Abstract
Background
There are four cell lineages derived from intestinal stem cells that are located at the crypt and villus in the mammalian intestine the non-secretory absorptive enterocytes, and the secretory cells, which include mucous-secreting goblet cells, regulatory peptide-secreting enteroendocrine cells and antimicrobial peptide-secreting Paneth cells. Although fibroblast growth factor (Fgf) signaling is important for cell proliferation and differentiation in various tissues, its role in intestinal differentiation is less well understood.
Methodology/Principal Findings
We used a loss of function approach to investigate the importance of Fgf signaling in intestinal cell differentiation in zebrafish; abnormal differentiation of goblet cells was observed when Fgf signaling was inhibited using SU5402 or in the Tg(hsp70ldnfgfr1-EGFP) transgenic line. We identified Fgfr2c as an important receptor for cell differentiation. The number of goblet cells and enteroendocrine cells was reduced in fgfr2c morphants. In addition to secretory cells, enterocyte differentiation was also disrupted in fgfr2c morphants. Furthermore, proliferating cells were increased in the morphants. Interestingly, the loss of fgfr2c expression repressed secretory cell differentiation and increased cell proliferation in the mibta52b mutant that had defective Notch signaling.
Conclusions/Significance
In conclusion, we found that Fgfr2c signaling derived from mesenchymal cells is important for regulating the differentiation of zebrafish intestine epithelial cells by promoting cell cycle exit. The results of Fgfr2c knockdown in mibta52b mutants indicated that Fgfr2c signaling is required for intestinal cell differentiation. These findings provide new evidences that Fgf signaling is required for the differentiation of intestinal cells in the zebrafish developing gut.
Introduction
In adult mammals, the epithelium of the small intestine comprises two structures: finger-like villi and pocket-like crypts of Lieberkühn. Intestinal stem cells are located at the bottom of the crypt. Crypts also contain transit amplifying progenitor cells. These proliferating cells differentiate, then migrate to villi and are removed at the top of the villi by apoptosis. There are four cell lineages that derive from intestinal stem cells: the non-secretory absorptive enterocytes, and secretory cells, which include mucous-secreting goblet cells, regulatory peptide-secreting enteroendocrine cells, and antimicrobial peptide-secreting Paneth cells [1], [2], [3], [4]. It has been reported that, unlike mammals, zebrafish do not possess crypts of Lieberkühn or Paneth cells [5].
Many signaling molecules regulate stem cell self-renewal, proliferation, and differentiation in the intestines [6], [7]. The Wnt pathway is important in controlling crypt cell proliferation. The crypt precursors of Tcf4 null mice exhibit decreased cell proliferation, and comprise various differentiated cells [8]. However, in mice that lack Apc expression (APCmin), crypt cells exhibit greater proliferation than they do in Tcf4 null mice, and in the deficient mice, these cells only differentiate to form Paneth cells [9], [10]. In Apc mutant zebrafish (Apcmcr), the enterocyte differentiation marker, intestinal fatty acid binding protein (ifabp), was failed to express [11], [12]. In bone morphogenetic protein receptor 1a (Bmpr1a) mutant mice or Noggin transgenic mice, the expansion of proliferating cells in the crypt results in intestinal polyposis [13], [14]. Three secretory cells are also reduced in Bmpr1a mutant mice [15]. Interestingly, Wnt signaling is highly activated in these Bmp pathway deficient mice. Additionally, Notch signaling is important for cell lineage commitment and proliferation. Notch1 and Notch2 double knockout mice exhibit complete conversion of proliferating crypt progenitors into post-mitotic goblet cells [16]. In deltaD (aeiAR33) and mind bomb (mibta52b) mutation zebrafish, secretory cells are also overproduced [17]. Interestingly, concerted activation of Notch and Wnt pathways are required for the maintenance of undifferentiated and proliferative cells in crypts. Hes1 is highly expressed in undifferentiated cells of Apcmin mice. Notch signaling inhibitor can induce reduction in the number of proliferated cells and increase differentiation into goblet cells in Apcmin mice [18].
Fibroblast growth factor (Fgf) signaling is involved in intestinal development and cell differentiation. There are 22 Fgfs and 4 Fgfrs in mice [19], [20]. Fgfr1∼3 has two isoforms, b and c, which result from alternative splicing. These two isoforms have different ligand-binding specificities [21]. Fgf10 signaling is required, in a dose-dependent manner for the survival and proliferation of colonic epithelia progenitor cells [22]. Overexpression of Fgf10 can attenuate stomach and duodenum cell differentiation [23], [24]. Goblet cells, but not Paneth cells or enteroendocrine cells, were increased in recombinant FGF7 protein treated rats [25]. Furthermore, the depth of the crypt and the numbers of proliferating cells were increased in Fgfr3 deficient mice but villi length and the distribution of differentiated intestinal cells were unaffected [26]. Nevertheless, a recent report indicated that Paneth cell differentiation is reduced in Fgfr3 deficient mice [27]. These evidences suggest that the Fgf signaling pathway has a regulatory role in cell differentiation in the gastrointestinal tract. However, few reports address how Fgf signaling controls intestinal cell differentiation.
Zebrafish offer many advantages for studying intestinal cell differentiation, they have rapid development and transparent embryos, and techniques for the manipulation their gene expression are well-established. Furthermore, the early-stage development of the zebrafish gastrointestinal tract has been well described [5], [28], [29]. Using Tg(hsp70l:dnfgfr1-EGFP) transgenic fish to inhibit Fgf signaling [30], we found that heat-shock treatments inhibited the differentiation of secretory cells. We further found that Fgfr2c signaling controls the differentiation of secretory and absorptive cells by regulation of progenitor cell proliferation. These findings provide new evidence that Fgf signaling is required for the differentiation of intestinal cells in the zebrafish developing gut.
Materials and Methods
Ethics Statement
All embryos were handled according to protocols approved by the Institutional Animal Care and Use Committee of Tzu Chi University, Hualien, Taiwan (approval ID: 99056).
Zebrafish
Zebrafish (Danio renio) were raised as described in the Zebrafish Book [31]. The AB wild type (WT) strain was used for morpholino injection and other experiments. The transgenic and mutant fish lines used in this study were Tg(hsp70l:dnfgfr1-EGFP)pd1, provided by Taiwan Zebrafish Core Facility at Academia Sinica, TZCAS, and mibta52b, respectively.
SU5402 Treatment and Heat Induction Experiments
SU5402 (Calbiochem, UK) was dissolved into DMSO. Three days post fertilization (dpf) embryos were placed in a 6-well plate and SU5402 solution (2.5 or 3.4 µM) was added. We used 0.025% DMSO solution as control. At 4 dpf, the solution was replaced with fresh solution of the same concentration. All treated embryos were harvested at 5 dpf.
Water was pre-warmed to 37°C. Zebrafish embryos received a single 37°C heat-shock treatment for one hour in an air incubator, and the water then left to cool to 28°C.
Microinjection
All morpholinos (MOs) were obtained from Gene Tools, LLC. MOs targeting fgfr2b, fgfr2c, fgfr4, and with a 5-base mismatch for fgfr2c MO were used in this study. MOs sequences were as follows: fgfr2b MO 5′-CGCTCCTGCTTTTTTACCTGGTATG-3′, fgfr2c MO 5′-AAGCAGTGGAAGGTGAGTTTATACC-3′, 5-base mismatch for fgfr2c MO 5′-AAcCAcTGcAAGGTcAcTTTATACC-3′ [32]; fgfr4-I1E2 MO 5′-ATATCTGCTGGAGTAAAAAATGAGG-3′ [33]. MOs (4 ng) were injected into 1–2 cell stage embryos.
