Summary
Productive intercellular delivery of cargo by secretory systems requires exquisite temporal and spatial choreography. Our laboratory has demonstrated that the hemolysin co-regulated secretion island I (HSI-I)-encoded type VI secretion system (H1-T6SS) of Pseudomonas aeruginosa transfers effector proteins to other bacterial cells. The activity of these effectors requires cell contact-dependent delivery by the secretion apparatus, and thus their export is highly repressed under planktonic growth conditions. Here we define regulatory pathways that orchestrate efficient secretion by this system. We identified a T6S-associated protein, TagF, as a posttranslational repressor of the H1-T6SS. Strains activated by TagF derepression or stimulation of a previously identified threonine phosphorylation pathway (TPP) share the property of secretory ATPase recruitment to the T6S apparatus, yet display different effector output levels and genetic requirements for their export. We also found that the pathways respond to distinct stimuli; we identified surface growth as a physiological cue that activates the H1-T6SS exclusively through the TPP. Coordination of posttranslational triggering with cell contact-promoting growth conditions provides a mechanism for the T6SS to avoid wasteful release of effectors.
Keywords: regulation, Pseudomonas, secretion system
Introduction
In Gram-negative bacteria six secretion systems (types I-VI) have been identified, with specialized functions ranging from general cellular maintenance and physiology to host and bacterial cell interactions (Economou et al., 2006). The type VI secretion system (T6SS) is found broadly among the Proteobacteria, including many important environmental and human-associated taxa (Schwarz et al., 2010a). These systems are encoded by gene clusters composed of 13 conserved genes (type six secretion, tss), and additional accessory genes (type VI secretion associated gene, tag) that vary in number and content (Boyer et al., 2009). Core components of the T6SS include ClpV, a AAA+ family ATPase, TssM, a homolog of the type IV secretion protein IcmF, and VgrG (valine glycine rich G) and Hcp (hemolysin co-regulated protein), two extracellular structural proteins (Bingle et al., 2008, Cascales, 2008, Aschtgen et al., 2010). Interestingly, several conserved components of the T6SS share sequence and structural similarity to bacteriophage tail and baseplate proteins (Kanamaru, 2009, Leiman et al., 2009, Mougous et al., 2006a, Pell et al., 2009). Based on this relatedness, the system has been proposed to function as an outward facing puncturing device at the surface of the cell.
The T6SS has been implicated in diverse processes including host-cell interactions (Ma et al., 2009, Shalom et al., 2007), biofilm formation (Aschtgen et al., 2008), and gene regulation (Weber et al., 2009, Jani & Cotter, 2010). Recent studies indicate that the T6SS plays a critical role in interbacterial interactions (Schwarz et al., 2010a). This was first reported in Pseudomonas aeruginosa, where it was observed that H1-T6SS-dependent export of a toxin, Tse2, can provide a fitness advantage to the organism when cultivated in direct contact with another P. aeruginosa strain lacking a Tse2-specific immunity protein, Tsi2 (Hood et al., 2010). Antibacterial activity has also been attributed to T6SSs of Burkholderia thailandensis (T6SS-1), Serratia marcescens and Vibrio cholerae (Vas), however in these instances the effector proteins involved have not been identified (MacIntyre et al., 2010, Schwarz et al., 2010b, Murdoch et al., 2011). Like other specialized secretion systems, expression of T6S and export of its effectors are stringently regulated (Bernard et al., 2010). For the H1-T6SS of P. aeruginosa, regulation at the transcriptional, posttranscriptional, and posttranslational levels has been studied. The system is transcriptionally repressed by the quorum sensing regulator, LasR (Lesic et al., 2009). The binding of LasR to HSI-I promoters has not been shown, leaving the mechanism of repression unknown. Regulation of the H1-T6SS at the posttranscriptional level is governed by the RNA-binding protein RsmA (Brencic & Lory, 2009, Goodman et al., 2004). The Rsm pathway appears to coordinate reciprocal regulation of factors important for planktonic and sessile modes of P. aeruginosa growth (Goodman et al., 2004, Yahr & Greenberg, 2004, Burrowes et al., 2006, Brencic et al., 2009). Consistent with the requirement for long-term cell contact in T6S-dependent effector delivery between cells, the system was found to be co-regulated by RsmA with factors required for adhesion between cells and to surfaces (Mougous et al., 2006a, Goodman et al., 2004, Ventre et al., 2006, Brencic & Lory, 2009). Furthermore, studies have found P. aeruginosa T6S proteins abundantly produced in biofilms relative to planktonic culture (Zhang et al., 2011, Sauer et al., 2002).
