Abstract
Graft-versus-host disease (GvHD) is a key contributor to the morbidity and mortality after allogeneic hematopoetic stem cell transplantation (HSCT). Regulatory Foxp3+ CD4+ T cells (Treg) suppress conventional T cell activation and can control GvHD. In our previous work, we demonstrate that a basic mechanism of Treg mediated suppression occurs by the transfer of cyclic adenosine monophosphate (cAMP) to responder cells. Whether this mechanism is relevant for Treg mediated suppression of GvHD is currently unknown. To address this question, bone marrow and T cells from C57BL/6 mice were transferred into lethally irradiated BALB/c recipients, and the course of GvHD and survival were monitored. Transplanted recipients developed severe GvHD that was strongly ameliorated by the transfer of donor Treg cells. Towards the underlying mechanisms, in vitro studies revealed that Treg communicated with DCs via gap junctions, resulting in functional inactivation of DC by a metabolic pathway involving cAMP that is modulated by the phosphodiesterase (PDE) 4 inhibitor rolipram. PDE2 or PDE3 inhibitors as well as rolipram suppressed allogeneic T cell activation, indirectly by enhancing Treg mediated suppression of DC activation and directly by inhibiting responder T cell proliferation. In line with this, we observed a cooperative suppression of GvHD upon Treg transfer and additional rolipram treatment. In conclusion, we propose that an important pathway of Treg mediated control of GvHD is based on a cAMP dependent mechanism. These data provide the basis for future concepts to manipulate allogeneic T cell responses to prevent GvHD.
Introduction
For patients with high risk hematological malignancies allogeneic hematopoetic stem cell transplantation (HSCT) is the only curative treatment option [1]. The therapeutic principle of HSCT relies on a graft versus leukemia (GvL) or graft versus tumor (GvT) effect generated by donor lymphocytes that specifically recognize and eliminate malignant cells in the recipient [2]. However, after HSCT additional immune responses may occur against healthy tissues creating graft-versus-host disease (GvHD), an important contributor to transplant related morbidity and mortality [3]. To improve the feasibility of HSCT, it will be crucial to gain the ability to guide immune responses in the desired way maintaining anti-viral and anti-tumor responses while controlling undesired responses, namely GvHD.
Naturally occuring regulatory T cells (Treg) are responsible for maintaining peripheral self tolerance [4], but they may also play a role in the failure to control tumor growth as Treg cell depletion can facilitate tumor rejection [5], [6]. In the context of HSCT, Treg cells have been shown to control GvHD [7]–[9], while on the other hand preserving GvT reactions [10]. However, the strategies to control effector T or Treg cell activity might be relevant since stringent inhibition of both may result in poor tumor outcome [9]. In addition, current clinical protocols demonstrate the feasibility and safety of Treg cell transfer in humans [11], possibly opening Treg based treatment options for patients beyond experimental settings in the near future. Hence, it is important to understand the relevant mechanisms of Treg mediated suppression in HSCT.
Donor T cells are activated by conventional CD11c+ dendritic cells (DC) in the HSCT recipient [12]–[14]. In this context, the blockade of costimulatory molecules induces transplantation tolerance that is mediated by T cell anergy or Treg subpopulations depending on the particular model used [15]–[17]. In general, Treg mediated suppression occurs by cytokine independent [18]–[20], but contact dependent ways, i. e. via the glucocorticoid induced tumor necrosis factor receptor (GITR) [20], CTLA-4 or membrane-bound TGF-β [21]. Beyond this, the transfer of cyclic adenosine monophosphate (cAMP) to target cells via gap junction intercellular communication (GJIC) is a key mechanism of Treg mediated suppression [22], [23], also important in Treg-DC interaction that occurs by a direct contact (cAMP) dependent and by a contact independent pathway [24].
To examine the suppressive mechanisms utilized by Treg cells in the context of HSCT, we investigated a MHC mismatched mouse model of acute GvHD. We confirm an important role for Treg cells in ameliorating GvHD and show that Treg cells communicate with DC via a GJIC and a cAMP dependent mechanism, resulting in the cooperative suppression of allogeneic MLR using the cAMP elevating drugs/phosphodiesterase (PDE) inhibitors. Conversely, we observed cooperative amelioration of GvHD by Treg transfer and treatment with the PDE inhibitor rolipram. These results suggest that multimodal strategies combining Treg cellular transfer with cAMP modulating drug therapies may be a new treatment strategy for acute GvHD. This may contribute to future concepts in improving the feasibility and efficacy of HSCT.
Materials and Methods
Reagents
Biotinylated anti-CD25 (7D4) was purchased from BD (Heidelberg, Germany), PE- conjugated streptavidin was obtained from Dianova (Hamburg, Germany), anti-PE beads were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). The following additional mAbs were used: anti-CD3 mAb (145-2C11) and anti-CD28 mAb (37.51). If required, mAbs were affinity purified using protein G–Sepharose (GE Healthcare, Munich, Germany). Mouse recombinant IL-2 was affinity purified. Rolipram was purchased from Sigma-Aldrich (Taufkirchen, Germany), Cilostazol was obtained from Tocris Biosciences (Bristol, UK), BAY-60-7550 was purchased from Santa Cruz Biotechnology (Santa Cruz, USA).
Mice
C57BL/6, BALB/c, and B6.SJL (CD45.1+) mice were obtained from Charles River Laboratories and bred in a specific pathogen-free colony in the animal facility of the JGU Mainz. All animal procedures were performed in accordance with the institutional guidelines and approved by the responsible national authority (National Investigation Office Rheinland-Pfalz, Appoval ID: AZ 23 177-07/G07-1-009).
Cell Purification and Culture
Splenic DC from BALB/c mice were purified by density centrifugation as described previously [25] or bone marrow derived DC (BMDC) generated from bone marrow as described previously [26] and used as stimulators for allogeneic mixed lymphocyte reactions (MLR). Briefly, bone marrow from 6–8 weeks old mice was cultured in Iscovés medium supplemented with 5% fetal calf serum (FCS, inactivated at 56°C), 1% glutamine, 1% sodium pyruvate and GM-CSF (50 ng/ml). These cells were typically >85% CD11c+ as determined by flow cytometry. Furthermore, CD11c+ cells were CD80low. Medium was changed on days 2 and 4. Cells were used for the experiments at day 6 as immature DC.
Responder T cells from C57BL/6 splenocytes were purified by anti-CD90.2 conjugated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer’s instructions. Treg depletion of CD90.2+ T cells was performed with biotinylated anti-CD25 (7D4) and magnetic streptavidin-conjugated microbeads (Miltenyi Biotec).