RT-PCR and qPCR Analyses
The whole gut was collected at 5 dpf using #5 forceps for RNA extractions. Total RNA was extracted using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA). DNase-I treated total RNA (5 µg) was used to generate cDNA using an ImProm-II kit (Promega, Madison, WI, USA). PCR primers used in this study are listed in Table S1.
For qPCR, reverse transcription reaction was performed using a GoScript kit (Promega, Madison, WI, USA). Expression level of pepT1 was detected according to the published primer sequences [34]. Expression of β-actin1 was detected as an internal control [35]. The primer sequence for pepT1 was F-AACACAAACATCAAGCAAACC and R-AACTACCAACCCTCAAGCCC. The primer sequence for β-actin1 was F-GCTGTTTTCCCCTCCATTGTT and R-TCCCATGCCAACCATCACT. The Maxima SYBR Green qPCR Master Mix (Fermentas International Inc., Ontario, Canada) system was used to quantify the transcripts.
Whole Mount in situ hybridization
The following in situ probes were used: agr2, glucagon, and pepT1 [34]; fgfr1–4 [36], and ifabp. The ifabp fragment was amplified by PCR and subcloned into pGEM-T vector (Promega). The DIG-labeled probes were generated by in vitro transcription using a DIG RNA labeling kit (Roche). For whole mount in situ hybridization, DIG-labeled probes were used to hybridize the embryos overnight at 65°C, and were then washed under high stringency condition. Embryos were treated with blocking buffer (Roche) and incubated with AP-conjugated anti-DIG antibody overnight at 4°C (1∶2000, Roche). Embryos were washed to remove excess antibody and then colored with NBT/BCIP (Roche).
Whole Mount Wheat Germ Agglutinin (WGA), Immunofluorescence Staining and BrdU Labeling
For WGA staining, embryos were fixed in 4% paraformaldehyde at 4°C overnight, and the yolks were removed before staining. Fixed embryos were washed three times for 5 min with PBST (containing 0.3% TritonX-100) and buffer B (10 mM HEPES and 0.15 M NaCl, pH = 7.5), and then incubated with rhodamine conjugated WGA (Vector Laboratories, 1∶100, in buffer B) at 4°C overnight. After washing with PBST (four times for 30 min), embryos were mounted in SlowFade Gold antifade reagent with DAPI (Molecular Probes, S-36938). We counted the WGA positive cells for all the embryos using ImageJ software.
For WGA and 2F11 double labeling, embryos were blocked in PBST, containing 4% BSA, for one hour after WGA staining. Embryos were then incubated in mouse monoclonal 2F11 antibody (1∶1000, a gift from Dr. Julian Lewis) at 4°C overnight. After washing with PBST as described above, embryos were incubated in goat anti-mouse IgG Alexa Fluor 488 (Molecular Probes, A-11029, 1∶100) at 4°C overnight. After washing with PBST, embryos were rinsed with PBS three times, and the embryos were nuclear counterstained with TOPRO-3 (Molecular Probes, T-3605, 1∶1000). Embryos were mounted in SlowFade Gold antifade reagent with DAPI.
For histone H3-phosphorylated (H3P) staining, we used H3P antibody (Santa Cruz, sc-8656-R, 1∶200) and goat anti-rabbit IgG Hilyte Fluor 488 (AnaSpec, 1∶100) or goat anti-rabbit IgG Alexa Fluor 546 (Molecular Probes, A-11035, 1∶200). Before blocking, embryos were rinsed twice with sodium citrate buffer (10 mM, pH = 6). Embryos then were incubated in citrate buffer at 95°C for 5 min.
For BrdU labeling, 3 or 5 dpf embryos were incubated in 10 mM BrdU solution at 6°C for 30 min. Embryos were then rinsed five times and incubated with Ringer’s solution (0.116 M NaCl, 1.8 mM CaCl2, 2.9 mM KCl, and 5 mM HEPES) at 28°C for 1 h. Antigen retrieval was performed using 2 N HCl at 37°C for 20 min. Monoclonal anti-BrdU antibody (Sigma, B2531; 1∶200) was used to detect labeled cells. Goat anti-mouse IgG Alexa Fluor 488 antibody was used as described above.
For counting cell number, longitudinal confocal images, including esophagus to anus, were collected using a LEICA TCS SP2 AOBS confocal microscope. Stacked images were analyzed with ImageJ software Plugin (Cell Counter). The WGA, 2F11, BrdU, and H3P positive cells in intestinal epithelial layer were counted manually using this software. The two-tailed Student’s t-test was used for analyzing changes in cell number.
Whole Mount TUNEL Assay
Fixed embryos with their yolk removed were incubated with proteinase K (100 µg/ml) at 28°C for 35 min. After rinsing with PBS, embryos were incubated with TUNEL reaction mixture from an In Situ Cell Death Detection Kit, TMR red (Roche) at 37°C for 1 h. Labeled embryos were washed with PBST four times for 15 min. Nuclear counterstaining and mounting was performed as described above.
Results
Secretory Cells were Reduced after Inhibition of Fgf Signaling
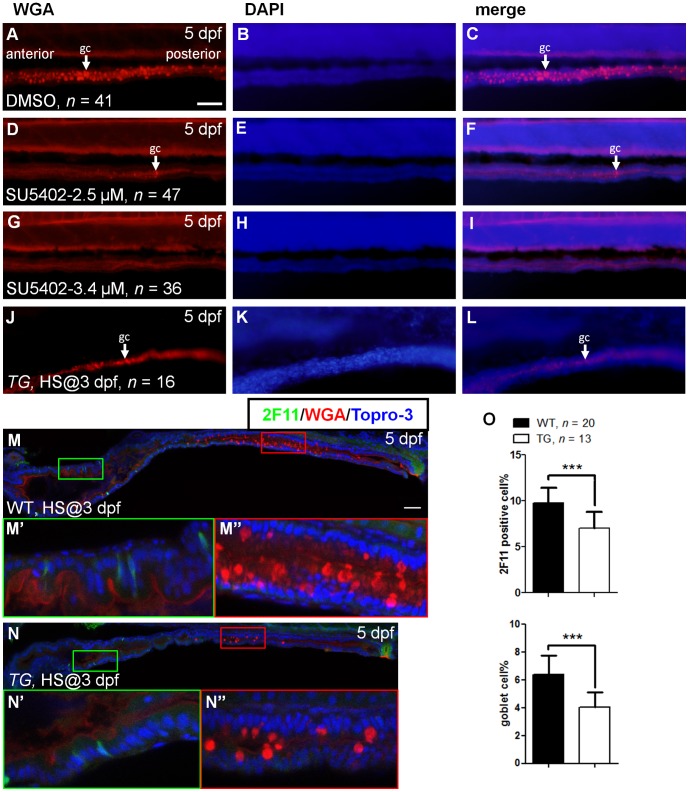
To determine the role of Fgf signaling in the differentiation of zebrafish intestinal cells, we treated wild type embryos with SU5402 and used the Tg(hsp70l:dnfgfr1-EGFP) fish line to inhibit Fgf signaling activity, respectively. In zebrafish embryos, intestinal tract compartmentalizes into three segments: intestinal bulb, mid-intestine, and posterior intestine. The mucin-secreting goblet cells are presented in the mid-intestine at 5 dpf [5]. We first analyzed the differentiation of goblet cells by using rhodamine conjugated WGA to stain the cells’ mucin [28]. In 5 dpf control embryos, goblet cells could be detected at the mid-intestine (Fig. 1A–1C, 93.0±3.4 goblet cells/embryo). When embryos were treated with SU5402 (2.5 µM) from 3 dpf, goblet cells were greatly reduced in number (Fig. 1D–1F, 3.3±0.9 goblet cells/embryo) at 5 dpf. There was a dosage dependent effect after treatment at the greater dose of 3.4 µM SU5402 (Fig. 1G–1I, 0.7±0.2 goblet cells/embryo). In addition to pharmacological inhibition, we also provided a genetic evidence using Tg(hsp70l:dnfgfr1-EGFP) fish line, which is widely used to block all Fgf signaling [30], [37], [38], [39], [40], [41], [42]. We have used this fish line to study Fgf signaling in liver homeostasis [43]. The Tg(hsp70l:dnfgfr1-EGFP) fish line showed a similar response when embryos were heat treated at 37°C for 1 hour at 3 dpf, and analyzed at 5 dpf. Goblet cells were greatly reduced in heat treated embryos (Fig. 1J–1L).