Our laboratory has found that the H1-T6SS is posttranslationally triggered by a threonine phosphorylation pathway (TPP) (Mougous et al., 2007, Kulasekara & Miller, 2007). Stimulation of the TPP results in export of extracellular structural components of the apparatus, including Hcp1, VgrG1, VgrG4, and substrates, including type VI secretion exported 1-3 (Tse1-3) (Hood et al., 2010). At least four proteins, TagR, PpkA, PppA and Fha1, all encoded within HSI-I, participate in the TPP. According to our current model, the H1-T6SS becomes activated by dimerization of PpkA, a membrane-spanning Hanks-type serine/threonine kinase. The environmental cue responsible for inducing PpkA dimerization is unknown; however, TagR, a periplasmic protein, functions upstream of PpkA and promotes activation of the kinase (Hsu et al., 2009). Dimerization of PpkA leads to autophosphorylation, which recruits Fha1 through interactions between the phosphorylated activation loop of the kinase and the Forkhead-associated (FHA) domain of Fha1. Fha1 associated with activated PpkA is phosphorylated by the kinase at Thr362 (p-Fha1), in turn promoting H1-T6SS activation by an unknown mechanism (Mougous et al., 2007). Fha1 resides in a complex with ClpV1, suggesting the possibility that recruitment of the ATPase to the apparatus could underlie activation. A PP2C family protein phosphatase, PppA, acts as an antagonist of the TPP by dephosphorylating Fha1, and, possibly, PpkA. Deletion of pppA results in constitutive secretion of Hcp1, VgrG1, VgrG4, and Tse1-3. The genes encoding known TPP components are found in a putative HSI-I operon that also contains four conserved T6S genes (tssJ1, K1, L1, M1) and four uncharacterized tag genes (tagQ, S, T, F) (Fig. 1A). Sequence analysis of the tag genes indicates that tagS and tagT encode proteins with homology to lipoprotein transport and sorting components, LolE and LolD, respectively (Narita & Tokuda, 2006). The tagQ gene encodes a predicted outer membrane lipoprotein, and tagF, a predicted cytoplasmic protein with unknown function.
Fig. 1.

The HSI-I genes, tagF and pppA, repress Hcp1 and Tse1 secretion. (A) Genomic organization of P. aeruginosa HSI-I. The gene encoding TagF is highlighted in purple. Genes colored in green are known components of the TPP and those colored in yellow are uncharacterized tag genes. (B and C) Hcp1 and Tse1 secretion is repressed by tagF and pppA. Western blot analysis of Hcp1–V and Tse1–V in supernatant (Sup) and cell-associated (Cell) fractions of the genetic backgrounds of P. aeruginosa indicated below the blots. Unless otherwise indicated, all blots in this and subsequent figures were probed with antibodies specific to the VSV-G epitope.
In this study, we sought to define additional regulatory elements of the H1-T6SS. Based on the proximity of tagQ, S, T, and F to genes encoding known posttranslational regulators of the system, and their variability amongst other T6SSs, we hypothesized that these genes also encode H1-T6SS regulators. In the course of this search, we identified TagF as a posttranslational regulatory protein that represses the activation of the H1-T6SS by a mechanism distinct from the TPP. Inactivation of tagF triggers Hcp1 secretion to levels observed in ΔpppA, however effector secretion is dampened in ΔtagF relative to a strain lacking pppA. Also, TagF regulates the activity of the H1-T6SS in a manner that does not require p-Fha1, PpkA, or other Tag proteins. Despite these differences, both the TPP and TagF-mediated activation pathways require Fha1 and recruit ClpV1 to the secretion apparatus. We also investigated the physiological significance of posttranslational regulation of the H1-T6SS. Interestingly, we discovered that the H1-T6SS is activated when P. aeruginosa is grown on a surface. This occurs via the TPP, as it requires PpkA and involves increased p-Fha1 levels.
