isolates or the isolates’ susceptibilities to penicillin G. In contrast, the frequency of the mefA gene presence and of serotypes 15B and 19F were statistically more common among PspA2 strains.
Conclusion
The vast majority of pneumococci isolated from the middle ear fluids, nasal discharges/sinus aspirates or pharyngeal secretions represented PspA families 1 and 2. Capsular serotypes were generally not exclusively associated with certain PspA families, although some capsular types showed a much higher proportion of either PspA1 or PspA2. A PspA-containing vaccine would potentially provide high coverage against pneumococcal infectious diseases because it would be cross-protective versus invasive disease with the majority of pneumococci infecting children and adults.
Introduction
Streptococcus pneumoniae (S. pneumoniae) is a major etiological agent causing various infectious diseases ranging from non-invasive diseases such as acute otitis media (AOM), rhinosinusitis and pneumonia to invasive disease such as sepsis and meningitis in young children and the elderly [1]–[4]. In recent decades, penicillin resistant S. pneumoniae (PRSP) have evolved at a rapid pace into a global problem [5]–[7]. The high prevalence of antimicrobial resistant pneumococci has further emphasized the importance of pneumococcal vaccines [8], [9].
Currently available pneumococcal vaccines are based on capsular polysaccharides. Although the 23-valent polysaccharide vaccine (23 PPV) is immunogenic and protective in most adults, it has been shown to be poorly efficacious in children younger than 2 years of age [10]. In contrast, the 7-valent pneumococcal conjugate vaccine (7 PCV) is highly efficacious at preventing bacteremic disease in children under 5 years of age [11]–[14]. Promising results regarding the prevention of pneumonia and AOM, reducing nasopharyngeal carriage of vaccine serotypes, and elicitation of herd immunity against vaccine serotypes have also been reported for PCV [15]. However, the protection is restricted to the limited number of the serotypes included in the vaccine. In recent years the protection afforded by the conjugate vaccine has begun to be eroded by an increasing frequency of infections with pneumococcal strains not covered by the vaccine [16], [17]. An ideal pneumococcal vaccine would be immunogenic in all young children, the age group for whom pneumococcal infection and mortality is the highest in the developing world. An ideal vaccine would also protect against pneumococci regardless of their capsular types [18].
Pneumococcal surface protein A (PspA) is an important virulent factor expressed by all pneumococci that is essential for full virulence in invasive disease, and contributes to colonization [19]–[21]. It is highly immunogenic and protective against invasive disease as well as nasal colonization in mice. Protective antibody to PspA is elicited in the alpha helical and proline-rich domains of PspA. Of the ∼300 amino acid alpha-helical domain protective, its 100 C-terminal amino acids, known as the clade/family-defining region are responsible for much of the elicited protection [22]–[25]. However, the N-terminal 100 amino acids are also protection-eliciting [24], [25]. All three protection-eliciting regions exhibit variability in their mosque sequence but contain many shared sequences some of which are highly conserved [22], [26]. These shared sequences outside the family-defining regions and shared sequence between families within the family-defining region explain why immunity to PspAs of one family can often elicit some or complete protection against strains expressing PspAs of other families. Since virtually all pneumococci have at least slightly different PspA sequences, we regard virtually all protection by antibody to PspA as cross-protection. Immunity to PspA is highly cross-protective against invasive disease [27], [28]. Thus, a vaccine containing at least three different PspAs should be able to provide redundant protection against all pneumococci. Both intranasal and humoral immunization with PspA can also protect against colonization [29]–[31]. Consequently, PspA is an attractive candidate antigen for the development of new effective vaccines [32].
Since capsular type distribution is not uniform world wide, it is important to know the overall distribution of the PspA family expressed in pneumococcal strains at multiple sites around the world to make sure the PspA molecules represented in a vaccine will be effective world wide [33]–[35]. In the present study, we evaluated the distribution of PspA family types among pneumococcal isolates from upper respiratory tract infections in Japan.
Materials and Methods
S. pneumoniae Strains
Between January and May 2003, the Japanese Society of Infectious Disease in Otorhinolaryngology conducted the fourth nationwide surveillance of the bacterial pathogens responsible for otorhinolaryngological infections. A total of 251 S. pneumoniae isolates were collected from 251 patients treated for the upper respiratory tract infections including AOM, rhinosinusitis and pharyngotonsillitis during these periods. All pneumococcal strains were identified by alpha-hemolysis and colony morphology on 5% sheep blood agar, Gram’s stained smear, optochin disk sensitivity, bile solubility, and the presence of ply gene by polymerase chain reaction (PCR). The patients ranged in age from 0 to 68 years old, with 125 females and 126 males. Among the isolates, 57 (22.7%) were from the middle ear fluids (MEFs), 88 (35.1%) were from nasal discharges or sinus aspirates and 106 (42.2%) were from the pharyngeal secretions (Table 1).
Table 1. Distribution of S. pneumoniae serotypes based on their susceptibilities to PCG.