Figure 1. Effects on cell differentiation after inhibition of Fgf signaling.
Five dpf embryos were stained with WGA after incubation in (A–C) DMSO, (D–F) SU5402 (2.5 µM), and (G–I) SU5402 (3.4 µM) at 3 dpf. (J–L) Three dpf Tg(hsp70l:dnfgfr1-EGFP) embryos were heat treated, then stained with WGA at 5 dpf. White arrow indicated the goblet cell (gc). DAPI nuclear counter stain showed the tissue structure. (M) Heat-treated WT embryos were used as controls. (N) Heat shocked transgenic embryos were double labeled with WGA and 2F11 antibody at 5 dpf. (M’,N’) The magnified image shows enteroendocrine and (M’’,N’’) goblet cells. (O) The bar charts show the percentage of 2F11 or WGA positive cells. All images were lateral view with anterior at left and posterior at right. Error bars indicate SD. Scale bars = 50 µm.
We examined whether the differentiation of enteroendocrine and goblet cells was affected in Fgf signaling inhibited embryos, by using 2F11 antibody to detect secretory cells [17]. After whole mount immunostain using 2F11 antibody, WGA staining, and Topro-3 nuclear counter staining, embryos were analyzed by confocal microscope. Scattered 2F11 positive cells were present in WT controls, particularly in the intestinal bulb (Fig. 1 M and 1 M’). As expected, goblet cells were present in the mid-intestine (Fig. 1 M and 1 M’’). After heat treatment of embryos from Tg(hsp70l:dnfgfr1-EGFP), scattered 2F11 positive cells were reduced in number (Fig. 1N and 1N’). Goblet cell numbers were greatly reduced (Fig. 1N and 1N’’). We further quantified the proportion of 2F11 and WGA positive cells in total intestinal epithelia. In control embryos, 9.8±1.6% of cells were positive for 2F11. Fewer 2F11 positive cells were observed in Fgf signaling inhibited embryos (7.0±1.8%, P<0.001; Fig. 1O). For goblet cells, 4.0±1.1% of WGA positive cells were present in Fgf signaling inactivated embryos, but were fewer than 6.4±1.4% in control embryos (P<0.001; Fig. 1O). These observations indicate that Fgf signaling is important for intestinal secretory cell differentiation.
Fgfr2c Signaling is Required for Secretory Cell Differentiation
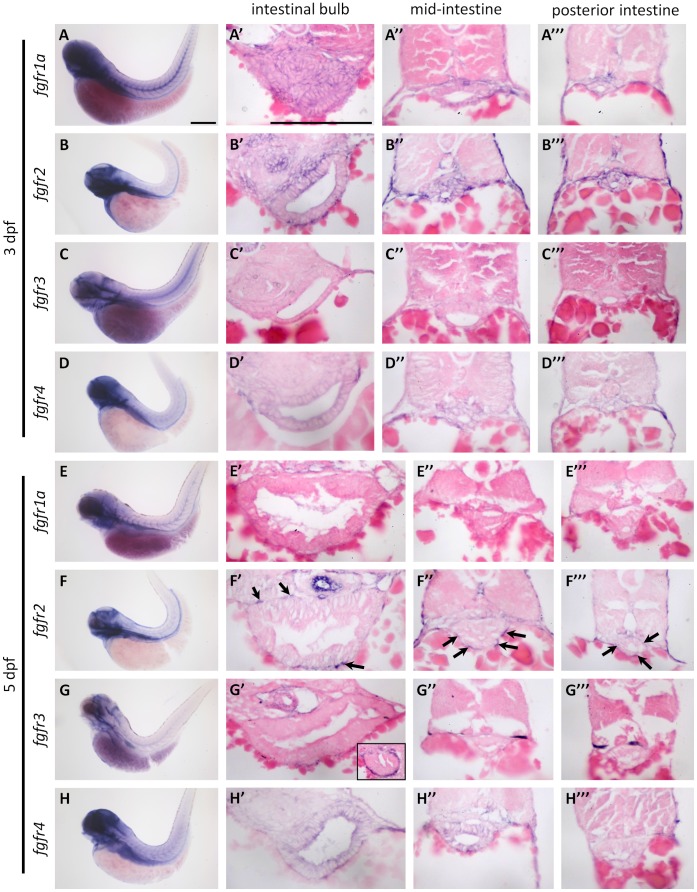
There are five fgf receptor genes, fgfr1a, fgfr1b, fgfr2, fgfr3, and fgfr4, in zebrafish [36], [44]. To identify receptors important for intestinal cell differentiation, we used RT-PCR to analyze fgfrs expression in the whole gut, including the esophagus, intestine, liver, and pancreas at 5 dpf. Except for fgfr1b, four receptors were detected in gut cDNA (Fig. S1A). We used whole mount in situ hybridization (WISH) to analyze the temporal and spatial expression pattern of the four receptors. Expression of fgfr1a, fgfr2, and fgfr4 were detected in the intestine at 3 dpf (Fig. 2A, 2B, and 2D). To analyze the cellular localization of these receptors, specimens were sectioned in paraffin. The fgfr1a and fgfr2 genes were expressed in the mesenchymal and epithelial layers of the intestinal bulb, mid-intestine, and posterior intestine at 3 dpf (Fig. 2A’-2A’’’ and 2B’-2B’’’). The fgfr4 gene was expressed in the epithelium in low levels at 3 dpf (Fig. 2D’-2D’’’). However, fgfr3 was not expressed in the intestine at 3 dpf (Fig. 2C–2C’’’) or 5 dpf (Fig. 2G–2G’’’), although many signals were present in mesenchymal cells of the esophagus at 5 dpf (inset in Fig. 2G’). Expression of fgfr1a was no longer expressed in intestine but in pancreatic islet at 5 dpf (Fig. 2E–2E’’’ and data not shown). Only fgfr2 and fgfr4 were expressed in the intestine at 5 dpf (Fig. 2F and 2H). The fgfr2 was strongly expressed in intestinal mesenchymal cells, but not in epithelium cells at 5 dpf (Fig. 2F’–2F’’’, arrows). Expression of fgfr4 was more intensive in the intestinal epithelium at 5 dpf than they were at 3 dpf (Fig. 2H’–2H’’’).
Figure 2. The expression pattern of zebrafish fgfr genes.
The expression of fgfr1a-4 was analyzed by WISH: (A–D) at 3 dpf and (E–H) at 5 dpf. WISH embryos were sectioned and analyzed for expression of fgfr1a-4 in: (A’–H’) the intestinal bulb, (A’’–H’’) the mid-intestine, and (A’’’–H’’’) the posterior intestine. Black arrows indicated the fgfr2 expression in mesenchymal cells. Scale bars = 200 µm.