Results
H1-T6SS activity is negatively regulated by TagF
In the course of our efforts to define the function of HSI-I genes, we noted that the putative HSI-I operon encoding PpkA, PppA, Fha1 and TagR, which constitute all known components of the TPP, also encodes additional non-conserved Tag proteins (TagQ, PA0070; TagS, PA0072; TagT, PA0073; TagF, PA0076) (Fig. 1A). Based on their genomic context and lack of conservation in other T6SSs (Boyer et al., 2009), we hypothesized that these genes also encode regulators of the H1-T6SS. To ascertain their role in regulating H1-T6SS activity, we introduced individual in-frame deletions of tagQ, S, T, and F into P. aeruginosa hcp1–V, and monitored the effect on Hcp1 secretion. This strain contains a chromosomal fusion of hcp1 to the vesicular stomatitis virus G protein (VSV-G) epitope tag (hcp1-V) (Mougous et al., 2006a). We also introduced deletions with known effects on Hcp1 secretion (ΔtagR, ΔppkA, ΔpppA, or ΔtssM1) into this genetic background as controls (Hsu et al., 2009, Mougous et al., 2006a, Mougous et al., 2007). Consistent with earlier findings, ΔpppA displayed high levels of Hcp1 secretion, whereas secretion from ΔtagR, ΔppkA, and ΔtssM1 did not exceed the wild-type (Fig. 1B). Interestingly, Hcp1 secretion from ΔtagF was increased relative to the wild-type strain. Deletions in tagQ, S, and T did not induce Hcp1 export, and none of the mutations significantly influenced cellular Hcp1 levels.
Hcp1 is considered a structural component of the T6SS; therefore, secretion of the protein is not necessarily indicative of an activated system. As an additional measure of H1-T6SS activation, we probed the effects of the same panel of deletions on the secretion of Tse1, a previously identified effector protein (Hood et al., 2010). While we observed reproducibly lower levels of secreted Tse1 from ΔtagF than from ΔpppA (discussed below), the strains showed a similar trend as that observed for Hcp1 secretion, suggesting that a tagF deletion results in constitutive activation of the H1-T6SS (Fig. 1C). Based on these data, we sought to further define the role of tagF in T6S regulation.
TagF posttranslationally represses the H1-T6SS
The tagF open reading frame is located immediately upstream of pppA in the genome of P. aeruginosa (Fig. 1A). To verify that the secretion phenotype of ΔtagF is not due to polar effects on pppA or other downstream genes, we genetically complemented the strain using an ectopic expression plasmid. Western blot analyses demonstrated that tagF expression returns secretion of Hcp1 and Tse1 to parental levels in the ΔtagF background (Fig. 2A). We further confirmed that Hcp1 and Tse1 export in ΔtagF occurs in a T6S-dependent manner. The addition of a clpV1 deletion to ΔtagF abrogated secretion of Hcp1 and Tse1 (Bonemann et al., 2009, Mougous et al., 2006a). Secretion of both proteins was restored by genetic complementation of the clpV1 gene (Fig. 2B).
Fig. 2.

TagF is a negative posttranslational regulator of the H1-T6SS. (A) Western blot results demonstrating Hcp1 and Tse1 secretion phenotypes of ΔtagF can be genetically complemented. (B) Hcp1 and Tse1 secretion in ΔtagF requires a functional H1-T6S apparatus. Western blot analysis of Hcp1–V and Tse1–V in supernatant (Sup) and cell-associated (Cell) fractions of the indicated genetic backgrounds of P. aeruginosa. (C and D) HSI-I expression is not altered by the deletion of tagF. β-galactosidase activity was measured from the indicated P. aeruginosa strains carrying a chromosomally-encoded lacZ translational fusion to fha1 (C) or tssA1 (D) (Brencic & Lory, 2009). Genes involved in the production of the Pel and Psl polysaccharides were deleted in the ΔretS background to prevent cell clumping during growth (Colvin et al., 2011, Ryder et al., 2007). Error bars represent standard deviation based on three independent replicates. Asterisks indicate statistically significant (P < 0.001) differences between ΔretS ΔpelA ΔpslD and wild-type or ΔtagF as determined by one-way ANOVA and Tukey’s post-hoc tests.
Possible explanations for the influence of TagF on Hcp1 and Tse1 export by the H1-T6SS apparatus include changes in the expression levels of these proteins, translational or transcriptional induction of the secretion apparatus as a whole, and posttranslational activation of the secretory apparatus. Overall levels of Hcp1 and Tse1 were unaltered in ΔtagF (Fig. 1B and C), suggesting that increased expression of these proteins does not underlie the secretion phenotype observed. To investigate whether TagF imparts a general influence on T6S expression levels, we employed translational lacZ fusion reporters to the fha1 and tssA1 genes, which are located immediately downstream of previously identified HSI-I promoters (Brencic & Lory, 2009). Inactivation of tagF did not influence the activities of these reporters; however, as described previously, both were strongly induced in a strain lacking a repressor of the Rsm pathway (RetS) (Fig. 2C and D) (Brencic & Lory, 2009). From these data we conclude that TagF represses activation of the H1-T6SS on a posttranslational level.