Treg were isolated as described previously [22] using biotin-conjugated anti-CD25 mAb (7D4), PE-conjugated streptavidin and magnetic anti-PE microbeads (Miltenyi). CD25 sort was performed twice. CD25+-enriched Treg cells were additionally depleted from CD8+ T cells, B cells and macrophages using anti-CD8, anti-B220 and anti-MAC1 Dynabeads (Dynal Biotech, Hamburg, Germany). The purity of the resulting CD25+ Foxp3+ Treg cells was typically >95%. In some experiments, Treg cells were preactivated by a combination of plate-bound anti-CD3 mAb (145-2C11; 3 µg/ml) and anti-CD28 mAb (37.51; 3 µg/ml) in presence of 1000 U/ml IL-2 (Proleukine from Novartis, Nuremberg, Germany). Cells were splitted on day 3 without further anti-CD3/anti-CD28 stimulation and harvested on day 5 and used as preactivated Treg (pre Treg). In some experiments, Treg cells were labelled with calcein (1 µM, from Molecular Probes, Eugene, Oregon, USA) for 30 min. at 37°C, and washed twice in medium before use. Where indicated the GJIC inhibitor GAP27 (300 µM, from Biozol, Eching, Germany) was added [22].
For MLR, 2×105 CFDA-SE labelled (1 µM, from Molecular Probes, Eugene, Oregon, USA) responder T cells per well were added to DC and/or Treg in titrated ratios as indicated and cultured for 4 days in 96 well plates before cell harvest. Cells were washed, labelled with specific mAbs and analyzed for CFDA-SE dilution as indicator for proliferation by flow cytometry. Alternatively, where indicated activation of responder T cells in MLR was assessed by 3H-thymidine incorporation as follows: unlabeled C57BL/6 T cells (CD90.2+ T cells purifed by magnetic sorting) were added to the MLR culture. 3H-thymidine (0.5 µCi/well) was added on day 3 of the culture, cells were harvested 18 h later for assement of 3H-thymidine uptake by β scintillation counting.
Where indicated, DC or Treg were fixed after 4 h of coincubation as follows: Cells were washed twice in PBS and suspended in 0.1% glutaraldehyde (Sigma-Aldrich, St. Louis, USA) in PBS. After 30 seconds lysine (0.2 M) and FCS were added to quench the reaction. The cells were washed twice in medium and added to secondary cultures as indicated.
Flow Cytometric Analyses and Calcein Transfer
After coculture cells were harvested, washed and stained for 30 minutes with antibodies. The following mAbs were used for analyses by flow cytometry: CD8 (clone 53-6.7), CD11c (N418), CD80 (16-10A1), CD86 (GL1), CD40 (3/23), B7-H1 (MIH5), B7-DC (122), MHCII (M5/114.15.2), CD90.2 (53-2.1), CD45.1 (clone A20; all from eBiosciences or BD Pharmingen, Hamburg, Germany). Viability was determined by propidium iodide.
For calcein transfer assays, calcein labelled pre Treg and BMDC were cocultured in a ratio of 5∶1 for 4 hours and subsequently harvested for FACS analysis.
Intracellular cAMP levels were assessed by a cAMP specific mAb (SMP486, Abcam, Cambridge, UK) [27] labelled with fluorescein (Lightning-Link fluorescein conjugation kit, Innova Biosciences, Cambridge, UK). After 4 h of coculture of preTreg and BMDC, the cell surface was stained with anti-CD11c and anti-CD90.2, cells were fixed with 4% PFA and permeabilized with saponine and subsequently stained with fluorescein-labled anti-cAMP or an fluorescein-labled isotype control.
All analyses were performed with a FACSCanto or a LSRII flow cytometer and FACSDiva software (BD) or FlowJo (Tree Star Inc.).
Purification of DC after Cocultures
After 4 h of coculture of preTreg and DC, cells were separated by anti-CD11c microbeads (Miltenyi Biotec) according to the manufacturers instructions. Purity of CD11c+ MHCII+ double positive cells was assessed by flow cytometry and was >97%.
Cyclic AMP ELISA
To assess cytosolic cAMP concentrations, DC were washed three times in PBS, lysed in 0.1 N HCl (1×107/ml) and a cAMP specific ELISA was performed (Direct cAMP EIA kit, Enzo Life Sciences, Lörrach, Germany). The resulting cAMP concentration (pmol/ml) was calculated for 1×106 cells.
IL-12 ELISA
Splenic DC were stimulated in triplicate wells (96-well plate) for 2 days as indicated. The cell free supernatants were collected and frozen at −20°C until required. The concentration of IL-12p40/p70 was assessed in the supernatants by sandwich ELISA (obtained from BD Pharmingen), according to the manufacturer’s instructions.
Bone Marrow Transplantations
Mice were transplanted following a standard protocol. At day −1 recipient animals received total body irradiation (TBI, 850 cGy for BALB/c) from 137Cs source (OB58-BA, Buchler, Braunschweig, Germany). On day 0, allogeneic donor T cell depleted bone marrow cells (TCD-BM; 5×106 cells per animal) and 5×105 CD90.2+ T cells (BM/T) were transferred by intravenous injection. Where indicated Treg cells (5×105 cells per animal) were co-injected. The animals were maintained under specific pathogen free conditions and received antibiotics (Sulfadoxin-Trimethoprim 1 g/ml in the drinking water) post transplantation.
Bone marrow cells were isolated from femura and tibiae, erythrocytes were lysed, T cells were depleted by using magnetic anti-CD90.2 microbeads (Miltenyi Biotec). Cells were washed twice and injected suspended in PBS.
Assessment of GvHD
The degree of systemic GvHD was examined at least every other day using a scoring system as described elsewhere [28]. The scoring system includes 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity. The scoring system was modified as pointed out in Table 1 using four instead three categories for weight loss to reduce animal suffering. Animals with a score of 2 or greater were inspected daily. To further limit animal suffering, mice with severe symptoms of GvHD, such as severe weight loss, reduced activity, hunched posture and scrubby fur texture as determined by clinical scores equal or greater than 6, were immediately euthanized by CO2, as required by the institutional animal ethics guide lines and the day subsequent to death determined as the following day.
Table 1. GvHD clinical Score.
| Score | Weight loss | Skin | Activity | Posture | Fur texture |
| 0 | <5% | normal | normal | normal | normal |
| 1 | 5–10% | flaked | reduced | hunched | scrubby |
| 2 | 10–20% | explicit fur loss | inactive | severely hunched | severely scrubby |
| 3 | >20% |
Mice were evaluated for clinical signs of GvHD twice weekly according to Table 1 for each category. Individual scoring points were cumulated. Mice were sacrificed when exceeding a cumulative score ≥6.
Histology
Mice were sacrificed on day 10 after transplantation, fur on the back was removed mechanically and biopsies of the skin were taken, fixed in 4% buffered formalin, parafine wax embedded, sectioned and stained with haematoxylin and eosin according to standard protocols.
Statistical Analysis
Statistical analyses comparing two groups of assumed Gaussian distribution were performed by a two-tailed Student’s t-test using GraphPad Prism (version 5.0a for Mac OSX, GraphPad Software, San Diego California USA, www.graphpad.com). Alternatively, a Mann-Whitney U-test was used as indicated for other distributions. For differences in survival, the indicated groups were compared and analyzed by Mantel-Cox test. For all analyses, p<0.05 was considered significant.