| Category | Sub-category | Total | Susceptibility to PCG (µg/ml) | p-value | ||
| PSSP | PISP | PRSP | DRSP v.s. PSSP | |||
| Gender | Female | 125 (49.8%) | 50 (19.9%) | 48 (19.1%) | 27 (10.8%) | p = 0.362 |
| Male | 126 (50.2%) | 43 (17.1%) | 56 (22.3%) | 27 (10.8%) | ||
| Age | 0–2 | 92 (36.7%) | 16 (6.4%) | 50 (19.9%) | 26 (10.4%) | p<0.001* |
| 3–5 | 37 (14.7%) | 16 (6.4%) | 17 (6.8%) | 4 (1.6%) | ||
| 6–12 | 25 (10%) | 18 (7.2%) | 7 (2.8%) | 0 (0%) | ||
| 13–20 | 7 (2.8%) | 4 (1.6%) | 2 (0.8%) | 1 (0.4%) | ||
| 21–50 | 72 (28.7%) | 30 (12.0%) | 22 (8.8%) | 20 (8.0%) | ||
| ≥51 | 18 (7.2%) | 9 (3.6%) | 6 (2.4%) | 3 (1.2%) | ||
| Origin | Middle ear fluids | 57 (22.7%) | 21 (8.4%) | 27 (10.8%) | 9 (3.6%) | p = 1.000 |
| Nasal discharge/Sinus aspirates | 88 (35.1%) | 37 (14.7%) | 34 (13.5%) | 17 (6.8%) | p = 0.273 | |
| Pharyngeal secretions | 106 (42.2%) | 35 (13.9%) | 43 (17.1%) | 28 (11.2%) | p = 0.291 | |
| Serotype | 1 | 2 (0.8%) | 1 (0.4%) | 0 (0%) | 1 (0.4%) | p = 1.000 |
| 3 | 14 (5.6%) | 13 (5.2%) | 1 (0.4%) | 0 (0%) | p<0.001 | |
| 4 | 1 (0.4%) | 0 (0%) | 0 (0%) | 1 (0.4%) | p = 1.000 | |
| 6A | 16 (6.4%) | 6 (2.4%) | 6 (2.4%) | 4 (1.6%) | p = 1.000 | |
| 6B | 37 (14.7%) | 14 (5.6%) | 17 (6.8%) | 6 (2.4%) | p = 1.000 | |
| 9V | 5 (2.0%) | 3 (1.2%) | 2 (0.8%) | 0 (0%) | p = 0.667 | |
| 14 | 20 (8.0%) | 2 (0.8%) | 13 (5.2%) | 5 (2.0%) | p = 0.002 | |
| 15B | 7 (2.8%) | 4 (1.6%) | 3 (1.2%) | 0 (0%) | p = 0.429 | |
| 19A | 5 (2.0%) | 4 (1.6%) | 1 (0.4%) | 0 (0%) | p = 0.064 | |
| 19F | 52 (20.7%) | 8 (3.2%) | 25 (10.0%) | 19 (7.6%) | p<0.001 | |
| 23F | 41 (16.3%) | 11 (4.4%) | 18 (7.2%) | 12 (4.8%) | p = 0.029 | |
| G23 | 6 (2.4%) | 4 (1.6%) | 2 (0.8%) | 0 (0%) | p = 0.198 | |
| Others | 45 (17.9%) | 23 (9.2%) | 16 (6.4%) | 6 (2.4%) | p = 0.040 | |
| Total | 251 (100%) | 93 (37.1%) | 104 (41.4%) | 54 (21.5%) | ||
G23: serogroup 23 strains except serotype 23F. PCG: penicillin G. PSSP: penicillin susceptible S. pneumoniae. PISP: penicillin intermediately resistant S. pneumoniae. PRSP: penicillin resistant S. pneumoniae. DRSP: PRSP+PISP. Others: serotypes not included in 23 PPV.
comparison between ≤2 y.o. vs. ≥3 y.o.
Susceptibility to penicillin G (PCG) was tested by a broth dilution standard method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). The CLSI published revised susceptibility breakpoints for penicillin and S. pneumoniae in 2008. The revised susceptibility breakpoint is ≤2 µg/ml for non-meningeal infections treated with parental penicillin. In this study, categorization of penicillin susceptibility according to the former CLSI guidelines was applied because most of the cases were treated with oral penicillin. Strains with MICs of PCG ≥2 µg/ml were interpreted as penicillin resistant S. pneumoniae (PRSP), strains with MICs from 0.1 to 1 µg/ml were classified as penicillin intermediate resistant S. pneumoniae (PISP), and strains with MICs ≤0.06 µg/ml were interpreted as penicillin susceptible S. pneumoniae (PSSP). During the assay, S. pneumoniae strains ATCC 49619 and ATCC BAA-334 were used as susceptible controls for quality assurance [36].
Serotype
All isolates were serotyped or serogrouped by the capsular quellung reaction method with pneumococcal capsule specific antisera (Statens Serum Institute, Copenhagen, Denmark), as recommended by the manufacturer. Strains of serotypes 4 (ATCC BAA-334) and 19F (ATCC 49619) obtained from the American Type Culture Collection 169 (ATCC, Manassas, VA, USA) were used for quality control in every reaction.
PspA Family Classification
PspAs were classified into three families by PCR. Briefly, genomic DNA was extracted from pneumococcal isolates as described and stored at 4°C [37]. PCR were carried out in a standard PCR mixture (QIAGEN, Valencia, CA, USA) of 25 µl containing 2.5 mM MgCl2, 200 µM dNTPs (each), 50 pmol of primers, and 2.5 U of Taq DNA polymerase. The oligonucleotide primers (LSM12, SKH63, SKH52, SKH41, SKH42, SKH02, ply1, and ply2) reported by Hollingshead et al were used in this study [37]. Primers for PspA family 1 (PspA1) and PspA family 2 (PspA2) were LSM12/SKH63 and LSM12/SKH52, respectively. Primers for PspA family 3 (PspA3) were SKH41 and SKH 42. Primers LSM12 and SKH02 were used for testing the presence of pspA gene. Primers ply1 and ply2 were used for testing the presence of the pneumolysin gene.
The PCR conditions were 95°C for 3 min; then 30 cycles of 95°C for 1 min, 62°C for 1 min and 72°C for 3 min, and finally 72°C for 10 min. The optimal annealing temperature was 62°C. The isolates that were not initially amplified were further processed with the same cycling pattern at an annealing temperature of 58°C, or, if that also failed, of 55°C. Isolate that were not typed after the lower annealing temperatures in the family 1, 2, and 3 tests were classified as nontypeable PspA (PspA NT). An additional two tests were used to verify that the PspA NT isolates were truly pneumococcal isolates. One test was for the presence of the pneumolysin gene and another test was for the presence of the pspA gene. A single isolate that was amplified by the ply primers and not amplified by any of the PspA primers was classified as PspA null.
Three microliters of the PCR products were loaded on 0.8% agarose gels, electrophoresed at 80 V for 1 h, and stained with 0.5 µg/ml ethidium bromide.
Statistical Analysis
All data were statistically analyzed by using Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA). A two tailed chi-square test or Fisher’s exact test (for small group sizes) was used for categorical variables to test the significance of differences between groups. A p-value of <0.05 was considered statistically significant. The odds ratio (OR) and 95% confidential intervals (CIs) of individual serotypes were calculated relative to all other serotypes in the samples.