TagF mediates activation of the H1-T6SS independently of p-Fha1
Next we considered whether TagF regulates the H1-T6SS via the TPP or independently of this pathway. Several lines of evidence suggest that TagF acts through the TPP. TagF orthologs are not located outside of T6S gene clusters, and within these clusters the gene is often found in a subgroup of locally syntenic genes that also includes orthologs of ppkA and pppA (Fig. S1). Furthermore, in several instances, including the T6S gene clusters of Agrobacterium tumefaciens, Nitrococcus mobilis and Rhizobium leguminosarum, tagF and pppA orthologs appear fused, thereby generating one open reading frame with apparent dual function (Fig. S1). Such fusion events are enriched among genes encoding interacting proteins or proteins participating in common pathways (Enright et al., 1999, Skrabanek et al., 2008). However, there is also strong genomic-based evidence arguing against TagF participation in the TPP. Most notably, tagF orthologs are sometimes present in T6S clusters lacking TPP components, including fha1 (Fig. S1).
Previous reports have shown that elevated levels of p-Fha1 lead to posttranslational activation of the H1-T6SS. Therefore, we postulated that if TagF directs activation of the H1-T6SS through the TPP, p-Fha1 levels might be elevated in a ΔtagF strain. To test this, we took advantage of an established SDS-PAGE mobility-based assay for monitoring p-Fha1 levels in P. aeruginosa (Mougous et al., 2007). Our analyses indicate that unlike ΔpppA, the ΔtagF strain does not possess p-Fha1 levels above those of the parental strain (Fig. 3A).
Fig. 3.

TagF-mediated activation of the H1-T6SS occurs independently of the TPP. (A) The deletion of tagF does not promote elevated levels of p-Fha1. Western blot analysis of Fha1–V in the indicated genetic backgrounds of P. aeruginosa. Phosphorylated Fha1 species (denoted by arrowheads) are separated from non-phosphorylated Fha1 by their electrophoretic mobility (Mougous et al., 2007). (B) Fha1, but not its phosphorylation, is required for TagF-mediated Hcp1 secretion. Western blot analysis of Hcp1–V secretion and cellular Fha1 levels in the indicated strains of P. aeruginosa. Expression plasmids used in each lane are indicated. Fha1 was detected with α-Fha1 antibodies. (C and D) HSI-I genes encoding TPP components and other tag genes are not required for Hcp1 and Tse1 secretion in P. aeruginosa strains lacking tagF. Western blot analysis of Hcp1–V and Tse1–V in the indicated P. aeruginosa strains. (ΔtagQ-ΔpppA, ΔtagQ ΔtagR ΔtagS ΔtagT ΔppkA ΔpppA; ΔtagQ-ΔtagF, ΔtagQ ΔtagR ΔtagS ΔtagT ΔppkA ΔpppA ΔtagF).
Based on our finding that p-Fha1 levels are unaffected by tagF deletion, we tested the hypothesis that TagF acts independently of the TPP, and thus, p-Fha1 should be dispensable in ΔtagF. We observed that fha1 deletion in the ΔtagF background inactivates the H1-T6SS as judged by Hcp1 secretion (Fig. 3B). This implies either that Fha1 must be phosphorylated at a basal level for TagF-mediated activation or that it functions in an important capacity independent of its phosphorylation. The latter is supported by genomic analyses, which found that one quarter of T6S gene clusters containing fha1 orthologs do not encode identifiable kinase or phosphatase genes (Boyer et al., 2009). To distinguish between the two possibilities, we conducted Δfha1 genetic complementation studies in the ΔtagF and ΔpppA backgrounds using wild-type fha1 or an allele encoding non-phosphorylatable Fha1 (fha1T362A). Wild-type fha1 restored secretion apparatus function in both backgrounds, however only ΔtagF Δfha1 was functional when complemented with fha1T362A (Fig. 3B). Together, these data conclusively demonstrate that Fha1 performs an important function in the T6SS independent of its phosphorylation state, and that TagF activation of the H1-T6SS proceeds independently of p-Fha1.