Results
Regulatory T cells Suppress Graft-versus-Host Disease
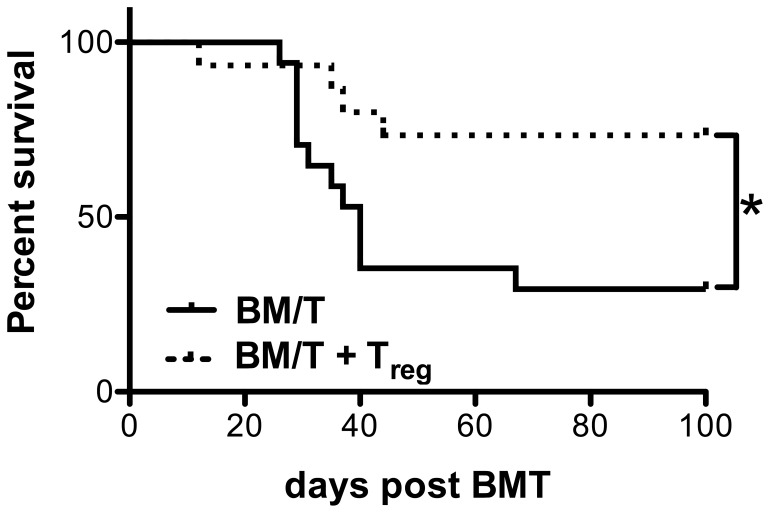
In HSCT, it has been clearly demonstrated that Treg cells can control GvHD [3], [7]. However, it is not clear how this is mediated in detail and multiple mechanisms of Treg mediated suppression have been described. To address this issue, we used a MHC mismatched HSCT model where BALB/c wild type hosts are lethally irradiated and transplanted with bone marrow and purified T cells from C57BL/6 mice. As depicted in Fig. 1, the animals succumbed to GvHD with a median survival of 40 days. Upon transfer of Treg cells, we observed protection from lethal GvHD in the transplanted animals (median survival not reached, p<0.001) which is consistent with previous reports [7].
Figure 1. Treg cells suppress GvHD.
BALB/c recipient mice were lethally irradiated (8,5 Gy) and received allogeneic C57BL/6 TCD-BM (5×106 cells) and Thy1.2+ T cells (5×105 cells, WT, black line, n = 17). A second group additionally received C57BL/6 Treg cells (5×105 cells, WT+WT Treg, broken line, n = 15). Survival was monitored for 100 days. Combined data from 3 independent experiments are shown. (*) indicates for P<0,05 according to a Mantel-Cox-Test.
Regulatory T cells Communicate with Allogeneic Dendritic Cells via Gap Junctions and Mediate a Suppressive Dendritic Cell Phenotype
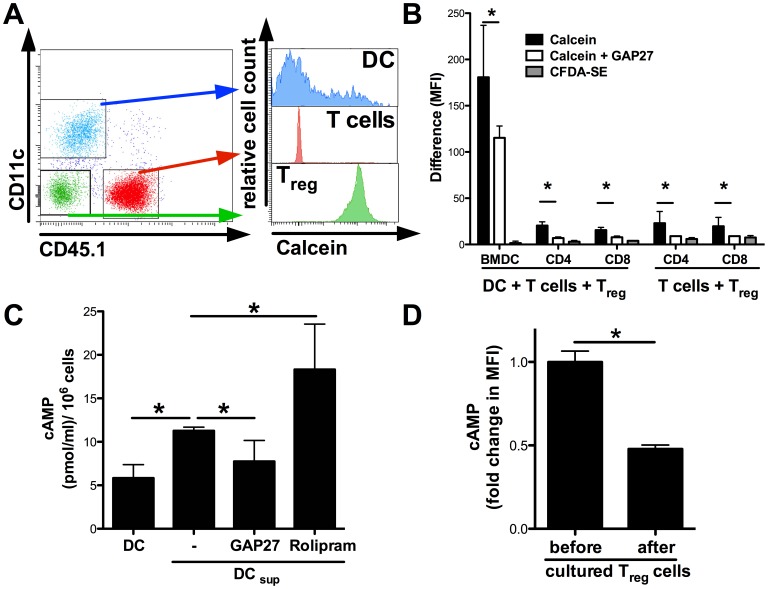
In our previous work, we have characterized the principle ability of Treg cells to inhibit T cell responses by suppression of DC activation. In continuation of this work, we were interested whether these mechanisms of Treg mediated inhibition of DC activation are also relevant for the suppression of allogeneic T cell activation and the initiation of GvHD. We and others have demonstrated before that Treg cells utilize a GJIC dependent pathway to suppress CD8+ T cell, CD4+ T cell and DC activation [22], [24], [29]. Therefore we asked for the relevant Treg interaction partners in the setting of alloreactive T cell activation. We used the fluorescent dye calcein that can be transferred from one cell to another only via GJIC [24], [30]. Treg cells were labelled with calcein and cocultured with DC and responder T cells for 4 hours. Subsequently, the amount of green fluorescent calcein was quantified in the lymphocyte or DC gate after exclusion of conjugates and doublets as illustrated in Fig. 2A. In the quantitative analysis (Fig. 2B), we detected a transfer of calcein from Treg to T cells. However, the relative amount transferred to DC was significantly higher. As a control, we co-incubated the cells with Treg cells that had been labelled with CFDA-SE, a green fluorescent dye that cannot be passed over from one cell to another by GJIC [29]. In this situation, we did not detect any transfer of fluorescence to DC or T cell populations (Fig. 2B, grey bars). The transfer of calcein was inhibited by the GJIC inhibitory peptide GAP27 (Fig. 2B, open bars) suggesting that Treg cells and DC truly communicate via GJIC also in the context of allogeneic T cell activation.
Figure 2. Treg cells communicate with allogeneic DC directly via cell-to-cell contact and GJIC dependent manner.
Bone marrow derived dendritic cells (BMDC from BALB/c mice, 3×104 per well) were cocultured with B6.SJL CD45.1+ T cells and C57BL/6 CD45.1− calcein-labelled pre Treg cells for 20 h in a 1∶1∶3 ratio. (A) Transfer of green fluorescent calcein after 20 h was analyzed by gating on CD45.1−CD11c+ DC, CD45.1−CD11c− Treg cells and CD45.1+CD11c− T cells, respectively. (B) Differences of median fluorescence intensity (MFI) among BMDC, CD4+ and CD8+ T cells was assessed. Where indicated Treg cells were preincubated with the gap junction inhibitor GAP27 (300 ng/ml). CFDA-SE (1 µM) labelled Treg cells were used as a control. (C) BALB/c BMDC were left untreated or cocultured with C57BL/6 pre Treg cells in an 1∶1 ratio in presence of soluble anti-CD3-mAb (3 µg/ml). Where indicated GAP27 (300 ng/ml) or rolipram (300 nM) were added. After 4 h suppressed DC (DC sup) were purified by CD11c specific MACS. Intracellular cAMP concentration was assessed by a specific ELISA after lysis of the cells. (D) Changes in cAMP levels after a 4 h coculture with BALB/c BMDC were mesured by flow cytometry in C57BL/6 preTreg. (*) indicates significant differences by Mann-Whitney U-test. The data shown are representative for 2 independent experiments in duplicate or triplicate wells.