Ethical Approval
The isolates used in this study are all clinical isolates obtained from patients with otorhinolaryngological infections as part of routine clinical diagnosis and management. The main ethical issue relates to specific consent for detailed characterization of an isolate from a clinical specimen taken from a patient on clinical ground. Because no information that would allow identification of the patients was collected in this study, this requirement was waived by the Institutional Review Board of the Ethical Committee of Wakayama Medical University. This study was therefore approved by the Institutional Review Board of the Ethical Committee of Wakayama Medical University.
Results
Distribution of Pneumococcal Serotypes Based on their Penicillin and Macrolide Susceptibilities
The distribution of S. pneumoniae serotypes based on their susceptibilities to PCG is listed in Table 1. Based on their susceptibility to PCG, the 251 pneumococcal isolates evaluated in this study were classified into three groups as follows: 93 (37.0%) PSSP, 104 (41.4%) PISP, and 54 (21.6%) PRSP. There were no significant differences in distributions of susceptibilities to PCG based on gender of the patients providing the strains, or based on the source of the isolates. Drug resistant S. pneumoniae (DRSP; PISP+PRSP) were frequently identified among children younger than 2 years old (OR 4.5, 95% CI 2.4–8.3, p<0.001).
The most common serotype was 19F (20.7%) followed by 23F (16.3%), 6B (14.7%), 14 (8.0%), 6A (6.4%) and 3 (5.6%). Among the serogroup 6 strains, we could not find the recently discovered serotype 6C and 6D strains. The distribution of S. pneumoniae serotypes based on their susceptibility to PCG was statistically significant (p<0.001). Serotype 3 (OR 25.5, 95% CI 3.3–198.6, p<0.001) was prevalent among the strains with MICs to PCG of ≤0.06 µg/ml. In contrast serotype 14 (OR 5.9, 95% CI 1.3–25.8, p = 0.002) and serotype 19F (OR 4.1, 95% CI 1.8–9.2, p<0.001) were frequently identified among DRSP strains. The isolated strains identified as serotypes 6A and 6B showed a broad spectrum of antibiotic resistance regardless of their susceptibility to PCG. The most common five serotypes (19F, 23F, 6B, 6A and 14) represented about 79.1% of the DRSP strains.
The distribution of S. pneumoniae serotypes based on their susceptibilities to macrolide is listed in Table 2. Based on the macrolide susceptibilities, the 251 isolates were classified into four groups as follows: 106 (42.2%) strains with the ermB gene, 75 (29.8%) strains with the mefA gene, 15 (6.0%) strains with both genes, and 55 (22.0%) strains without both genes. There were no significant differences in distributions of macrolide resistant traits based on the gender or based on the source of the isolates or age of the patients.
Table 2. Distribution of S. pneumoniae serotypes based on their macroride-resistant traits.
| Category | Sub-category | Total | Macrolide resistance genes | p-value | |||
| ermB | mefA | ermB+mefA | None | MLR v.s. MLS | |||
| Gender | Female | 99 (39.4%) | 51 (20.3%) | 39 (15.5%) | 9 (3.6%) | 26 (10.4%) | p = 0.761 |
| Male | 97 (38.6%) | 55 (21.9%) | 36 (14.3%) | 6 (2.4%) | 29 (11.6%) | ||
| Age | 0–2 | 76 (30.3%) | 39 (15.5%) | 32 (12.7%) | 5 (2.0%) | 16 (6.4%) | p = 0.208* |
| 3–5 | 28 (11.2%) | 14 (5.6%) | 12 (4.8%) | 2 (0.8%) | 9 (3.6%) | ||
| 6–12 | 14 (5.6%) | 11 (4.4%) | 3 (1.2%) | 0 (0%) | 11 (4.4%) | ||
| 13–20 | 4 (1.6%) | 3 (1.2%) | 0 (0%) | 1 (0.4%) | 3 (1.2%) | ||
| 21–50 | 58 (23.1%) | 29 (11.6%) | 22 (8.8%) | 7 (2.8%) | 14 (5.6%) | ||
| ≥51 | 16 (6.4%) | 10 (4.0%) | 6 (2.4%) | 0 (0%) | 2 (0.8%) | ||
| Origin | Middle ear fluids | 46 (18.3%) | 34 (13.5%) | 11 (4.4%) | 1 (0.4%) | 11 (4.4%) | p = 0.264 |
| Nasal discharge/Sinus aspirates | 65 (25.9%) | 35 (13.9%) | 25 (10.0%) | 5 (2.0%) | 23 (9.2%) | p = 0.716 | |
| Pharyngeal secretions | 85 (33.9%) | 37 (14.7%) | 39 (15.5%) | 9 (3.6%) | 21 (8.4%) | p = 0.539 | |
| Serotype | 1 | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 0 (0%) | 1 (0.4%) | p = 0.391 |
| 3 | 10 (4.0%) | 9 (3.6%) | 1 (0.4%) | 0 (0%) | 4 (1.6%) | p = 0.514 | |
| 4 | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 0 (0%) | 0 (0%) | p = 0.515 | |
| 6A | 13 (5.2%) | 8 (3.2%) | 4 (1.6%) | 1 (0.4%) | 3 (1.2%) | p = 1.000 | |
| 6B | 27 (10.8%) | 17 (6.8%) | 7 (2.8%) | 3 (1.2%) | 10 (4.0%) | p = 0.397 | |
| 9V | 4 (1.6%) | 3 (1.2%) | 0 (0%) | 1 (0.4%) | 1 (0.4%) | p = 1.000 | |
| 14 | 17 (6.8%) | 10 (4.0%) | 6 (2.4%) | 1 (0.4%) | 3 (1.2%) | p = 0.579 | |
| 15B | 6 (2.4%) | 5 (2.0%) | 0 (0%) | 1 (0.4%) | 1 (0.4%) | p = 1.000 | |
| 19A | 1 (0.4%) | 1 (0.4%) | 0 (0%) | 0 (0%) | 4 (1.6%) | p = 0.009 | |
| 19F | 49 (19.5%) | 15 (6.0%) | 30 (12.0%) | 4 (1.6%) | 3 (1.2%) | p = 0.001 | |
| 23F | 36 (14.3%) | 19 (7.6%) | 14 (5.6%) | 3 (1.2%) | 5 (2.0%) | p<0.001 | |
| G23 | 5 (2.0%) | 4 (1.6%) | 1 (0.4%) | 0 (0%) | 1 (0.4%) | p = 0.147 | |
| Others | 26 (10.4%) | 15 (6.0%) | 10 (4.0%) | 1 (0.4%) | 19 (7.6%) | p = 1.000 | |
| Total | 196 (78.1%) | 106 (42.2%) | 75 (29.9%) | 15 (6.0%) | 55 (21.9%) | p = 0.001 | |
G23: serogroup 23 strains except serotype 23F. PCG: penicillin G. PSSP: penicillin susceptible S. pneumoniae. PISP: penicillin intermediately resistant S. pneumoniae. PRSP: penicillin resistant S. pneumoniae. DRSP: PRSP+PISP. Others: serotypes not included in 23 PPV.