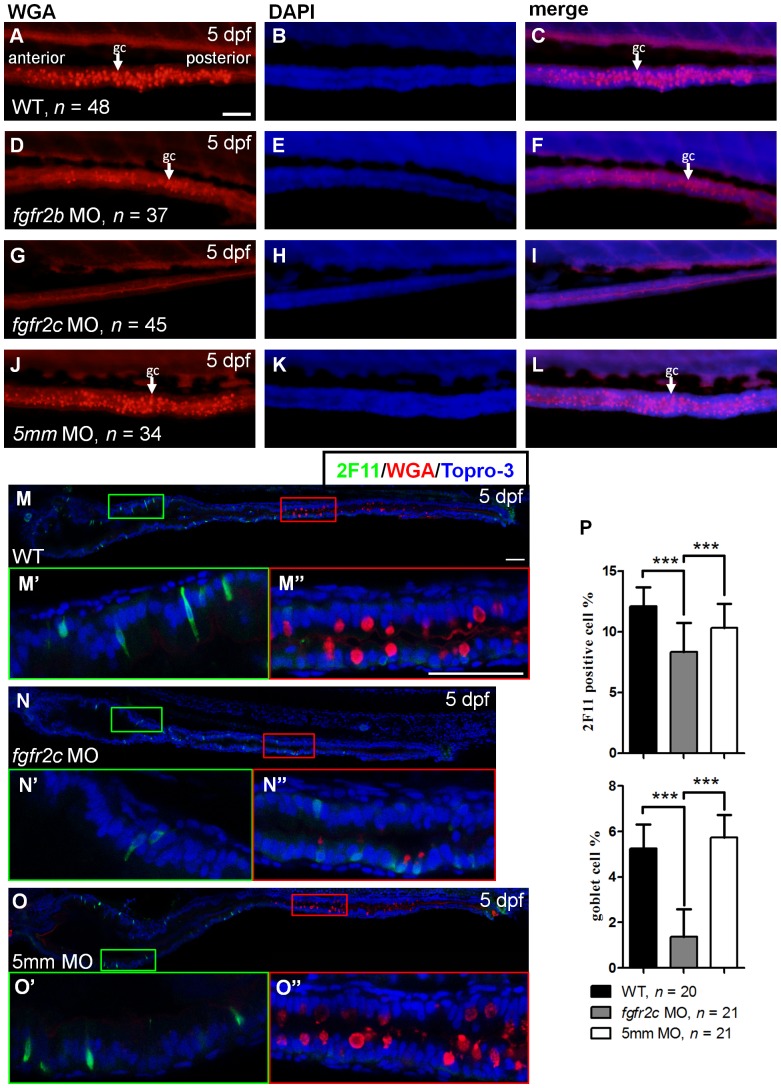
Because fgfr2 was the only receptor that was strongly expressed in the intestine at both 3 and 5 dpf, we analyzed whether intestinal cell differentiation was disrupted in Fgfr2 signaling inactive embryos. Two fgfr2 isoforms, fgfr2b and fgfr2c, have been identified [36]. We used morpholino antisense oligonucleotides to knockdown gene expression in zebrafish embryos. Two morpholinos, which respectively, target fgfr2b and fgfr2c, have been used by us to study zebrafish embryo development. One fgfr2c-5 mm morpholino, which contained 5 mismatch bases in fgfr2c morpholino sequence, was used as a control [32]. We studied intestinal cell differentiation in the morpholino-injected morphants. The number of goblet cells was reduced in fgfr2b morphants compared to WT (Fig. 3A–3F). Moreover, goblet cell differentiation was almost completely absent from the fgfr2c morphants (Fig. 3G–3I). Goblet cell was normally differentiated in fgfr2c-5 mm morphants (Fig. 3J–L).
Figure 3. The secretory cell differentiation of fgfr2c morphants.
WGA staining was performed on 5 dpf (A–C) WT embryos, (D–F) fgfr2b, (G–I) fgfr2c, and (J–L) fgfr2c-5 mm morphants. White arrows indicated goblet cell (gc). Double labelling using 2F11 antibody and WGA in (M) WT embryos, (N) fgfr2c morphants, and (O) fgfr2c-5 mm morphants. The magnified image shows (M’–O’) enteroendocrine cells and (M’’–O’’) goblet cells. Topro-3 was used for nuclear counter staining (blue). (P) The bar charts show the percentages of 2F11 or WGA positive cells. All images were lateral view with anterior at left and posterior at right. Error bars indicate SD. Scale bars = 50 µm.
We quantified the proportion of secretory cell in fgfr2b and fgfr2c morphants after 2F11 immunostaining and WGA staining. Goblet cell numbers were slightly reduced in the fgfr2b morphants, similar to our WGA staining results (Fig. S1B–1D; 5.4±1.45% in WT and 3.7±1.05% in morphants, P<0.001). The percentage of 2F11 positive cells was not reduced in fgfr2b morphants (Fig. S1B–1D; 10.9±2.33% in WT and 11.4±1.64% in morphants, P = 0.7995). In comparison with fgfr2b morphants, secretory cell differentiation was more significantly inhibited in fgfr2c morphants. Goblet cells were greatly reduced in number in fgfr2c morphants (Fig. 3M–3P; 1.37±1.21% in fgfr2c morphants, 5.72±1.00% in fgfr2c-5 mm morphants, and 5.25±1.06% in WT, P<0.001). The amount of 2F11 positive cells was reduced in fgfr2c morphants compared with control embryos (Fig. 3M–3P; 8.32±2.41% in fgfr2c morphants, 10.34±1.97% in fgfr2c-5 mm morphants, and 12.08±1.58% in WT, P<0.001). Consistent with the results for Tg(hsp70l:dnfgfr1-EGFP) embryos, these findings indicated that Fgfr2c signaling is important for secretory cell differentiation.
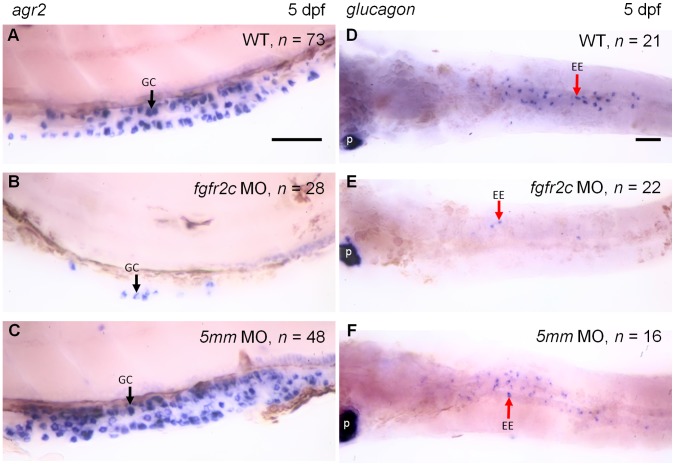
The loss of WGA signal might also be resulted from downregulation of mucin production. We used a goblet cell specific marker gene, anterior gradient 2 (agr2) to analyze goblet cell differentiation [45], [46]. The WISH results showed that agr2 expressing cells were presented in mid-intestine of control embryos (Fig. 4A and 4C). In fgfr2c morphants, agr2 expressing cells were dramatically reduced in number (Fig. 4B). These results indicated that goblet cell differentiation was inhibited in fgfr2c morphants.
Figure 4. The expression of agr2 and glucagon in fgfr2c morphants.
WISH of agr2 (A–C, lateral view) and glucagon (D–F, ventral view) were used to analyze goblet cell and enteroendocrine differentiation, respectively, in 5 dpf embryos. Black arrows indicated the goblet cells (gc) and red arrows indicated the enteroendocrine cells (ee). p: Pancreatic alpha cells. Scale bars = 50 µm.