To directly address whether this contact also affects the levels of intracellular cAMP in DC, we coincubated DC with Treg cells for 4 h and separated the cells afterwards by magnetic sorting. Subsequently, we used a cAMP specific ELISA to quantify the amounts of cAMP in DC [22], [24]. As depicted in Fig. 2C, DC harbour low levels of intracellular cAMP at baseline. Consistent with our previous results in the syngenic setting [24], cAMP levels in DC were significantly increased upon contact with Treg cells. Importantly, the increase in cAMP after contact with Treg cells was partly inhibited in the presence of the GJIC inhibitor GAP27 further supporting the relevance of a GIJC dependent pathway in this situation. Moreover, intracellular cAMP was even further increased in the presence of the PDE4 inhibitor rolipram. In addition, we analyzed the intracellular cAMP content in Treg cells before or after coincubation with DC by flow cytometry [27] and found that the cAMP levels in Treg cells were significantly decreased after coculture, further supporting the idea of cAMP transfer to DC (Fig. 2D).
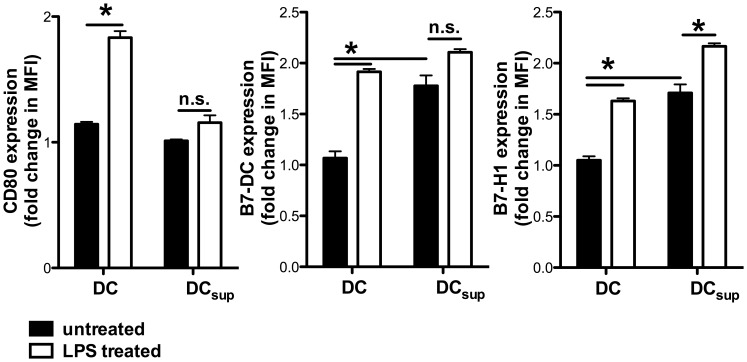
As a functional consequence of the contact with Treg cells, DC downregulate the costimulatory molecule CD80 while upregulating the inhibitory molecules B7-H1 and B7-DC (Fig. 3 and Fig. S1), as also demonstrated by us previously in a syngenic setting [24]. Taken together, our results suggest that Treg cells induce a suppressive DC phenotype in a cell contact dependent manner that involves cAMP.
Figure 3. Treg cells induce a suppressive DC phenotype.
BALB/c BMDC were left untreated or stimulated with LPS (100 ng/ml) and cocultured with C57BL/6 Treg cells in an 1∶1 ratio (DC sup). For optimal Treg stimulation, a soluble anti-CD3-mAb (3 µg/ml) was added. After 4 h expression of CD80, B7-H1 and B7-DC was determined by flow cytometry gating on CD11c+ MHCII+ cells. All depicted results were assayed in triplicate wells and are representative of three independent experiments. (*) indicates significant differences by Mann-Whitney test; n.s. – no significant differences.
Inhibition of Phosphodiesterases and Regulatory T cells Cooperatively Suppress Allogeneic Mixed Lymphocyte Reactions
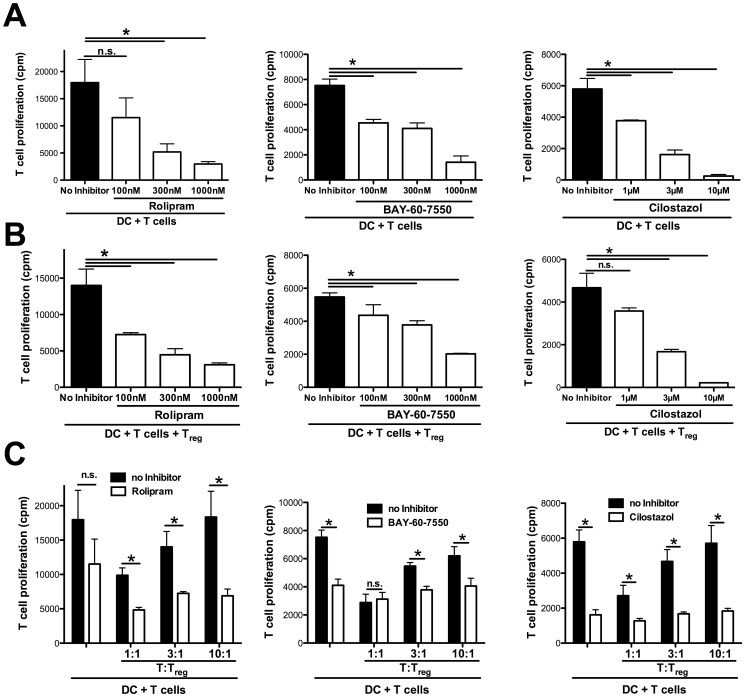
We have previously shown that the transfer of cAMP via GJIC from Treg to recipient cells is important for Treg mediated suppression. To enhance this metabolic pathway, the intracellular break down of cAMP may be blocked by inhibitors of PDEs, such as the PDE4 inhibitor rolipram which leads to an enhanced efficacy of Treg mediated suppression in vitro and also in vivo, as recently demonstrated in an asthma model [23]. Beyond PDE4, also other PDEs can affect Treg functionality as recently demonstrated by Feng and coworkers who find a pivotal role for the inhibition of PDE3 in allograft rejection [31].
Therefore we evaluated the role of inhibitors for PDE2 (BAY-60-7550), PDE3 (Cilostazol) or PDE4 (rolipram) in the suppression of allogeneic T cell responses in the presence or absence of Treg cells. As shown in Fig. 4A, the additional presence of each of these inhibitors suppressed MLR induced T cell proliferation in a concentration dependent manner, even when not adding Treg cells. Nevertheless, when Treg cells were added to the MLR, T cell proliferation was further suppressed, either titrating the respective inhibitors (Fig. 4B) or the number of Treg cells in the culture (Fig. 4C).
Figure 4. Rolipram enhances the suppressive capacities of Treg cells in vitro.
(A, B) BALB/c splenic DC (3×104 per well) were cocultured with allogeneic C57BL/6 Thy1.2+ T cells (1×105 per well) with titrated amounts of the PDE4 inhibitor rolipram (100 to 1000 nM), PDE2 inhibitor BAY-60-7550 (100 to 1000 nM) or PDE3 inhibitor Cilostazol (1 µM to 10 µM) for 3 days. Cell proliferation was determined after 3 days by 3H-thymidine incorporation. (B) DC (3×104 per well) were cocultured with allogeneic C57BL/6 Thy1.2+ T cells (1×105 per well) as in (A), but C57BL/6 Treg cells (3×104 per well) were added. (C) DC (3×104 per well) were cocultured with allogeneic C57BL/6 Thy1.2+ T cells (1×105 per well) and C57BL/6 Treg cells in indicated ratios for 3 days. Where indicated PDE4 inhibitor rolipram (100 nM), PDE2 inhibitor BAY-60-7550 (300 nM) or PDE3 inhibitor (3 µM) was added and proliferation was assessed after 3 days by 3H-thymidine incorporation. All depicted results were assayed in triplicate wells and are representative of three independent experiments. (*) indicates significant differences by Mann-Whitney U-test; n.s. – no significant differences.