To further probe the relationship between TagF and TPP-mediated T6S activation, we conducted epistasis experiments monitoring Hcp1 and Tse1 secretion. Immediately upstream of Fha1 in the TPP is PpkA, which catalyzes Fha1 phosphorylation and is essential for T6S-apparatus assembly in a constitutively active background (ΔpppA) (Hsu et al., 2009). While ppkA is essential for Hcp1 and Tse1 secretion in the ΔpppA background, we found that the gene is dispensable for these functions in ΔtagF (Fig. 3C). Further upstream of PpkA in the TPP is TagR. This protein appears to regulate the kinase, as inactivation of tagR decreases p-Fha1 levels, and ectopic expression of PpkA overrides the requirement for tagR in H1-T6SS activation (Hsu et al., 2009). Three additional tag genes flank tagR (tagQ, S, and T), and have been postulated to also participate in the TPP. Remarkably, deletion of the four tag genes in conjunction with deletions of ppkA and pppA, did not influence Tse1 or Hcp1 secretion via the H1-T6SS in the ΔtagF background. In contrast, the H1-T6SS failed to export these proteins when tagF was present in this background (Fig. 3D). Together, these data show that the tagF deletion is epistatic to deletions in genes encoding the TPP core component PpkA and the TPP positive regulatory protein TagR. This suggests that TagF represses activation of the H1-T6SS by acting independently of the TPP. The role of tagQ, S, and T remains unknown, however our results show that these genes also encode proteins that are dispensable for H1-T6SS effector secretion in a P. aeruginosa background lacking tagF.
The TagF and TPP-mediated pathways differentially influence Hcp1 and Tse1 secretion
The finding that a deletion in tagF alleviates the requirement for ppkA, suggests that TagF acts either downstream or independently of the TPP. To investigate this, we conducted quantitative analyses comparing Hcp1 and Tse1 secretion from strains with H1-T6S derepressed in either of the pathways (ΔtagF or ΔpppA) or both (ΔtagF ΔpppA). Only if the pathways act independently or partially independently would we anticipate observing additive or synergistic effects on secretion. Surprisingly, we found that the double deletion of pppA and tagF had differential effects on Hcp1 and Tse1 secretion relative to the single deletion strains. Levels of secreted Hcp1 were similar in the three deletion strains, whereas the deletion of the regulators influenced Tse1 export in an additive fashion (Fig. 4). The observation that one output of the pathways-effector secretion-is induced additively upon their activation suggests that TagF does not act purely by repressing downstream of the TPP. Furthermore, our quantitative analyses proved consistent with our qualitative observation that more Tse1 is exported by ΔpppA than ΔtagF (Fig. 1C and Fig. 2A). This is noteworthy, as this difference in output is also inconsistent with a simple epistatic relationship between the pathways.
Fig. 4.

Hcp1 and Tse1 secretion are differentially regulated by the TPP and TagF-mediated pathways. (A-D) Representative Western blots of Hcp1–V (A) and Tse1–V (C) secretion from the indicated genetic backgrounds of P. aeruginosa. (B and D) Quantitative analyses of Hcp1 (B) and Tse1 (D) secretion based on band densitometry from independent Western blot experiments similar to those shown in A and C, respectively. Data are normalized to ΔpppA ΔtagF. Error bars represent standard deviation based on three independent replicates. Asterisks indicate secretion is significantly less than the ΔpppA ΔtagF background based on ANOVA and Tukey’s post-hoc test (P < 0.05).
The TPP and TagF-mediated activation pathways converge on ClpV1 recruitment
Using fluorescence microscopy (FM), our laboratory previously demonstrated that a chromosomally encoded functional green fluorescent protein fusion to the H1-T6SS ATPase ClpV1 (ClpV1–GFP) is recruited to secretion foci in the cell upon activation of the apparatus via p-Fha1 (Mougous et al., 2007). The formation of these foci is thought to reflect maturation of the secretion system, as their presence and intensity correlates with the level of secreted Hcp1 and deletion of essential apparatus components results in their dissolution. Notably, while recruitment of ClpV1 to the H1-T6S apparatus is enhanced by Fha1 phosphorylation, the physical interaction between these proteins is phosphorylation independent (Hsu et al., 2009). Thus, we reasoned that if TagF-mediated activation converges on the TPP downstream of p-Fha1, ClpV1 recruitment to the H1-T6S-apparatus should also be observed in the ΔtagF strain. To test this hypothesis, we introduced clp–gfp to the ΔtagF background and examined the cells by FM. The absence of tagF promoted ClpV1–GFP recruitment to an extent similar to that observed in the ΔpppA control, and ectopic expression of tagF in the ΔtagF background fully complemented the phenotype (Fig. 5). The formation of these foci required ppkA in the ΔpppA background, but not the ΔtagF background. This mirrors the requirement for ppkA with regard to Tse1 and Hcp1 secretion in these two backgrounds, and indicates that the formation of foci correlates with a functional active secretory apparatus (Fig. 5). Further consistent with the functional significance of ClpV1-GFP foci, we found that a strain lacking both pppA and tagF displayed higher levels of ClpV1-GFP foci than those with individual deletions in these genes. Therefore, ClpV1 recruitment to foci is proportional to effector export from these strains. Differences in the number of ClpV1-GFP foci-positive cells among the strains were not due to altered levels of the ClpV1 protein (Fig. S2). Based on these data, we conclude that TagF and TPP-mediated T6S activation share a convergence in mechanism at the level of ClpV1–GFP recruitment to the secretion apparatus. Interestingly, previous work from our laboratory had led us to conclude that PpkA is an essential structural component of the H1-T6SS (Hsu et al., 2009). Our current work clearly indicates that the structural requirement for PpkA is conditional on TagF. This in part reconciles the observation that PpkA orthologs are absent from the majority of identified T6SSs (Mougous et al., 2007).