We further used glucagon probe to analyze the differentiation of enteroendocrine [5], [34], [47]. The signal could be detected in pancreatic alpha cells in fgfr2c morphants and control embryos (Fig. 4D–4F). In contrast, glucagon-expressing cells were greatly reduced in intestine of fgfr2c morphants compared with control embryos (Fig. 4D–4F, arrow). Taken together, the differentiation of both secretory cell types was disrupted in fgfr2c morphants.
Enterocyte Differentiation was Disrupted in fgfr2c Morphants
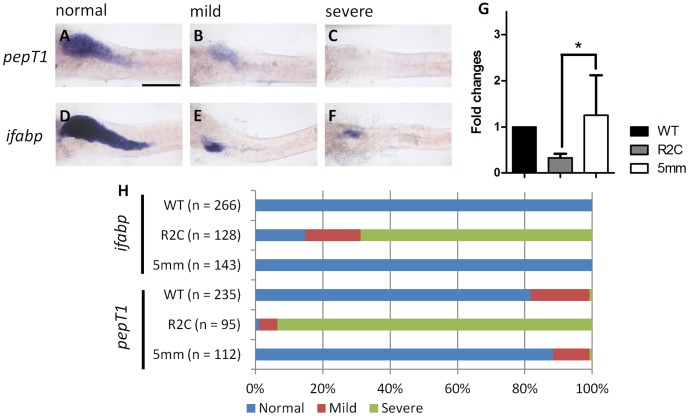
We wondered whether the differentiation of absorptive cells was normal in fgfr2c morphants. We analyzed the expression of the gene for intestinal fatty acid binding protein ifabp, and oligopeptide transporter pepT1. These two genes were expressed in the differentiated enterocytes [34]. According to the expression pattern in 5 dpf embryos, three different levels of pepT1 and ifabp gene expression were classified (Fig. 5A–5F): level 1; expression in the whole intestinal bulb (normal; Fig. 5A and 5D). Level 2; expression in the whole intestinal bulb, but at reduced levels (mild; Fig. 5B and 5E). Level 3; expression only in the anterior intestinal bulb or no expression (severe; Fig. 5C and 5F). The ifabp expression level was normal in all WT embryos (Fig. 5H). For pepT1 expression, 81.7% WT embryos were normal. However, 17.4% expressed mild and 0.9% expressed severe patterns (Fig. 5H). The expression of pepT1 and ifabp in fgfr2c-5 mm morphants was similar to that of WT embryos (Fig. 5H). However, severe reduction of the enterocyte population was found in most fgfr2c morphants. We observed 14.8% morphants with normal, 16.4% morphants with mild, and 68.8% morphants exhibiting severe ifabp expression pattern. Reduction in pepT1 expression was even more obvious. Only 1.1% morphants exhibited the normal pepT1 pattern. Mild and severe patterns were observed in 5.3% and 91.6% morphants, respectively (Fig. 5H). The results of qPCR also showed the expression level of pepT1 was reduced in the fgfr2c morphants compared with control embryos (Fig. 5G). Therefore, we conclude that the differentiation of absorptive enterocytes was also affected in fgfr2c morphants.
Figure 5. Absorptive cell differentiation in fgfr2c morphants.
The pepT1 and ifabp WISH were used to analyze enterocyte differentiation in 5 dpf embryos. Three different signal levels were classified as (A,D) normal, (B,E) mild, and (C,F) severe. (G) qPCR analysis of the pepT1 expression level in the fgfr2c morphants and control embryos. Data were normalized with β-actin1 and expressed as fold-induction relative to wild type embryos. Error bars indicate SD. *indicates P<0.05. (H) The bar charts show the percentages of three expression levels of these two genes in WT embryos, and fgfr2c and fgfr2c-5 mm morphants. Scale bar = 200 µm.
Proliferating Cells were Increased in fgfr2c Morphants
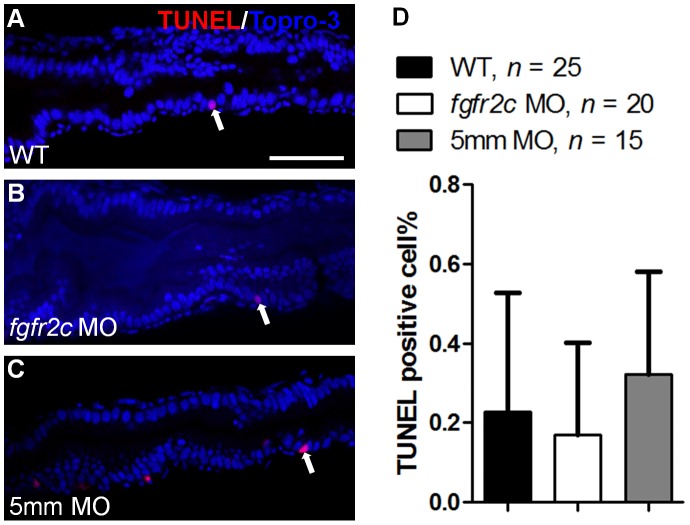
Because differentiation of both secretory and absorptive cells in the intestine was disrupted, we wondered whether cell death was altered in fgfr2c morphants. TUNEL assay indicated that only very few cell deaths occurred in 5 dpf WT embryos, and fgfr2c and fgfr2c-5 mm morphants (0.2±0.3%, 0.2±0.2%, and 0.3±0.3%, respectively; Fig. 6). Thus, the observed reduction in cell differentiation was not caused by cell death.
Figure 6. Cell death in fgfr2c morphants.
TUNEL assay was performed on (A) WT embryos, (B) fgfr2c morphants, and (C) fgfr2c-5 mm morphants at 5 dpf. TUNEL-positive cells (red) were detected in developing gut (indicated by white arrows). Topro-3 was used for nuclear counter staining (blue). (D) The bar charts show the percentages of TUNEL-positive cells in WT embryos, and fgfr2c and fgfr2c-5 mm morphants. Error bars indicate SD. Scale bar = 50 µm.
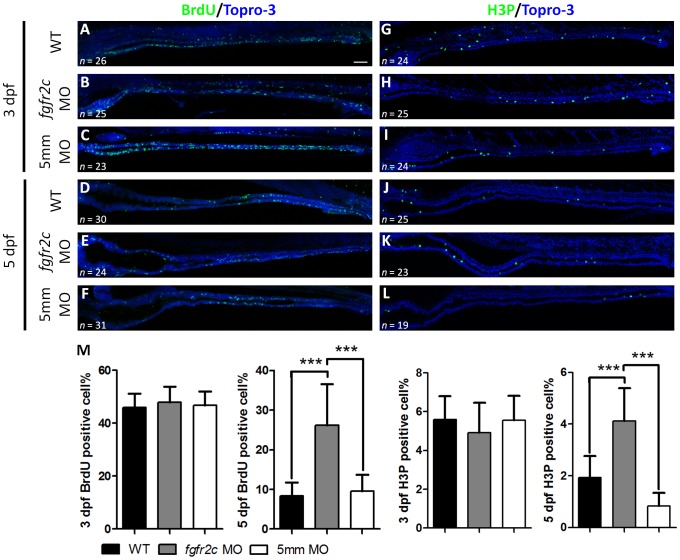
We analyzed the cell proliferation status in fgfr2c morphants. BrdU incorporation was used to detect the proportion of S-phase cells, and H3P antibody was used to detect M-phase cells, in 3 and 5 dpf embryos. Normally proliferating cells are densely scattered throughout the intestine at 52–74 hours post fertilization (hpf) and reduced in number at 74–120 hpf [5]. We observed 45.9±5.3% S-phase, and 5.6±1.6% M-phase cells in the intestine of WT embryos at 3 dpf (Fig. 7A and 7G). The proportion of cycling cells present in fgfr2c-5 mm morphants at 3 dpf was similar to that for WT (S phase: 46.9±5.1%, and M phase: 5.6±1.3%; Fig. 7C and 7I). The percentages of proliferating cells in fgfr2c morphants were similar to those of the control embryos at 3 dpf (S phase: 47.6±5.8% and M phase: 4.9±1.6%; Fig. 7B, 7H, and 7 M). The cycling cells of WT embryos were significantly reduced in number at 5 dpf (S phase: 8.4±3.4% and M phase: 1.9±0.8%; Fig. 7D and 7J). A similar reduction occurred in fgfr2c-5 mm morphants at 5 dpf (S phase: 9.6±4.2% and M phase: 0.8±0.5%; Fig. 7F and 7L). Although cycling cells were reduced at 5 dpf compared to 3 dpf in fgfr2c morphants, the proportion of proliferating cells was still much greater than that of control embryos (S phase: 26.2±10.3% and M phase: 4.1±1.3%, P<0.001; Fig. 7E, 7K, and 7M). In summary, cycling cells were unchanged at 3 dpf but increased in number at 5 dpf after inhibition of Fgfr2c signaling.