Since we found Treg dependent as well as independent suppression of MLR by all tested PDE inhibitors, these results suggest that cAMP elevating drugs and Treg cells have cooperative effects despite the fact that PDE isoforms differentially expressed, i. e. PDE3b being highly repressed by Treg cells [32]. This indicates that these drugs mediate suppression of MLR by multiple mechanisms.
Rolipram Enhances Treg Function and Suppresses DC and Responder T cell Activation
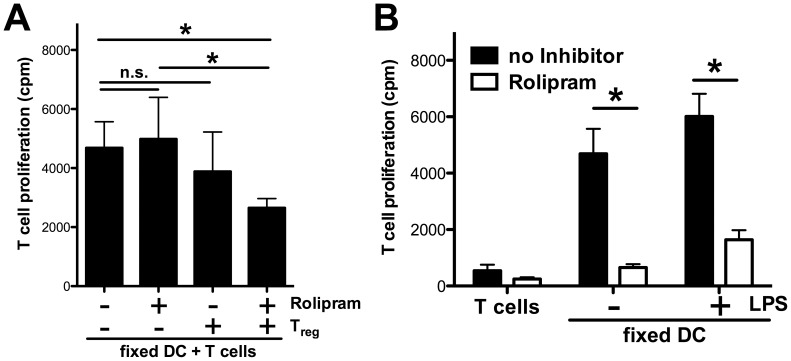
Because we observed similar effects by all PDE inhibitors, we decided to focus our further studies for the underlying mechanisms on the PDE4 inhibitor rolipram where we have observed cooperative effects with Treg cells in an asthma model [23]. We asked whether rolipram directly suppresses the activation of responder T cells or indirectly, i. e. by modulating the Treg/DC interaction. Firstly, we incubated DC in the presence or absence of rolipram and found that IL-12 production was diminished in escalating doses of rolipram, while we did not observe a significant impact on the activation (CD80, CD86 or CD40) phenotype or viability (Fig. S2). Next, we cocultured DC with allogeneic Treg cells in the additional absence or presence of rolipram and fixed the cells after 4 h with glutaraldehyde, as illustrated in Fig. S3A. Subsequently, the cells were cultured with allogeneic responder T cells, again in the absence or presence of rolipram. As shown in Fig. S3B, fixed DC induced the activation of allogeneic T cells, although markedly less than viable DC depicted in comparison. Nevertheless, preactivation of DC with the TLR4 agonist LPS resulted in a significant increase in T cell activation that was suppressed in the presence of Treg cells (Fig. S3C). It is important to notice that the 4 h coincubation period of viable DC with Treg before fixation was necessary and sufficient for the impairment of subsequent allogeneic T cell activation since coincubation of DC with fixed Treg cells had no impact on the MLR (Fig. S3D).
To further support a role for cAMP in this situation, we added the PDE4 inhibitor rolipram into the MLR culture. Under these stringent conditions, we did not observe any direct suppressive effect of rolipram on DC in terms of inhibition of T cell activation (Fig. 5A). Upon contact of DC with Treg alone, this brief incubation period also did not result in significant suppression of T cell activation. In contrast, only in the presence of rolipram and Treg cells the capability of DC to induce allogeneic T cell activation was significantly suppressed.
Figure 5. Rolipram suppresses MLR directly by inhibiting T cell prolifation and indirectly by modulating DC/Treg interaction.
(A) BALB/c BMDC were cultured in absence or presence of C57BL/6 Treg cells (1∶1 ratio) and rolipram (300 nM) for 4 h. Anti-CD3 (3 µg/ml) was added to every well. After fixation cells were cocultured with C57BL/6 Thy1.2+ T cells in a 10∶1 ratio for 3 days. Proliferation was determined by 3H-thymidine incorporation. (B) BALB/c BMDC were stimulated with LPS (100 ng/ml) for 4 h or left untreated and subsequently fixed with glutaraldehyde. C57BL/6 Thy1.2+ CD25− T cells were cocultured with LPS stimulated or unstimulated fixed BMDC (1×104 per well) for 3 days in a 10∶1 ratio. All depicted results were assayed in triplicate wells and are representative of three independent experiments. (*) indicates significant differences by Mann-Whitney U-test; n.s. – no significant differences.
To clarify whether rolipram also directly affects the activation of responder T cells, we coincubated fixed DC with allogeneic responder T cells in the additional presence or absence of rolipram demonstrating that rolipram also directly inhibited allogeneic T cell proliferation (Fig. 5B). These results indicate that rolipram suppresses MLR by at least three distinct mechanisms: the inhibition of IL-12 production by DC, the direct inhibition of allogeneic T cell proliferation and a Treg/DC dependent pathway involving cAMP that mediates a diminished alloreactive capacity of DC.
Rolipram and Regulatory T cells Cooperatively Ameliorate Acute Graft-versus-Host Diseases
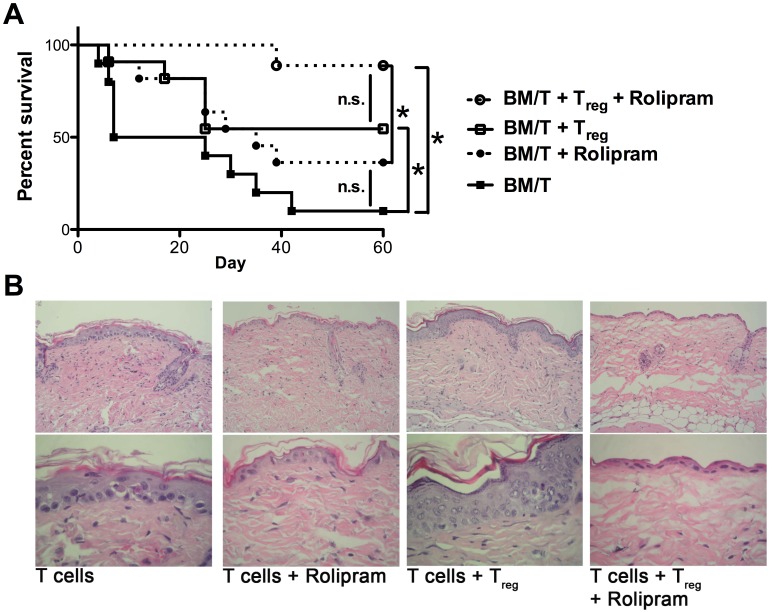
To see whether these results obtained by in vitro experiments are also relevant in vivo, we performed allogeneic BMT, similar to the above described experiments, in the absence or presence of Treg cells and additionally treated the mice with the PDE4 inhibitor rolipram. In contrast to the experiments shown in Fig. 1, we used C57BL/6 derived CD90.2+ T cells that had been Treg depleted using a CD25 specific antibody (clone 4D7) for transplantation in order to exclude rolipram driven effects on Treg cells present in the CD90.2+ T cell graft. As shown in Fig. 6A and Fig. S4, in mice transplanted with Treg depleted T cells alone we observed acute and lethal GvHD (filled squares, median survival 7 days) comparable to the experiments shown in Fig. 1. Upon the additional transfer of Treg cells (open squares, median survival 29 days), the onset of GvHD was delayed, but we observed an increased mortality (compare Fig. 1: median survival not reached), most likely due to a durable protective effect by an increased number of Treg cells in the initial (not CD25 depleted) transplantation setting. Interestingly, the mice treated with the PDE4 inhibitor rolipram alone showed a prolonged median survival (Fig. 6, filled circles, median survival 25 days). However, only the group of mice that received Treg and rolipram was almost completely protected from lethal GvHD (open circles, median survival not reached). Additional histology skin sections as a classical target organ showed typical GvHD findings, i.e. focal spongiosis of the epidermis associated with only scant lymphoid cell infiltration of the dermis and scattered apoptotic keratinocytes, that appeared less prominent in mice that had received Treg and rolipram (Fig. 6B).