Fig. 5.

ClpV1 recruitment to the H1-T6S-apparatus is triggered by TagF-mediated activation. (A) Increased ClpV1–GFP recruitment to the H1-T6S-apparatus is promoted by a tagF deletion and occurs independent of the kinase (ΔtagF ΔppkA) as observed by the formation of punctate foci using fluorescence microscopy (indicated by white triangles). The lipophilic dye, TMA-DPH, was used to visualize bacterial membranes. (B) Quantitative analysis of ClpV1–GFP foci positive cells in the indicated backgrounds (parental background PAO1 clpV1–gfp). Error bars represent standard deviation based on three independent replicates. Asterisks indicate the number of ClpV1–GFP foci per cell are statistically higher than the parental or lower than ΔpppA ΔtagF based on ANOVA and Tukey’s post-hoc test (P < 0.002).
The H1-T6SS is activated by surface growth
The H1-T6SS efficiently targets Tse2 to other P. aeruginosa cells. If recipient cells lack the Tse2-specific immunity protein, Tsi2, their fitness is decreased relative to donors actively exporting Tse2 through a functional H1-T6S apparatus (Hood et al., 2010). Here, we used the self-targeting capability of the H1-T6SS as a means to probe the functional consequences of mutations that posttranslationally activate the H1-T6SS (ΔpppA and ΔtagF).
The influence of regulatory mutations on intercellular effector targeting by the H1-T6SS was assessed by measuring the fitness of donor strains competed against a susceptible recipient (Δtse2 Δtsi2). Interbacterial transfer of effectors by the T6SS requires intimate cell contact; therefore, competition assays were performed between strains co-inoculated on a solid growth substrate. When competed in this manner, the parental donor strain displayed a 3.2-fold fitness advantage relative to the recipient. This effect was T6S-dependent, as the deletion of clpV1 in the donor strain negatively impacted its fitness advantage. Donor strains lacking pppA or tagF showed a fitness advantage comparable to the parental. All strains displayed equal fitness when cultivated in liquid media, consistent with the known requirement for intimate cell contact in T6S-dependent fitness changes (Fig. 6A) (Hood et al., 2010, Schwarz et al., 2010b).
Fig. 6.

The H1-T6SS is posttranslationally activated by the TPP when P. aeruginosa is grown on a surface. (A) Deletions in pppA or tagF do not influence T6S-dependent fitness. Results of growth competition assays conducted on a solid surface (open circles) or in liquid media (filled circles) between the indicated donor strains and a susceptible recipient (Δtse2 Δtsi2). Asterisks indicate a competitive index that is statistically lower than the parental strain based on ANOVA followed by Tukey’s post-hoc test (P < 0.05). (B) p-Fha1 levels are increased when P. aeruginosa is grown on a surface. Arrowheads denote p-Fha1 species. For each strain, the percentage of total Fha1–V in the p-Fha1–V form based on band densitometry is provided. Data are normalized to ΔppkA. Error provided is standard deviation based on three independent replicates. (C) Secretion of Hcp1 by the H1-T6SS is triggered by surface growth. Western blot analysis of secreted and cell-associated Hcp1–V.
The finding that ΔpppA and ΔtagF did not increase T6S-dependent fitness of P. aeruginosa was unexpected, and led us to hypothesize that – unlike in our secretion assays – the apparatus is posttranslationally activated under conditions of the competition experiments. To investigate further the posttranslational activation state of the H1-T6SS under competition assay conditions, we probed the requirement for ppkA in the fitness advantage of ΔpppA against recipient bacteria. We reasoned that if the TPP was triggered, deletion of the kinase – even in an activated ΔpppA background – should inactivate the system. However, if TagF-mediated repression was fully relieved (as in ΔtagF), a strain lacking both ppkA and pppA should remain functional, as was observed in secretion and apparatus assembly assays (Fig. 3D and Fig. 5). Interestingly, the ΔpppA ΔppkA strain does not possess a T6S-dependent fitness advantage over recipient bacteria. This is not attributable to inactivation of the apparatus due to the loss of PpkA, as ΔtagF ΔppkA retained the full fitness advantage of the parental strain (Fig. 6A). In total, these results suggest that the H1-T6SS is activated posttranslationally by the TPP under the conditions of our in vitro growth competition assay.