Figure 7. Cell proliferation in fgfr2c morphants.
The S-phase proliferating cells of WT, fgfr2c, and fgfr2c-5 mm morphants were labeled with BrdU at (A–C) 3 dpf and (D–F) 5 dpf. H3P antibody was used to label M-phase cells at (G–I) 3 dpf and (J–L) 5 dpf. (M) Topro-3 was used for nuclear counter staining (blue). The bar charts show the percentages of proliferating cells. Error bars indicate SD. Scale bar = 50 µm.
Disruption of Cell Differentiation and Proliferation in mibta52b Mutants with fgfr2c Knockdown
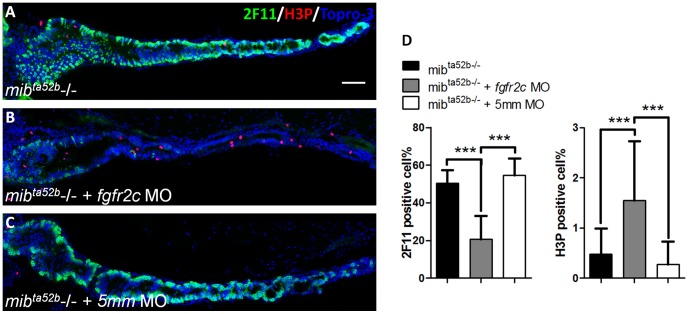
It seemed likely that proliferating progenitor cells in fgfr2c morphants could not exit from cycling progression to undergo terminal differentiation. To test this hypothesis, we used the mibta52b mutant to investigate the cell differentiation and proliferation. It was reported that Delta-Notch signaling is inhibited in mibta52b mutant fish and differentiation of almost all intestinal cells is toward the secretory cell lineage [17], [47]. If Fgfr2c signaling were important for cell cycle exit and terminal cell differentiation, then 2F11 positive secretory cells should be reduced and M-phase cycling cells should be increased in mibta52b mutants injected with fgfr2c morpholino. We observed a large ratio of 2F11 positive cells in mibta52b and mibta52b mutants injected with fgfr2c-5 mm morpholino at 5 dpf (50.5±6.8% and 54.6±9.3%, respectively; Fig. 8A and 8C). Less than one percent of H3P positive cycling cells could be detected in mibta52b and mibta52b mutants injected with fgfr2c-5 mm morpholino at 5 dpf (0.5±0.5% and 0.3±0.5%, respectively; Fig. 8A and 8C). There was a large reduction in secretory cells and an increased number of cycling cells in mibta52b mutants injected with fgfr2c morpholino compared with control embryos at 5 dpf (2F11∶20.7±12.5%, P<0.001; H3P: 1.6±1.2%, P<0.001; Fig. 8B and 8D). We noticed that these H3P positive cells were not co-localized with the 2F11 signal. This indicated that proliferating cells were not differentiated secretory cells, but that they might be progenitor cells. Therefore, it is likely that intestinal proliferating progenitor cells cannot exit from cell cycling and are unable to differentiate further, in embryos that lack Fgfr2c signaling.
Figure 8. The secretory cell differentiation of mibta52b mutants after injection with fgfr2c morpholino.
The 2F11 (green) and H3P (red) antibodies were used to label the secretory and proliferating cells respectively, in (A) mibta52b mutant, (B) mutant injected with fgfr2c morpholino, and (C) mutant injected with fgfr2c-5 mm morpholino at 5 dpf. Topro-3 was used for nuclear counter staining (blue). (D) The bar charts show the percentages of secretory and proliferating cells. Error bars indicate SD. Scale bar = 50 µm.
Discussion
Intestinal progenitor cells are highly proliferative. It is important to understand what signaling factor(s) control these cycling cells’ differentiation to various mature intestinal epithelial cells, and how this is accomplished. In this study, we noted a novel role of Fgfr2c signaling in the gut epithelium. In the absence of Fgfr2c signaling, the differentiation of secretory and absorptive cells was inhibited, and cell proliferation was increased. Inhibition of Fgfr2c signaling in mibta52b mutant embryos showed a similar effect. These results suggest that Fgfr2c signaling promotes progenitor cell’s exit from cell cycling, and their differentiation to various intestinal cell types.
In mice, Fgfr1–3 is expressed in the developing gastrointestinal tract, including the forestomach, small intestine, colon, and cecum [22], [24], [27], [48], [49], [50]. Although few reports indicate that Fgfr4 is expressed in gut tissue during developmental stages in mice, we found of Fgfr4 in the stomach and intestine using the Genepaint database, which contains in situ hybridization data [51]. Fgfr2b −/− mice exhibit decreased differentiation gastric mucosa, and reduced numbers of proliferating epithelial cells [52]. Fgfr3 −/− mice showed significantly reduced numbers of clonogenic stem cells and Paneth cells in the small intestine [27]. However, we found no reports that either Fgfr1 or Fgfr4 signaling regulates intestinal development and cell differentiation in mice.
We used zebrafish to study the function of Fgf signaling in the gut. Only fgfr2 and fgfr4 genes were strongly expressed in the intestine at 5 dpf, in which most of the observed cell differentiation occurred. In zebrafish, the fgfr1 gene is duplicated to fgfr1a and fgfr1b [44]. RT-PCR did not reveal any evidence for the expression of fgfr1b at 5 dpf. Although fgfr1a was expressed in intestinal cells at 3 dpf, we did not detect its expression in intestinal mesenchymal or epithelial cells at 5 dpf. The fgfr3 gene was not expressed in intestinal cells at 3 and 5 dpf. These expression patterns, and that for fgfr3 in particular, were different from those reported for mice [27], [50].
In this study, we observed expression of fgfr4 in the intestinal epithelial cells at both 5 dpf and adult fish (Fig. 2 and Fig. S2B). We administered fgfr4 morpholino to verify whether or not Fgfr4 signaling affected cell differentiation. The results of ifabp WISH showed that loss of fgfr4 expression does not disrupt intestinal cell differentiation (data not shown). Enterocyte expression of Fgfr4 is important for bile acid homeostasis [53]. Thus, we suggest that Fgfr4 signaling might have the similar function in the zebrafish intestine.