comparison between ≤2 y.o. vs. ≥3 y.o.
The distribution of S. pneumoniae serotypes based on their macrolide resistant traits is also statistically significant (p = 0.001). The mefA gene was most prevalent among isolates typed as serotype 19F (OR 4.7, 95% CI 2.5–8.9, p<0.001). Strains of the most predominant six serotypes (19F, 23F, 6B, 6A, 14, and 3) represented 60.6% of the total strains and about 77.6% of the strains with macrolide resistant genes.
Distribution of PspA Families Based on their Serotypes and Penicillin Susceptibilities
Among the 251 pneumococci isolates studied, the 49.4% were identified as belonging to family 2 (PspA2), and 44.6% to family 1 (PspA1). Thus, 94.0% of the isolates included in this study were PspA1- or PspA2-positive isolates. Eight isolates (3.2%) classified into PspA family 3 (PspA3). Four isolates (1.6%) were classified as PspA NT. Three isolates (1.2%) was identified as a PspA null strain.
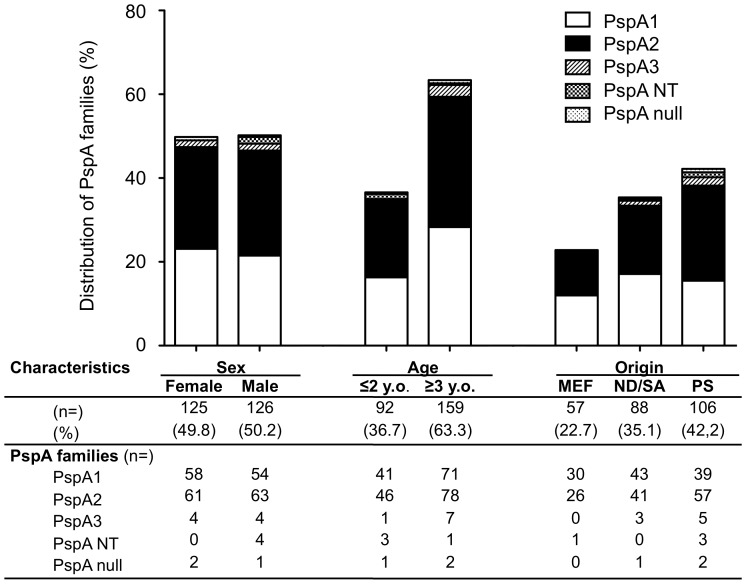
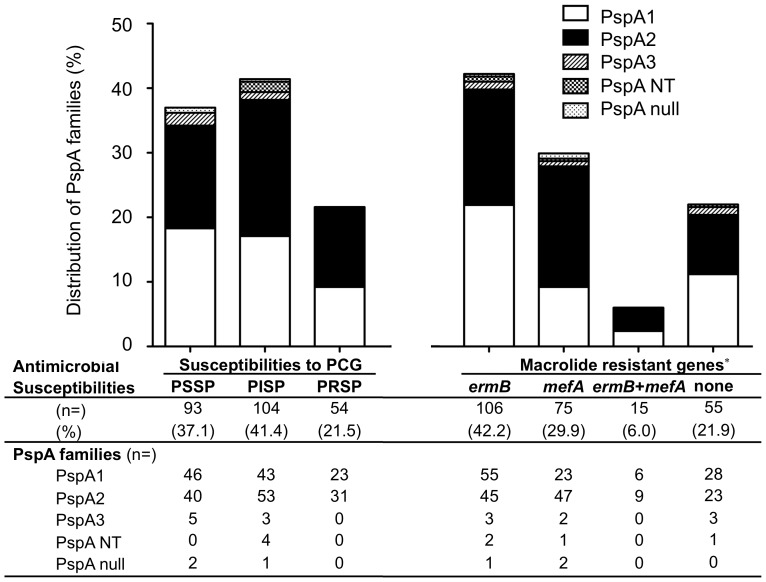
Because the vast majority of PspA families were identified as PspA1 or PspA2, we further evaluated the distributions of PspA1 and PspA2 by the other parameters. There were no significant differences in the distributions of PspA1 and PspA2 based on the age and gender of the patients, the origin of the isolates (Fig. 1.). Although there were no significant differences in the distribution of PspA1 and PspA2 based on the isolates’ susceptibilities to PCG, PspA2 were expressed at a higher frequency among the strains with the mefA gene (OR 2.4, 95% CI 1.4–4.1, p = 0.003) than the population of strains in general (Fig. 2.).
Figure 1. Distribution of PspA familes based on sex, age and origin of pneumococci.
MEF: middle ear fluid, ND/SA: nasal discharge/sinus aspirate, PS: pharyngeal secretion. Each numbers shows numbers of isolates and percentage shows in parenthesis. There is no significant differences in PspA family distribution based on sex, age and origin of isolates.
Figure 2. Distribution of PspA familes based on antimicoribila suscptibilities.
PSSP: penicillin susceptible S. pneumoniae, PISP: Penicillin intermediately resistant S. pneumoniae, PRSP: penicillin resistant S. pneumoniae. Each numbers shows numbers of isolates and percentage shows in parenthesis. *p<0.05.