Next we sought to directly measure the activation state of the H1-T6SS under growth competition assay conditions. We considered three substantive differences between the conditions of our secretion and competition assays that might underlie the apparent change to the activation state of the H1-T6SS: 1) the time period over which the two experiments were conducted (secretion, 4 hr; competition, 18 hr), 2) the number of discrete P. aeruginosa genetic backgrounds present, and 3) propagation in liquid (secretion assay) versus solid (competition assay) media. Multiple lines of evidence indicated that the latter difference was most likely the key determinant of activation of the secretion system under competition conditions. In particular, we noted that all T6SS-dependent interactions observed to date require intimate cell contact. The release of effectors into the milieu is not productive, which is exemplified in the observation that strains bearing mutations leading to strong constitutive activation of the H1-T6SS have no fitness advantage over highly susceptible recipients in liquid cultures (Hood et al., 2010, Russell et al., 2011). Thus, it is logical that bacteria should repress T6S during planktonic growth and relieve this repression when grown in an environment containing a high density of closely interacting bacteria that could be effectively targeted.
If surface growth was responsible for H1-T6SS posttranslational activation under competition conditions, we would expect to observe changes in substrate secretion and p-Fha1 levels in the wild-type background relative to those observed in liquid secretion assays performed under otherwise similar conditions (see Methods). Densitometry analysis of p-Fha1 levels observed by Western blot indicated that growth on a surface strongly promotes Fha1 phosphorylation; approximately 20.1% of the protein was phosphorylated after growth on a solid surface, whereas the phosphorylated species was only 4.3% of total Fha1 in liquid grown cells (Fig. 6B). Enhanced Fha1 phosphorylation incurred during surface growth required PpkA, consistent with our model of posttranslational activation by the TPP.
To probe more directly the activation state of the H1-T6SS during growth on a solid substrate, we measured Hcp1 secretion from surface grown cells. For this experiment, it was necessary to place cells harvested from the substratum into liquid medium for a brief period to allow the accumulation of secreted proteins. H1-T6S-dependent secretion of Hcp1 was greatly increased by surface growth of P. aeruginosa. Indeed, levels of the secreted protein were comparable between parental and constitutively triggered backgrounds (Fig. 6C). It is noteworthy that the TPP and TagF-mediated pathways appear to have no role in promoting H1-T6SS activation beyond that observed during surface growth. This finding explains our aforementioned observation that wild-type, ΔpppA, and ΔtagF strains display equal T6S-dependent fitness (Fig. 6A). In addition, our finding that ΔtagF is activated through the TPP during surface growth (p-Fha1 levels equal to wild-type), suggests that the fitness advantage exhibited by this mutant is phenotypically equivalent to ΔtagF ΔpppA.
Discussion
The results of our current study lead us to propose a new model of posttranslational activation of the H1-T6SS (Fig. 7). As in previous models, Fha1 remains a pivotal protein in the activation process. However, it is now clear that ClpV1 recruitment to the apparatus and apparatus activation can occur via Fha1 phosphorylation-dependent (TPP-mediated) and independent processes (TagF-mediated). We propose that distinct inputs received by the cell regulate these two convergent mechanisms of H1-T6SS activation. Our data argue that TPP-dependent activation, which requires PpkA, is stimulated by a cue deriving from the physiological changes encountered during surface growth. On the other hand, phosphorylation-independent activation, which is strongly repressed by TagF and does not require PpkA in any capacity, appears not to occur under such circumstances. One appealing hypothesis is that TagF posttranslational derepression of the H1-T6SS is modulated by sensing of non-P. aeruginosa bacterial species. Our current study was limited to analyses of H1-T6SS activation during intraspecies growth competition experiments (Fig. 6A). Intriguingly, we note that in addition to the P. aeruginosa H1-T6SS, two other bacterial cell-targeting T6SSs, B. thailandensis T6SS-1 and the T6SS of S. marcescens, appear to utilize TagF proteins. P. aeruginosa and B. thailandensis encode six additional T6SSs – none of which have been implicated in interbacterial interactions. Among these, only B. thailandensis T6SS-6 possesses a tagF ortholog (Hood et al., 2010, Schwarz et al., 2010b). The vas system of V. cholerae complicates this simple correlation between bacterial cell targeting and the presence of TagF. This system appears to target both bacteria and eukaryotic cells and does not possess a tagF gene (MacIntyre et al., 2010).