Figure 6. Rolipram enhances suppressive capacities of Treg cells in vivo and ameliorates GvHD.
(A) BALB/c mice were lethally irradiated (8,5 Gy) and received either TCD bone marrow (5×106 cells) and Thy1.2+ CD25− T cells (5×105 cells) from C57BL/6 donors (n = 10, filled squares), Thy1.2+ CD25− T cells plus Treg cells (1∶1 ratio, n = 10, open squares), Thy1.2+ T cells plus rolipram (n = 10, filled circles) or Thy1.2+ T cells plus Treg cells and rolipram (n = 9, open circles). Rolipram (0.3 mg/kg) was injected i.p. on days 0 to 20 once per day. Results show the combined survival data from 2 independent experiments (* p<0.0005 by Mantel-Cox test). (B) Transplantation and rolipram treatment were performed as descibed above, but the mice were sacrificed on day 10. Representative skin sections from the back were stained with haematoxylin and eosin. The upper pictures are showing an overview (original magnification 200×), the lower pictures are showing a detailed view (original magnification 630×).
Collectively, these results confirm and extend the concept that Treg mediated suppression of T cell responses involves a cAMP dependent pathway, which can be manipulated by drugs like rolipram.
Discussion
Utilizing donor derived Treg cells to control GvHD has a great potential to decrease the morbidity and mortality after allogeneic HSCT. The basic capability of Treg cells for this has been demonstrated in experimental settings [7], [10], [11] and is confirmed by our results. However, along with current studies that provide evidence for the clinical feasibility of Treg based immunotherapy [11], it becomes increasingly urgent to clarify the relevant mechanisms of Treg mediated suppression in the context of GvHD in order to be able to define the risks, limits and potentials of such treatments.
Multiple Treg subpopulations and mechanisms of Treg mediated suppression have been described [33], [34]. Inducible Treg cells can suppress GvHD in an antigen specific manner, as recently demonstrated [35]. However, in our current work we followed up on previous observations demonstrating that a major mechanism of suppression by naturally occurring Treg cells is mediated by a pathway involving the transfer of cAMP via GJIC. Current concepts of GvHD priming highlight the role of host derived DC populations in the initiation phase of GvHD [3], [12], [13] and the interaction with Treg cells in this context [36]. Concerning the potential mechanisms of Treg mediated suppression in the context of GvHD, we have recently demonstrated a contact and cAMP dependent pathway of Treg mediated suppression of CD4+ T cells [22]. This metabolic pathway is also important for the suppression of DC activation as shown by us in vitro [24] as well as in vivo as demonstrated in a model for contact hypersensitivity [29] and asthma [23]. Therefore we sought to evaluate this cAMP dependent pathway in the context of allogeneic T cell activation. We found that indeed Treg cells preferentially make contact with DC and transfer the small fluorescent dye calcein as surrogate for cAMP to DC rather than directly to effector T cells. In line with this and consistent with our previous results obtained in the syngeneic setting [24], DC downregulate costimulatory molecules coincidently with elevated levels of cAMP that can be blocked in part by the GJIC inhibitor GAP27. The significance of this mechanism in suppression of GvHD is underpinned by Yi et al. demonstrating that the interaction of host APC with Treg cells via CD80/B7H1 is important for Treg expansion post HSCT [37]. To evaluate the functional contribution of cAMP in this situation, we used three different PDE inhibitors, all confirming previous results obtained with rolipram for enhanced cAMP levels and suppressive function of Treg cells in vitro and also in vivo [22], [23]. Apparently, all PDE inhibitors used had suppressive effects on MLR despite differential PDE expression patterns among T cell populations [32] and distinct specificity of the inhibitors arguing for a general effect by this class of inhibitors. However, our studies using viable or fixed DC as stimulators clearly show that rolipram affects three distinct mechanisms that may be relevant in the suppression of MLR: a direct effect inhibiting IL-12 production by DC, another direct effect on responder T cell proliferation and interestingly, also an indirect effect that enhances the suppressive effect of Treg cells. This particular mechanism is mediated by interfering with the activation of DC and their subsequent ability to prime alloreactive T cells in a cAMP dependent manner. We are unable to differentiate whether the elevated levels of cAMP in DC are truely achieved by the transfer via GJIC as suggested by our calcein assay and the decreased cAMP levels in suppressed DC in the presence of the GJIC inhibitor. On the other hand, the GJIC inhibitory peptide GAP27 can not inhibit all potential gap junction variants with equal efficiency [38]. Alternatively, Treg cells may trigger other surface receptors, such as A2A or A2B receptors that in turn activate cAMP dependent signalling pathways [39]. However, using the A2A receptor inhibitor SCH58261 in an MLR, we did not observe any enhancing or inhibitory effects on T cell proliferation (data not shown) arguing against a major role of this particular mechanism in the context of allogeneic T cell activation.
Conversely, our data allow the conclusion that Treg/DC communications require live cell interactions as fixed Treg cells are unable to confer the suppressive phenotype (Fig. S3D). Importantly, under our experimental conditions where Treg cells had been depleted from the T cell graft before transplantation (Fig. 6), we merely observed a moderate delay in the course of GvHD as illustrated by the survival plots when Treg cells were transplanted alone. The same is true for rolipram alone in this situation, suggesting that the pronounced direct effect of rolipram on responder T cell proliferation might not be the most relevant mechanism of rolipram in suppression of GvHD in vivo. On the contrary, the fact that we observe nearly full protection in the group of Treg transfer and rolipram suggests that a mechanism involving the cAMP dependent Treg/DC interaction is more important in the in vivo situation. However, we can not exclude off-target effects of rolipram in vivo, i.e. on PDE3 which is important for the induction of Treg cells in the context of allograft rejection [31]. Here, we are unable to differentiate whether rolipram affects transplanted Treg cells or mediates the induction of Treg cells which is a limitation of our study.
Never the less, our findings are corroborated by the recent work of Klein and coworkers who demonstrate the cAMP in human Treg cells is essential for Treg mediated suppression in general and also in the context of GvHD using a humanized mouse model [40]. Finally, our results fit well with previous data from O’Shaughnessy et al. who demonstrate that elevated cAMP levels in CD4+ T cells are protective in GvHD [41]. We can now extend this notion by showing that this protection involves the enhanced suppressive action of Treg cells indicating that one important pathway of Treg mediated suppression is via a metabolic pathway involving cAMP. This pathway can be modulated by drugs like the phosphodiesterase inhibitor rolipram.