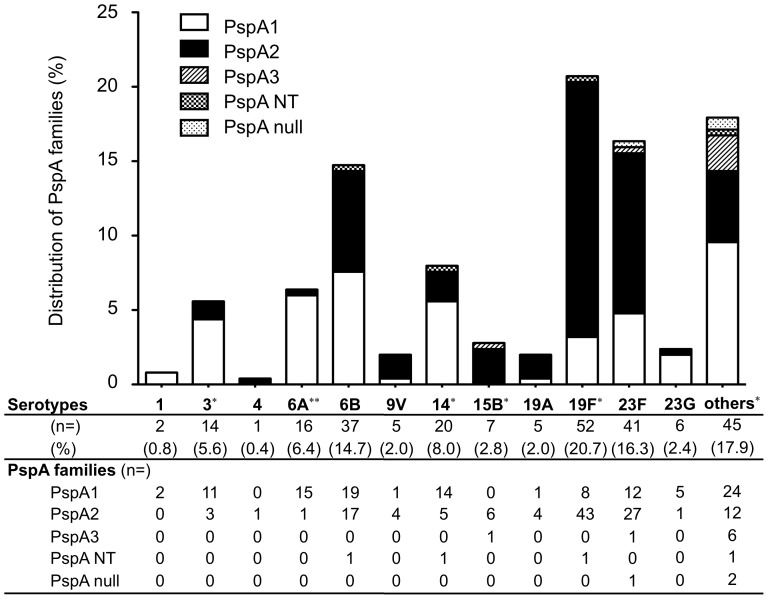
The distribution of PspA families based on their serotypes is shown in Fig. 3. The differences in distribution of PspA1 and PspA2 isolates based on their pneumococcal serotype were statistically significant (p = 0.013). Serotype 19F (OR 6.9, 95% CI 3.1–15.5, p<0.001), and serotype 15B (OR 12.3, 95% CI 0.7–221.8, p = 0.031) frequently expressed PspA2. Serotype 3 (OR 4.4, 95% CI 1.2–16.2, p = 0.025), serotype 6A (OR 19.0, 95% CI 1.7–146.6, p<0.001) and serotype 14 (OR 3.4, 95% CI 1.2–9.8, p = 0.029) tended to express PspA1. Serotypes 6B contained equal numbers of PspA1 and PspA2 isolates. In spite of these statistical differences in PspA family frequency among the different capsular types, representative capsular types (except for serotype 15B) had isolates of both PspA families. Thus, in general the capsular types were found not to be restricted to particular PspA families and PspA families were not restricted to particular capsular types.
Figure 3. Distribution of PspA familes based on pneumococcal serotypes.
G23: serogroup 23 strains except serotype 23F. Others: serotypes not included in 23 PPV. Each numbers shows numbers of isolates and percentage shows in parenthesis. *p<0.05, **p<0.01.
Coverage of Pneumococcal Vaccine Formulas
The coverage and 95% CI of pneumococcal vaccine formulas according to serotypes and PspA families are listed in Table 3. The total serotype coverage of the 7-valent, 10-valent (10 PCV), 13-valent (13 PCV), and 23-valent pneumococcal vaccines were 62.2%, 62.9%, 76.9% and 70.5%, respectively. The coverage of DRSP by the 7-valent, 10-valent, 13-valent, and 23-valent pneumococcal vaccines were 74.7%, 75.3%, 82.9% and 78.5%, respectively. The total coverage of S. pneumoniae with either the mefA gene or the ermB gene by the 7-valent, 10-valent, 13-valent, and 23-valent pneumococcal vaccines were 68.4%, 68.9%, 81.1%, and 74.5%, respectively. The percentages of total pneumococcal isolates, DRSP, and macrolide resistant S. pneumoniae (MRSP) having either mefA or ermB gene strains that would be covered by a PspA vaccine including the PspA1 and PspA2 families were 94.0%, 94.9%, and 94.4%, respectively. Consequently, the serotype coverage by a PspA vaccine was higher than the serotype coverage provided by the current 7 PCV, 10 PCV, 13 PCV, and 23 PPV vaccines (p<0.001).
Table 3. Serotype coverage of pneumococcal vaccine formulas among S. pneumoniae isolates from upper respiratory tract infections in Japan.
| Vaccine formulations | Number and percentable coverage of S. pneumoniae | |||||
| DRSP (n = 158) | MRSP (n = 196) | Total (n = 251) | ||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| 7-valent (4,6B,9V,14,18C,19F,23F) | 118 (74.7%) | 67.9%−81.5% | 134 (68.4%) | 61.9%−74.9% | 156 (62.2%) | 56.2%−68.2% |
| 10-valent (1,4,5,6B,7F,9V,14,18C,19F,23F) | 119 (75.3%) | 68.6%−82.0% | 135 (68.9%) | 62.4%−75.4% | 158 (62.9%) | 57.0%−68.9% |
| 13-valent (1,3,4,5,6A,6B,7F,9V,14,18C,19A,19F,23F) | 131 (82.9%) | 77.0%−88.8% | 159 (81.1%) | 75.6%−86.6% | 193 (76.9%) | 71.7%−82.1% |
| 23-valent (1,2,3,4,5,6B,7F,8,9V,9N,10A,11A,12F,14,15B,17F,18C,19A,19F,20,22F,23F,33F) | 124 (78.5%) | 72.1%−84.9% | 146 (74.5%) | 68.4%−80.6% | 177 (70.5%) | 64.9%−76.2% |
| PspA (PspA1 and PspA2) | 150 (94.9%) | 91.5%−98.4% | 185 (94.4%) | 91.2%−97.6% | 236 (94.0%) | 91.1%−97.0% |
DRSP: drug resistant S. pneumoniae (PISP+PRSP). MRSP: macrolide resistant S. pneumoniae.
Discussion
PspA consists of five domains including a signal peptide, alpha-helical charged region, a proline-rich domain, a choline-binding domain consisting of ten amino acids repeats, and a C-terminal amino acid tail [37]–[39]. Depending on the divergence of nucleotide sequences in the alpha-helical charged region, PspA is classified into three families, with no more than 50% sequence divergence within each family. The three PspA families are made up of six PspA clades that diverge from each other by no more than 20% sequence identity within each clade; family 1 (clades 1 and 2), family 2 (clades 3, 4, and 5), and family 3 (clade 6) [38], [40], [41].