The expressions of the two Fgfr2 isoforms are highly regional specific. Fgfr2b and Fgfr2c are limited to epithelial and mesenchymal cells, respectively [54]. The fgfr2 probe we used was full length over 4 kb cDNA for fgfr2c [36]. However, this probe was not specific to fgfr2c because there is only a 140 base difference between fgfr2b and fgfr2c in the full length region. We found that zebrafish fgfr2 was expressed in both epithelial and mesenchymal cells of the intestine at 3 dpf, but that it was only expressed in intestinal mesenchymal cells at 5 dpf. This indicated that fgfr2b might only express in epithelial cells at 3 dpf, and that expression of fgfr2c is sustained in mesenchymal cells from 3 to 5 dpf. The fgfr2 signal was restricted in mesenchymal cells of adult intestine tissue (Fig. S2A). We concluded that the major fgfr2 isoform expressed in zebrafish intestine was fgfr2c, and that this gene specifically expressed in mesenchymal cells. However, Fgfr2b is reported to be the major isoform expressed in the developing gastrointestinal tract in mice [24], [48], [49]. Thus, Fgfr2 signaling may regulate intestinal cell proliferation and cell differentiation in different ways. In mice, Fgfr2b signaling acted autonomously in intestinal epithelium cells. Because fgfr2c was expressed in the mesenchymal cells of zebrafish intestine, other paracrine signal(s), regulated directly or indirectly by Fgfr2c may exist to control epithelial differentiation.
Mesenchymal-epithelial interactions are important for intestinal cell proliferation and differentiation. Many signaling factors are associated with these interactions, including Hedgehog (Hh) and Bmp factors [1]. The expression of Ihh and Shh could be detected in small intestinal epithelial cells and their receptors were observed in intestinal mesenchymal cells of mice; when Hh signaling was disrupted, the small intestinal epithelial cells were hyperproliferative, and these proliferating cells were negative for enterocyte differentiation [55]. Furthermore, with the exception of the proliferating zone, Bmp4 was expressed in the mesenchymal layer of the small intestine, and Bmpr1a was expressed in the epithelial cells. In Bmpr1a conditional knockout mice, proliferating cells were generally increased in number [14]. In zebrafish, mesenchymal-epithelial interactions also regulate gut epithelial development. MiR-145 is expressed in gut mesenchymal cells in zebrafish, while mesenchymal expressed miR-145 directly controls gata6 expression in the gut [56]. Recently reports suggest that miR-145 regulates paracrine signaling to control gut development [57]. We found that fgfr2c is expressed in the mesenchymal layer, thus, some Fgf ligands must exist in the epithelial or mesenchymal layers of the zebrafish gut. Using RT-PCR, we identified putative Fgfr2c ligands (fgf1, fgf2, fgf4, fgf17 and fgf24) that were expressed in 5 dpf zebrafish gut (data not shown), but we still do not know which specific Fgf ligands interact with Fgfr2c.
Wnt signaling is critical for intestine development. When Wnt ligands are present, cytosolic β-catenin proteins are not degraded by the destruction complex. Stable β-catenin proteins then translocate into the nucleus and bind with T cell factor (Tcf) transcription factors. This newly formed complex has transcriptional activity and can drive Wnt signaling target genes, including c-Myc and cyclin D, to promote cell cycle progression [6], [9]. In the fgfr2c morphant intestine, there were more proliferating cells present than there were in control embryos. We used β-catenin antibody to verify whether Wnt signaling is activated in the intestinal epithelial cells of fgfr2c morphants; only membrane accumulated β-catenin was detected, and there were no differences in the β-catenin localization pattern between fgfr2c morphants and the control embryos (data not shown). Thus, we still do not know whether Wnt signaling is activated in intestinal epithelial cells of fgfr2c morphants.
In conclusion, we found that Fgfr2c signaling derived from mesenchymal cells is important for regulating the differentiation of zebrafish intestine epithelial cells by promoting cell cycle exit. The results of Fgfr2c knockdown in mibta52b mutants indicated that Fgfr2c signaling is required for intestinal cell differentiation. However, the mechanism by which mesenchymal cells control the behaviors of epithelial cell needs investigation. Furthermore, although the expression of fgfr2 could be detected in the mesenchymal cells of adult zebrafish intestines, we do not know whether Fgfr2c signaling directs differentiation in adult fish.
Supporting Information
The expression of fgfrs and the secretory cell differentiation of fgfr2b morphants. (A) fgfr1a, fgfr2, fgfr3 and fgfr4 gene were analyzed in 5 dpf zebrafish gut tissue by RT-PCR. (C) WT embryos and (D) fgfr2b morphants were double labeled using 2F11 antibody and WGA. The magnified image shows (C’–D’) enteroendocrine cells and (C”–D”) goblet cells. DAPI was used for nuclear counter staining (blue). (B) The bar charts show the percentages of 2F11 and WGA positive cells. Error bars indicate SD. Scale bar = 50 µm.
(TIF)
The expression of fgfr2 and fgfr4 in adult zebrafish intestine. Section in situ hybridization was used to analyze the expression of fgfr2 and fgfr4 genes. (A) fgfr2 was detected in the lamina propria, and (B) fgfr4 was expressed mainly in the epithelial layer of the intestine. Scale bar = 50 µm.
(TIF)
Primer list for RT-PCR analysis.
(DOC)
Acknowledgments
We would like to thank Dr. J. Lewis for gifting the 2F11 antibody, Dr. S. P. Hwang for a gift of the agr2 and pepT1 probes, and Dr. K. Yamasu for donating the fgfr1–4 probes. We also thank the Taiwan Zebrafish Core Facility at Academia Sinica, which is supported by a National Science Council (NSC) grant number 100-2321-B-001-030, for providing the glucagon probe and the Tg(hsp70:dnfgfr1-egfp) fish. We also thank Dr. S. P. Hwang for providing many useful comments on this work.
Funding Statement
This work was supported by grants from the National Science Council (NSC100-2311-B-320-001), and Tzu Chi University (TCMRC-P-99013-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7: 349–359. [DOI] [PubMed] [Google Scholar]
- 2. Radtke F, Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307: 1904–1909. [DOI] [PubMed] [Google Scholar]
- 3. Sancho E, Batlle E, Clevers H (2003) Live and let die in the intestinal epithelium. Curr Opin Cell Biol 15: 763–770. [DOI] [PubMed] [Google Scholar]
- 4. van Es JH, Clevers H (2005) Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med 11: 496–502. [DOI] [PubMed] [Google Scholar]
- 5. Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, et al. (2005) Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 286: 114–135. [DOI] [PubMed] [Google Scholar]
- 6. Scoville DH, Sato T, He XC, Li L (2008) Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864. [DOI] [PubMed] [Google Scholar]
- 7. Yeung TM, Chia LA, Kosinski CM, Kuo CJ (2011) Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci 68: 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, et al. (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383. [DOI] [PubMed] [Google Scholar]
- 9. Andreu P, Colnot S, Godard C, Gad S, Chafey P, et al. (2005) Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 10. Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, et al. (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18: 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faro A, Boj SF, Ambrosio R, van den Broek O, Korving J, et al. (2009) T-cell factor 4 (tcf7l2) is the main effector of Wnt signaling during zebrafish intestine organogenesis. Zebrafish 6: 59–68. [DOI] [PubMed] [Google Scholar]
- 12. Mahmoudi T, Boj SF, Hatzis P, Li VS, Taouatas N, et al. (2010) The leukemia-associated Mllt10/Af10-Dot1l are Tcf4/beta-catenin coactivators essential for intestinal homeostasis. PLoS Biol 8: e1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, et al. (2004) De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303: 1684–1686. [DOI] [PubMed] [Google Scholar]
- 14. He XC, Zhang J, Tong WG, Tawfik O, Ross J, et al. (2004) BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 36: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 15. Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N (2007) Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896. [DOI] [PubMed] [Google Scholar]
- 16. Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, et al. (2008) Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, et al. (2005) Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132: 1093–1104. [DOI] [PubMed] [Google Scholar]
- 18. van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, et al. (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963. [DOI] [PubMed] [Google Scholar]
- 19. Bottcher RT, Niehrs C (2005) Fibroblast growth factor signaling during early vertebrate development. Endocr Rev 26: 63–77. [DOI] [PubMed] [Google Scholar]
- 20. Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2: REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149. [DOI] [PubMed] [Google Scholar]
- 22. Sala FG, Curtis JL, Veltmaat JM, Del Moral PM, Le LT, et al. (2006) Fibroblast growth factor 10 is required for survival and proliferation but not differentiation of intestinal epithelial progenitor cells during murine colon development. Dev Biol 299: 373–385. [DOI] [PubMed] [Google Scholar]
- 23. Nyeng P, Bjerke MA, Norgaard GA, Qu X, Kobberup S, et al. (2011) Fibroblast growth factor 10 represses premature cell differentiation during establishment of the intestinal progenitor niche. Dev Biol 349: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyeng P, Norgaard GA, Kobberup S, Jensen J (2007) FGF10 signaling controls stomach morphogenesis. Dev Biol 303: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Housley RM, Morris CF, Boyle W, Ring B, Biltz R, et al. (1994) Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest 94: 1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arnaud-Dabernat S, Yadav D, Sarvetnick N (2008) FGFR3 contributes to intestinal crypt cell growth arrest. J Cell Physiol 216: 261–268. [DOI] [PubMed] [Google Scholar]
- 27. Vidrich A, Buzan JM, Brodrick B, Ilo C, Bradley L, et al. (2009) Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol 297: G168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallace KN, Akhter S, Smith EM, Lorent K, Pack M (2005) Intestinal growth and differentiation in zebrafish. Mech Dev 122: 157–173. [DOI] [PubMed] [Google Scholar]
- 29. Wallace KN, Pack M (2003) Unique and conserved aspects of gut development in zebrafish. Dev Biol 255: 12–29. [DOI] [PubMed] [Google Scholar]
- 30. Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD (2005) Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132: 5173–5183. [DOI] [PubMed] [Google Scholar]
- 31.Westerfield M (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). : Univ. of Oregon Press, Eugene.