Taken together, our results provide the basis for an advanced understanding of Treg mediated suppression of GvHD that may allow the establishment of combined approaches incorporating adoptive immunotherapy with targeted drug treatments to overcome the current limitations of allogeneic HSCT.
Supporting Information
Treg cells induce a suppressive DC phenotype. (A) Gating strategy to discriminate between BMDC and pre Treg after coculture. (B) BALB/c BMDC were left untreated (DC, filled grey area) or cultured with C57BL/6 pre Treg cells (DCsup, solid black line) in a 1∶1 ratio. Where indicated LPS (100 ng/ml) was added to the culture (solid black line). For optimal pre Treg stimulation soluble anti-CD3-mAb (3 µg/ml) was added. After 4 h expression of CD80, B7-H1 and B7-DC was determined by flow cytometry.
(TIFF)
Rolipram affects LPS induced IL-12 production by DC, but not the activation phenotype. BALB/c splenic DC were left untreated (black bars) or stimulated with LPS (100 ng/ml, white bars) over night in presence of different concentrations of the PDE4 inhibitor rolipram (100 to 1000 nM). (A, B, C) Expression of the activation markers CD80, CD86 and CD40 was assessed by flow cytometry. (D) Release of IL-12 into the media was measured by a specific ELISA after 2 days of incubation. (E) Viability of the DC was determined after over night incubation by staining with propidium iodide (PI) and flowcytometric determination of the percentage of PI negative cells.
(TIFF)
Fixed BMDC are sufficient stimulators in MLR. (A) Scheme of the experimental setup. (B) C57BL/6 Thy1.2+ T cells were left untreated, cocultured with fixed BALB/c BMDC or viable BALB/c BMDC (1×104 per well) in a 10∶1 ratio for 3 days. Proliferation was determined by 3H-thymidine incorporation. (C) BALB/c BMDC were stimulated with LPS (100 ng/ml) in absence or presence of C57BL/6 pre Treg cells in a 1∶1 ratio for 4 h with soluble anti-CD3 (3 µg/ml). After fixation cells were cultured with viable C57BL/6 Thy1.2+ T cells in a 10∶1 T/DC ratio for 3 days. Proliferation was determined by 3H-thymidine incorporation. (D) BALB/c BMDC were left alone or cocultured with C57BL/6 Treg in a 1∶1 ratio for 4 h with soluble anti-CD3 (3 µg/ml). Without separation the cells were subsequently fixed and used as stimulators for C57BL/6 T cells (T/DC 10∶1) for 3 days. As a additional control C57BL/6 T cells were stimulated with viable BALB/c DC alone or together with fixed C57BL/6 Treg in the same ratios as stated above. Proliferation was determined by 3H-thymidine incorporation. All depicted results were assayed in six replicate wells and are representative for two independent experiments. (*) indicates significant differences by Mann-Whitney test. n.s. – no significant differences.
(TIFF)
Rolipram enhances suppressive capacities of Treg cells in vivo . BALB/c mice were lethally irradiated (8,5 Gy) and received either TCD bone marrow (5×106 cells) and Thy1.2+ CD25− T cells (5×105 cells) from C57BL/6 donors (n = 10, filled squares), Thy1.2+ T cells plus Treg cells (1∶1 ratio, n = 10, open squares), Thy1.2+ T cells plus rolipram (n = 10, filled circles) or Thy1.2+ T cells plus Treg cells and rolipram (n = 9, open circles). Rolipram (0,3 mg/kg) was injected i.p. on days 0 to 20 once a day. Results show the combined scoring data evaluated according to the clinical scoring system from 2 independent experiments.
(TIFF)
Acknowledgments
The authors express their gratitude to Annekatrin Klaric, Andrea Drescher and Giusy Carlino for excellent technical assistance.
Funding Statement
This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.P.R. and H.S. (Ra988/4-2), SFB TR52 TPA1 (T.B. and E.S.), the GRK 1043 International Graduate School of Immunotherapy (E.S. and T.B.), “Forschungszentrum Immunologie (FZI)” of the University medical center (H.S., E.S. and T.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Doehner H, Estey EH, Amadori S, Appelbaum FR, Buechner T, et al. (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115: 453–474 doi:10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2. KOLB H, SCHATTENBERG A, GOLDMAN J, HERTENSTEIN B, JACOBSEN N, et al. (1995) Graft-Versus-Leukemia Effect of Donor Lymphocyte Transfusions in Marrow Grafted Patients. Blood 86: 2041–2050. [PubMed] [Google Scholar]
- 3. Ferrara JLM, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373: 1550–1561 doi:10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakaguchi S (2004) Naturally Arising CD4+ Regulatory T Cells for Immunologic Self-Tolerance and Negative Control of Immune Responses. Ann Rev Immunol 22: 531–562. [DOI] [PubMed] [Google Scholar]
- 5. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, et al. (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59: 3128–3133. [PubMed] [Google Scholar]
- 6. Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, et al. (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 194: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 196: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vela-Ojeda J, Montiel-Cervantes L, Granados-Lara P, Reyes-Maldonado E, García-Latorre E, et al. (2010) Role of CD4+CD25+ highFoxp3+CD62L+ Regulatory T Cells and Invariant NKT Cells in Human Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells and Development 19: 333–340 doi:10.1089/scd.2009.0216. [DOI] [PubMed] [Google Scholar]
- 9. Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, et al. (2006) Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108: 390–399 doi:10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, et al. (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9: 1144–1150 doi:10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 11. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117: 1061–1070 doi:10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, et al. (1999) Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285: 412–415. [DOI] [PubMed] [Google Scholar]
- 13. Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, et al. (2004) Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol 172: 7393–7398. [DOI] [PubMed] [Google Scholar]
- 14. Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, et al. (2002) Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med 8: 575–581 doi:10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 15. Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, et al. (2004) Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood 103: 4336–4343 doi:10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 16. Zhai Y, Meng L, Gao F, Wang Y, Busuttil RW, et al. (2006) CD4+ T regulatory cell induction and function in transplant recipients after CD154 blockade is TLR4 independent. J Immunol 176: 5988–5994. [DOI] [PubMed] [Google Scholar]
- 17. Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M (2005) Early regulation of CD8 T cell alloreactivity by CD4+CD25- T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol 35: 2679–2690 doi:10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 18. Fahlen L (2005) T cells that cannot respond to TGF- escape control by CD4+CD25+ regulatory T cells. J Exp Med 201: 737–746 doi:10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kearley J, Barker JE, Robinson DS, Lloyd CM (2005) Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 202: 1539–1547 doi:10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McHugh R, Whitters M, Piccirillo C, Young D, Shevach E, et al. (2002) CD4(+)CD25(+) immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16: 311–323. [DOI] [PubMed] [Google Scholar]