Despite the great variation in the sequences of PspA, mouse and humans antibodies against PspA can be cross-reactive and cross protective against invasive disease in mice [27], [28]. The serologic cross-reactivity of PspA has been found to be strongly associated with PspA, but not restricted to family [42], [43]. Even so these antibodies can be more cross-protective than their level of cross-reactivity might suggest. Immunization of adult humans and mice with a PspA family 1 produced antibodies that could protect mice from infection with strains of PspA families 1 or 2 and from infections with strains of 3 different capsular types [42], [44], [45]. In addition successful fusion proteins have been made between family 1 and family 2 PspAs that can elicit antibody in mice protect against challenge strains of both PspA families [46]. In this study we focused on the distribution of PspA families among clinical isolates in Japan.
The Japanese strains were evenly distributed over family 1 and family 2. The proportions of the different PspA families can vary somewhat among countries. Hollingshead et al reported that the majority of PspAs in a collection of strains from Alabama fell into family 1 [37]. A study on invasive pneumococcal strains isolated from children less than 5 years of age in Colombia showed that 62.5% and 35.0% of strains belonged to families 1 and 2, respectively [43]. In Argentina 54.4% and 41.6% of the strains belonged to family 1 and family 2, respectively, with only 4.0% of the strains isolated from children being unclassifiable [47]. In Brazil, 50.5% of the isolates belonged to family 1, 43.2% were members of family 2, and 6.3% were not classified [48], [49]. In contrast, the high prevalence of PspA family 2 among pneumococci isolated from invasive pneumococcal diseases has been reported from Spain, Poland, Canada, Sweden, Germany, the USA, and France [37], [50]–[53]. A recent study of pneumococci isolates from nasopharyngeal carriage in Finnish children showed a prevalence of PspA family 1 and family 2 that was similar to our results [54]. The vast majority of pneumococci isolated from the middle ear fluid or nasopharyngeal secretion samples of the Finnish children less than 2 years old were from PspA families 1 and 2 [54]. Prior to our study, there had been a few reports of the PspA family distribution among pneumococci in Japan or any other countries in Asia [55], [56].
In contrast to the similar frequencies of PspA1 and PspA2 in Japan the frequency of different capsular serotypes was highly variable with 19F, 23F, 14, 6A, 6B, and 3 being the predominant common capsular types we observed which together accounted for 71.7% of the pneumococci isolates in this study. However, the PspA family distribution varied somewhat among serotypes. Earlier studies found that both PspA families occurred within the most common capsular serotypes, but that some serotypes were associated more strongly with one PspA family than the other [57], [58]. The capsular serotypes most strongly associated with a certain PspA family are 9N, 9V, 11A, 14, and 23F, whereas serotypes 6A, 6B, 19A, and 19F were equally associated with PspA families 1 and 2. This was most dramatic for the 24 different 23F isolates which were 25% PspA1 and 75% PspA2. In a study in France 37 different 23F strains were examined; 92% were PspA1 and 8% were PspA2 [54]. These findings indicate that there can be variations of distributions in PspAs in different geographic different regions although serotypes do not necessarily globally associate with certain PspAs. In some regions some capsular serotypes associated with a certain PspA family might be heavily clonal.
Based on the previously published information on PspA family distribution, there is still little information about the relationship between PspA families and antimicrobial-susceptibilities. In Japan, the rate of antimicrobial-resistant S. pneumoniae has increased continually since around 1990 and was about 49.0% between 1998 and 2000 [59], [60]. As documented in previous reports, penicillin-resistant strains were frequently identified among children younger than 2 years old [61]. In our previous study most of the serotype 19F and 23F strains were classified as either PISP or PRSP, while all of serotype 3 stains were classified as PSSP in middle ear isolates [62]. In this study, PRSP strains consisted equally of family 1 and 2 PspA. This means that a PspA-based vaccine would show a higher coverage of PRSP compared to the polysaccharide-based vaccines that have been available in the market.
Previous studies showed that PspA clades were independent of capsular serotypes [49], [50], [51]. Pneumococci of the same serotype were associated with different PspA clades from the same or a different family. This means that PspA-containing vaccines may be able to improve the protective efficacy of pneumococcal vaccines compared with the currently available serotype-based vaccines and may be able to avoid the serotype replacement that has been observed with conjugate vaccines [16]. The coverage of serotypes and PRSP by the 7 PCV was reported to be 62.8% and 88.0% for middle ear isolates, respectively. A PspA-based vaccine that contained representatives of PspA families 1 and 2 would potentially provide a high coverage rate because it would be cross-protective against invasive disease caused by the bulk of pneumococci infecting children and adults. It will be important however that data relating to both serotype and antibiotic resistance, similar to those reported here for Japan, should be collected in other geographical areas. Such a study would help to determine if a vaccine covering PspA families 1 and 2 would be appropriate for the geographic region in question.
In conclusion, even conjugate vaccine formulations with 13 pneumococcal capsular polysaccharides will not reach the coverage of 90% or more achieved by a vaccine containing family 1 and 2 PspA. The addition of PspA to the existing conjugate vaccine formulations may be a possible alternative for future development of pneumococcal vaccine.
Acknowledgments
We cordially thank the Surveillance Subcommittee, the Japan Society for Infectious Diseases in Otolaryngology, the 80 university hospitals, the affiliated hospitals, and the general practitioners who provided clinical specimens for this nationwide surveillance. We greatly thank Miss Yuki Tatsumi (Department of Otolaryngology-Head and Neck Surgery, Wakayama Medical University, Wakayama, Japan) for her technical assistance and Dr. Akihito Wada (National Institute of Infectious Disease, Tokyo, Japan) for evaluating serotype 6C and 6D.