- 32. Liu DW, Hsu CH, Tsai SM, Hsiao CD, Wang WP (2011) A variant of fibroblast growth factor receptor 2 (Fgfr2) regulates left-right asymmetry in zebrafish. PLoS One 6: e21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakayama Y, Miyake A, Nakagawa Y, Mido T, Yoshikawa M, et al. (2008) Fgf19 is required for zebrafish lens and retina development. Dev Biol 313: 752–766. [DOI] [PubMed] [Google Scholar]
- 34. Chen YH, Lu YF, Ko TY, Tsai MY, Lin CY, et al. (2009) Zebrafish cdx1b regulates differentiation of various intestinal cell lineages. Dev Dyn 238: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 35. Paffett-Lugassy N, Hsia N, Fraenkel PG, Paw B, Leshinsky I, et al. (2007) Functional conservation of erythropoietin signaling in zebrafish. Blood 110: 2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tonou-Fujimori N, Takahashi M, Onodera H, Kikuta H, Koshida S, et al. (2002) Expression of the FGF receptor 2 gene (fgfr2) during embryogenesis in the zebrafish Danio rerio. Gene Expr Patterns 2: 183–188. [DOI] [PubMed] [Google Scholar]
- 37. Esain V, Postlethwait JH, Charnay P, Ghislain J (2010) FGF-receptor signalling controls neural cell diversity in the zebrafish hindbrain by regulating olig2 and sox9. Development 137: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hochmann S, Kaslin J, Hans S, Weber A, Machate A, et al. (2012) Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS One 7: e30365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kan NG, Junghans D, Izpisua Belmonte JC (2009) Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J 23: 3516–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein C, Mikutta J, Krueger J, Scholz K, Brinkmann J, et al. (2011) Neuron navigator 3a regulates liver organogenesis during zebrafish embryogenesis. Development 138: 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, et al. (2006) A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127: 607–619. [DOI] [PubMed] [Google Scholar]
- 42. Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, et al. (2007) Bmp and Fgf signaling are essential for liver specification in zebrafish. Development 134: 2041–2050. [DOI] [PubMed] [Google Scholar]
- 43.Tsai SM, Liu DW, Wang WP (2012) Fibroblast growth factor (Fgf) signaling pathway regulates liver homeostasis in zebrafish. Transgenic Res. [DOI] [PubMed]
- 44. Rohner N, Bercsenyi M, Orban L, Kolanczyk ME, Linke D, et al. (2009) Duplication of fgfr1 permits Fgf signaling to serve as a target for selection during domestication. Curr Biol 19: 1642–1647. [DOI] [PubMed] [Google Scholar]
- 45. Chen YC, Lu YF, Li IC, Hwang SP (2012) Zebrafish Agr2 is required for terminal differentiation of intestinal goblet cells. PLoS One 7: e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shih LJ, Lu YF, Chen YH, Lin CC, Chen JA, et al. (2007) Characterization of the agr2 gene, a homologue of X. laevis anterior gradient 2, from the zebrafish, Danio rerio. Gene Expr Patterns 7: 452–460. [DOI] [PubMed] [Google Scholar]
- 47. Zecchin E, Filippi A, Biemar F, Tiso N, Pauls S, et al. (2007) Distinct delta and jagged genes control sequential segregation of pancreatic cell types from precursor pools in zebrafish. Dev Biol 301: 192–204. [DOI] [PubMed] [Google Scholar]
- 48. Burns RC, Fairbanks TJ, Sala F, De Langhe S, Mailleux A, et al. (2004) Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev Biol 265: 61–74. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Stappenbeck TS, White AC, Lavine KJ, Gordon JI, et al. (2006) Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development 133: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vidrich A, Buzan JM, Ilo C, Bradley L, Skaar K, et al. (2004) Fibroblast growth factor receptor-3 is expressed in undifferentiated intestinal epithelial cells during murine crypt morphogenesis. Dev Dyn 230: 114–123. [DOI] [PubMed] [Google Scholar]
- 51. Visel A, Thaller C, Eichele G (2004) GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res 32: D552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spencer-Dene B, Sala FG, Bellusci S, Gschmeissner S, Stamp G, et al. (2006) Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology 130: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 53. Sinha J, Chen F, Miloh T, Burns RC, Yu Z, et al. (2008) beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol 295: G996–G1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP (2009) ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 33: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, et al. (2005) Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132: 279–289. [DOI] [PubMed] [Google Scholar]
- 56. Zeng L, Carter AD, Childs SJ (2009) miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci U S A 106: 17793–17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zeng L, Childs SJ (2012) The smooth muscle microRNA miR-145 regulates gut epithelial development via a paracrine mechanism. Dev Biol 367: 178–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of fgfrs and the secretory cell differentiation of fgfr2b morphants. (A) fgfr1a, fgfr2, fgfr3 and fgfr4 gene were analyzed in 5 dpf zebrafish gut tissue by RT-PCR. (C) WT embryos and (D) fgfr2b morphants were double labeled using 2F11 antibody and WGA. The magnified image shows (C’–D’) enteroendocrine cells and (C”–D”) goblet cells. DAPI was used for nuclear counter staining (blue). (B) The bar charts show the percentages of 2F11 and WGA positive cells. Error bars indicate SD. Scale bar = 50 µm.
(TIF)
The expression of fgfr2 and fgfr4 in adult zebrafish intestine. Section in situ hybridization was used to analyze the expression of fgfr2 and fgfr4 genes. (A) fgfr2 was detected in the lamina propria, and (B) fgfr4 was expressed mainly in the epithelial layer of the intestine. Scale bar = 50 µm.
(TIF)
Primer list for RT-PCR analysis.
(DOC)