- 21. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, et al. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322: 271–275 doi:10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 22. Bopp Becker, Klein Klein-Heßling, Palmetshofer, et al (2007) Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 204: 1303–1310 doi:10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, et al. (2009) Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol 182: 4017–4024 doi:10.4049/jimmunol.0803310. [DOI] [PubMed] [Google Scholar]
- 24. Fassbender M, Gerlitzki B, Ullrich N, Lupp C, Klein M, et al. (2010) Cyclic adenosine monophosphate and IL-10 coordinately contribute to nTreg cell-mediated suppression of dendritic cell activation. Cell Immunol 265: 91–96 doi:10.1016/j.cellimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 25. Ruedl C, Rieser C, Böck G, Wick G, Wolf H (1996) Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur J Immunol 26: 1801–1806 doi:10.1002/eji.1830260821. [DOI] [PubMed] [Google Scholar]
- 26. Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, et al. (2006) Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 108: 544–550 doi:10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 27. Vaeth M, Schliesser U, Müller G, Reissig S, Satoh K, et al. (2012) Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 109: 16258–16263 doi:10.1073/pnas.1203870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rowe V, Banovic T, MacDonald KP, Kuns R, Don AL, et al. (2006) Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood 108: 2485–2492 doi:10.1182/blood-2006-04-016063. [DOI] [PubMed] [Google Scholar]
- 29. Ring S, Karakhanova S, Johnson T, Enk AH, Mahnke K (2010) Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8(+) T cells. J Allergy Clin Immunol 125: 237–246.e237 doi:10.1016/j.jaci.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 30. Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, et al. (2005) Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434: 83–88 doi:10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 31. Feng GG, Nadig SNS, Bäckdahl LL, Beck SS, Francis RSR, et al. (2011) Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Science Translational Medicine 3: 83ra40–83ra40 doi:10.1126/scitranslmed.3002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, et al. (2007) Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445: 771–775 doi:10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 33. Bluestone JA, Abbas AK (2003) Opinion-Regulatory Lymphocytes: Natural versus adaptive regulatory T cells. Nat Rev Immunol 3: 253–257 doi:10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 34. Shevach EM (2006) From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25: 195–201 doi:10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35. Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM (2011) Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med 208: 2489–2496 doi:10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tawara I, Shlomchik WD, Jones A, Zou W, Nieves E, et al.. (2010) A Crucial Role for Host APCs in the Induction of Donor CD4+CD25+ Regulatory T Cell-Mediated Suppression of Experimental Graft-versus-Host Disease. J Immunol. doi:10.4049/jimmunol.1001625. [DOI] [PMC free article] [PubMed]
- 37. Yi T, Li X, Yao S, Wang L, Chen Y, et al. (2011) Host APCs Augment In Vivo Expansion of Donor Natural Regulatory T Cells via B7H1/B7.1 in Allogeneic Recipients. The Journal of Immunology 186: 2739–2749 doi:10.4049/jimmunol.1002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans WH, Boitano S (2001) Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 29: 606–612. [DOI] [PubMed] [Google Scholar]
- 39. Sitkovsky MV (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 30: 102–108 doi:10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40. Klein M, Vaeth M, Scheel T, Grabbe S, Baumgrass R, et al. (2012) Repression of cyclic adenosine monophosphate upregulation disarms and expands human regulatory T cells. The Journal of Immunology 188: 1091–1097 doi:10.4049/jimmunol.1102045. [DOI] [PubMed] [Google Scholar]
- 41. O'Shaughnessy MJ, Chen Z-M, Gramaglia I, Taylor PA, Panoskaltsis-Mortari A, et al. (2007) Elevation of intracellular cyclic AMP in alloreactive CD4(+) T Cells induces alloantigen-specific tolerance that can prevent GVHD lethality in vivo. Biol Blood Marrow Transplant 13: 530–542 doi:10.1016/j.bbmt.2007.01.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treg cells induce a suppressive DC phenotype. (A) Gating strategy to discriminate between BMDC and pre Treg after coculture. (B) BALB/c BMDC were left untreated (DC, filled grey area) or cultured with C57BL/6 pre Treg cells (DCsup, solid black line) in a 1∶1 ratio. Where indicated LPS (100 ng/ml) was added to the culture (solid black line). For optimal pre Treg stimulation soluble anti-CD3-mAb (3 µg/ml) was added. After 4 h expression of CD80, B7-H1 and B7-DC was determined by flow cytometry.
(TIFF)
Rolipram affects LPS induced IL-12 production by DC, but not the activation phenotype. BALB/c splenic DC were left untreated (black bars) or stimulated with LPS (100 ng/ml, white bars) over night in presence of different concentrations of the PDE4 inhibitor rolipram (100 to 1000 nM). (A, B, C) Expression of the activation markers CD80, CD86 and CD40 was assessed by flow cytometry. (D) Release of IL-12 into the media was measured by a specific ELISA after 2 days of incubation. (E) Viability of the DC was determined after over night incubation by staining with propidium iodide (PI) and flowcytometric determination of the percentage of PI negative cells.
(TIFF)
Fixed BMDC are sufficient stimulators in MLR. (A) Scheme of the experimental setup. (B) C57BL/6 Thy1.2+ T cells were left untreated, cocultured with fixed BALB/c BMDC or viable BALB/c BMDC (1×104 per well) in a 10∶1 ratio for 3 days. Proliferation was determined by 3H-thymidine incorporation. (C) BALB/c BMDC were stimulated with LPS (100 ng/ml) in absence or presence of C57BL/6 pre Treg cells in a 1∶1 ratio for 4 h with soluble anti-CD3 (3 µg/ml). After fixation cells were cultured with viable C57BL/6 Thy1.2+ T cells in a 10∶1 T/DC ratio for 3 days. Proliferation was determined by 3H-thymidine incorporation. (D) BALB/c BMDC were left alone or cocultured with C57BL/6 Treg in a 1∶1 ratio for 4 h with soluble anti-CD3 (3 µg/ml). Without separation the cells were subsequently fixed and used as stimulators for C57BL/6 T cells (T/DC 10∶1) for 3 days. As a additional control C57BL/6 T cells were stimulated with viable BALB/c DC alone or together with fixed C57BL/6 Treg in the same ratios as stated above. Proliferation was determined by 3H-thymidine incorporation. All depicted results were assayed in six replicate wells and are representative for two independent experiments. (*) indicates significant differences by Mann-Whitney test. n.s. – no significant differences.
(TIFF)
Rolipram enhances suppressive capacities of Treg cells in vivo . BALB/c mice were lethally irradiated (8,5 Gy) and received either TCD bone marrow (5×106 cells) and Thy1.2+ CD25− T cells (5×105 cells) from C57BL/6 donors (n = 10, filled squares), Thy1.2+ T cells plus Treg cells (1∶1 ratio, n = 10, open squares), Thy1.2+ T cells plus rolipram (n = 10, filled circles) or Thy1.2+ T cells plus Treg cells and rolipram (n = 9, open circles). Rolipram (0,3 mg/kg) was injected i.p. on days 0 to 20 once a day. Results show the combined scoring data evaluated according to the clinical scoring system from 2 independent experiments.
(TIFF)