Funding Statement
This work was supported by national grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (no. 22791624). The PspA work of David E. Briles and Susan K Hollingshead is supported by a National Institutes of Health (NIH) grant (Grant No. R01-AI021458) and Bill & Melinda Gates Foundation Grant (Grant No. 37863). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Greenwood B (1999) The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci 354: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hollingshead SK, Briles DE (2001) Streptococcus pneumoniae: new tools for an old pathogen. Curr Opin Microbiol 4: 71–77. [DOI] [PubMed] [Google Scholar]
- 3.Musher DM, Breiman RF, Tomasz A (2000) Streptococcus pneumoniae: at the threshold of the 21st century. In: Tomaz A editor. Streptococcus pneumoniae: molecular biology and mechanisms of disease: Mary Ann Liebert, Inc., 485–491.
- 4. Yamanaka N, Hotomi M, Billal DS (2008) Clinical bacteriology and immunology in acute otitis media in children. J Infect Chemother 14: 180–187. [DOI] [PubMed] [Google Scholar]
- 5. Appelbaum PC (1992) Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis 15: 77–83. [DOI] [PubMed] [Google Scholar]
- 6. Breiman R, Butler JC, Tenover FC, Elliot J, Facklam RR (1994) Emergence of drug-resistant pneumococcal infections in the United States. JAMA 271: 1831–1835. [PubMed] [Google Scholar]
- 7. Klugman KP (1990) Pneumococcal resistance to antibiotics. Clin Microbiol Rev 3: 171–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dagan R, Klugman KP (2008) Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis 8: 785–795. [DOI] [PubMed] [Google Scholar]
- 9. Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, et al. (2000) Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 343: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 10. Ortqvist A, Hedlund J, Burman LA, Elbel E, Höfer M, et al. (1998) Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet 351: 399–403. [DOI] [PubMed] [Google Scholar]
- 11. Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, et al. (2005) Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 12. Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, et al. (2009) Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 360: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, et al. (2006) Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 295: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 14. Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, et al. (2006) Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 368: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 15. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, et al. (2001) Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 344: 403–409. [DOI] [PubMed] [Google Scholar]
- 16. Klugman KP (2009) The significance of serotype replacement for pneumococcal disease and antibiotic resistance. Adv Exp Med Biol 634: 121–128. [DOI] [PubMed] [Google Scholar]
- 17. Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia J, et al. (2008) Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis 46: 174–182. [DOI] [PubMed] [Google Scholar]
- 18.Razzaque R, Shah P, Watt JM, Mirza S, Coats MT, et al. (2012) Intranasal immunization of mice with neuraminidase A elicits virtually complete protection against nasopharyngeal colonization and subsequent invasion by Streptococcus pneumoniae. Infect Immun In submission.
- 19. Briles DE, Yother J, McDaniel LS (1988) Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae . Rev Infect Dis 10: S372–S374. [DOI] [PubMed] [Google Scholar]
- 20. Ren B, Li J, Genschmer K, Hollingshead SK, Briles DE (2012) The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro. Clin Vaccine Immunol 19: 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogunniyi AD, Lemessurier KS, Graham RM, Watt JM, Briles DE, et al. (2007) Contribution of pneumolysin, PspA, and PspC (CbpA) to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun 75: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hollingshead SK, Becker RS, Briles DE (2000) Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae . Infect Immun 68: 5889–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coral MCV, Fonseca N, Castaneda E, Di Fabio JL, Hollingshead SK, et al. (2001) Families of pneumococcal surface protein A (PspA) of Streptococcus pneumoniae invasive isolates recovered from Colombian children. Emerging Infectious Diseases 7: 832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDaniel LS, Sheffield JS, Swiatlo E, Yother J, Crain MJ, et al. (1992) Molecular localization of variable and conserved regions of pspA, and identification of additional pspA homologous sequences in Streptococcus pneumoniae . Microb Pathog 13: 261–269. [DOI] [PubMed] [Google Scholar]
- 25. Roche H, Hakansson A, Hollingshead SK, Briles DE (2003) Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect Immun. 71: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks-Walter A, McDaniel LS, Hollingshead SK, Crain MJ, Briles DE (1995) RFLP of the pspA gene from different isolates of Streptococcus pneumoniae reveal families of pspA. 35th ICAAC, San Franscisco, CA.: ASM Press.
- 27. Briles DE, Hollingshead SK, Nabors GS, Paton JC, Brooks-Walter A (2000) The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae . Vaccine 19: S87–S95. [DOI] [PubMed] [Google Scholar]
- 28. Briles DE, Hollingshead SK, King J, Swift A, Braun PA, et al. (2000) Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis 182: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 29. Briles DE, Ades E, Paton JC, Sampson JS, Carlone GM, et al. (2000) Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae . Infect Immun 68: 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, et al. (2010) Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol 185: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 31. Ferreira DM, Oliveira ML, Moreno AT, Ho PL, Briles DE, et al. (2010) Protection against nasal colonization with Streptococcus pneumoniae by parenteral immunization with a DNA vaccine encoding PspA (Pneumococcal surface protein A). Microb Pathog 48: 205–213. [DOI] [PubMed] [Google Scholar]
- 32.Briles DE, Paton JC, Swiatlo E, Crain MJ (2006) Pneumococcal Vaccines. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram Positive Pathogens. 2nd ed: ASM Press. 289–298.
- 33. Franco CM, Andrade AL, Andrade JG, Almeida e Silva S, Oliveira CR, et al. (2010) Survey of nonsusceptible nasopharyngeal Streptococcus pneumoniae isolates in children attending day-care centers in Brazil. Pediatr Infect Dis J 29: 77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shouval DS, Greenberg D, Givon-Lavi N, Porat N, Dagan R (2009) Serotype coverage of invasive and mucosal pneumococcal disease in Israeli children younger than 3 years by various pneumococcal conjugate vaccines. Pediatr Infect Dis J 28: 277–282. [DOI] [PubMed] [Google Scholar]
- 35. Chiba N, Morozumi M, Sunaoshi K, Takahashi S, Takano M, et al. (2010) Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol Infect 138: 61–68. [DOI] [PubMed] [Google Scholar]
- 36. Weinstein MP, Klugman KP, Jones RN (2009) Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis 48: 1596–1600. [DOI] [PubMed] [Google Scholar]
- 37. Hollingshead SK, Baril L, Ferro S, King J, Coan P, et al. (2006) Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol 55: 215–221. [DOI] [PubMed] [Google Scholar]
- 38. Hollingshead SK, Becker R, Briles DE (2000) Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae . Infect Immun 68: 5889–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Briles DE, Tart RC, Swiatlo E, Dillard JP, Smith P, et al. (1998) Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin Microbiol Rev 11: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jedrzejas MJ, Hollingshead SK, Lebowitz J, Chantalet L, Briles DE, et al. (2000) Production and characterization of the functional fragment of pneumococcal surface protein A. Arch Biochem Biophys. 373: 116–125. [DOI] [PubMed] [Google Scholar]
- 41. Jedrzejas MJ, Lamani E, Becker RS (2001) Characterization of selected strains of pneumococcal surface protein A. J Biol Chemi. 276: 33121–33128. [DOI] [PubMed] [Google Scholar]
- 42. Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, et al. (2000) Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18: 1743–1754. [DOI] [PubMed] [Google Scholar]
- 43. Vala Coral MC, Fonseca N, Castaneda E, Di Fabio JL, Hollingshead SK, et al. (2001) Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates form Colombian children. Emerg Infect Dis 7: 832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tart RC, McDaniel LS, Ralph BA, Briles DE (1996) Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis 173: 380–386. [DOI] [PubMed] [Google Scholar]
- 45. Briles DE, Hollingshead SK, King J, Swift A, Braun PA, et al. (2000) Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis 182: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 46. Darrieux M, Miyaji EN, Ferreira DM, Lopes LM, Lopes AP, et al. (2007) Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect Immun 75: 5930–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mollerach M, Regueira M, Bonofiglio L, Callejo R, Pace L, et al. (2004) Streptococcus pneumoniae Working Group: Invasive Streptococcus pneumoniae isolates from Argentinean children: serotypes, families of pneumococcal surface protein A (PspA) and genetic diversity. Epidemiol Infect 132: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brandileone MC, Andrade AL, Teles EM, Zanella RC, Yara TI, et al. (2004) Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 28: 3890–3896. [DOI] [PubMed] [Google Scholar]
- 49. Pimenta FC, Ribeiro-Dias F, Brandileone MCC, Miyaji EN, Leite LCC, et al. (2006) Genetic diversity of PspA types among nasopharyngeal isolates collecting during an ongoing surveillance study of children in Brazil. J Clin Microbiol 44: 2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beall B, Gherardi G, Fracklam RR, Hollingshead SK (2000) Pneumococcal pspA sequence types of prevalent multiresistant pneumococcal strains in the United States and of internationally disseminated clones. J Clin Microbiol 38: 3663–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heeg C, Franken C, van der Linden M, Ai-Lahlam A, Reiner RR (2007) Genetic diversity of pneumococcal surface protein A of Streptococcus pneumoniae meningitis in German children. Vaccine 25: 1030–1035. [DOI] [PubMed] [Google Scholar]
- 52. Rolo D, Ardanuy C, Fleites A, Martín R, Liñares J (2009) Diversity of pneumococcal surface protein A (PspA) among prevalent clones in Spain. BMC Microbiol 9: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sadowy E, Skoczynska A, Fiett J, Gniadkowski M, Hryniewicz W (2006) Multilocus sequence types, serotypes, and variants of the surface antigen PspA in Streptococcus pneumoniae isolates from meningitis patients in Poland. Clin Vaccine Immunol 13: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Melin MM, Hollingshead SK, Briles DE, Hanage WP, Lahdenkari M, et al. (2008) Distribution of pneumococcal surface protein A families 1 and 2 among Streptococcus pneumoniae isolates from children in finland who had acute otitis media or were nasopharyngeal carriers. Clin Vaccine Immunol 15: 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Imai S, Ito Y, Ishida T, Hirai T, Ito I, et al. (2011) Distribution and clonal relationship of cell surface virulence genes among Streptococcus pneumoniae isolates in Japan. Clin Microbiol Infect 17: 1409–1414. [DOI] [PubMed] [Google Scholar]
- 56. Ito Y, Osawa M, Isozumi R, Imai S, Ito I, et al. (2007) Pneumococcal surface protein A family types of Streptococcus pneumoniae from community-acquired pneumonia patients in Japan. Eur J Clin Microbiol Infect Dis 26: 739–742. [DOI] [PubMed] [Google Scholar]
- 57. Crain MJ, Turner JS, Robinson DA, Coffey TJ, Brooks-Walter A, et al. (1996) Evidence for the simultaneous expression of two PspAs by a clone of capsular serotype 6B Streptococcus pneumoniae . Microb Pathog 21: 265–275. [DOI] [PubMed] [Google Scholar]
- 58. Robinson DA, Turner JS, Facklam RR, Parkinson AJ, Breiman RF, et al. (1999) Molecular characterization of a globally distributed lineage of serotype 12F Streptococcus pneumoniae causing invasive disease. J Infect Dis 179: 414–422. [DOI] [PubMed] [Google Scholar]
- 59. Niki Y, Hanaki H, Matsumoto T, Yagisawa M, Kohno S, et al. (2009) Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2007: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother 15: 156–167. [DOI] [PubMed] [Google Scholar]
- 60. Niki Y, Hanaki H, Yagisawa M, Kohno S, Aoki N, et al. (2008) The first nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy. Part 1: a general view of antibacterial susceptibility. J Infect Chemother 14: 279–290. [DOI] [PubMed] [Google Scholar]
- 61. Hotomi M, Billal DS, Shimada J, Suzumoto M, Yamauchi K, et al. (2006) High prevalence of Streptococcus pneumoniae with mutations in pbp1a, pbp2x, and pbp2b genes of penicillin-binding proteins in the nasopharynx in children in Japan. ORL J Otorhinolaryngol Relat Spec 68: 139–145. [DOI] [PubMed] [Google Scholar]
- 62. Hotomi M, Billal DS, Kamide Y, Kanesada K, Uno Y, et al. (2008) Serotype distribution and penicillin resistance of Streptococcus pneumoniae isolates from middle ear fluids of pediatric patients with acute otitis media in Japan. J Clin Microbiol 46: 3808–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]