Fig. 7.
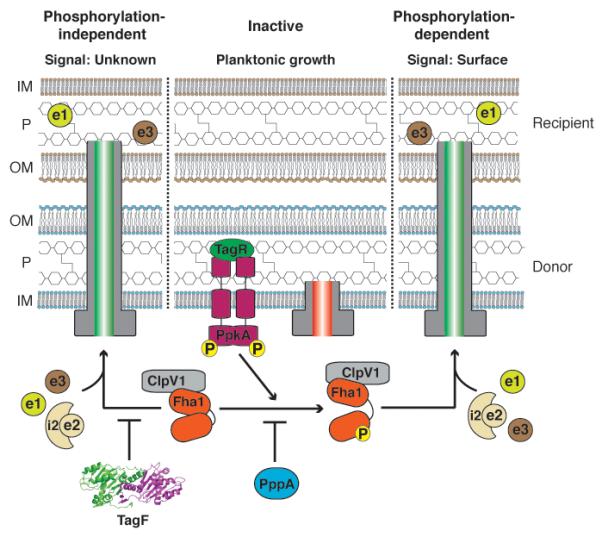
Model of H1-T6SS posttranslational regulatory pathways. The schematic depicts the interface between P. aeruginosa (donor, blue) and a competing Gram-negative bacterium (recipient, brown). Inner membrane (IM), outer membrane (OM), and periplasm (P) of the cells are shown. Emphasis is given to peptidoglycan (interconnected hexagon chains), the target of Tse1 and Tse3 (Russell et al., 2011). Based on data from this study and previous studies, we propose two pathways for H1-T6SS-activation. During planktonic growth, the H1-T6S apparatus is not extended (red tube) and Tse1-3 (e1-3) secretion is repressed. Upon surface growth, the TPP is stimulated (right). This leads to PpkA activation, p-Fha1 formation, ClpV1 recruitment, extension of the apparatus (green tube), and effector delivery into recipient bacterial cells. Relevant phosphorylation events are indicated (yellow spheres). The H1-T6SS can also be activated by derepression of TagF through an unknown signal (left). When TagF repression is relieved, non-phosphorylated Fha1 activates the apparatus by a mechanism also involving ClpV1 recruitment.
At this point, we cannot exclude the assertion that TagF prevention of phosphorylation-independent T6S activation is static (structural) rather than dynamic (regulatory). For example, TagF may act simply as a barrier that prevents non-phosphorylated Fha1 or downstream H1-T6S-components from activating the H1-T6SS. One compelling argument against this model is that TagF appears in many T6SSs that lack Fha1, PpkA and PppA orthologs (Fig. S1) (Boyer et al., 2009). This strongly suggests that phosphorylation-independent posttranslational activation mechanisms for T6S are likely to exist. In addition, this observation leads us to speculate that TagF and TPP-mediated activation converge downstream of Fha1. Another indication that TagF is part of a dynamic regulatory pathway derives from our comparison of Hcp1 and Tse1 secretion from P. aeruginosa strains with H1-T6S activity derepressed as the result of mutations in one versus both pathways. We observed Hcp1 secretion is enhanced equally by individual deletions in tagF and pppA, and that in combination the deletions do not display an additive effect on secretion of the protein. On the contrary, the absence of the negative regulators triggers Tse1 secretion to different extents, and, in combination, the mutations display an additive effect on the export of this effector and apparatus assembly. This latter result argues that TagF is not simply a downstream repressor of the TPP and suggest that inputs received from TagF and the TPP are used to tune effector export by the H1-T6SS.
The X-ray crystal structure of TagF was recently determined as part of a structural genomics effort (Fig. S3) (Filippova et al.). In the structure, TagF is found to associate as a homodimer with nearly identical monomers composed of α and β elements assembled as a three-layer sandwich (α-β-α). The TagF monomer is not closely related to other solved structures, however it bears some similarity to the N-terminal regulatory domain of the eukaryotic SNARE protein, Sec22b (DALI, r.m.s.d. = 3.3 Å over 87 residues; Z score = 3.9) (Fig. S4) (Rossi et al., 2004, Holm & Rosenstrom, 2010). SNARE proteins mediate vesicle trafficking and membrane fusion; therefore, it is unlikely that direct functional parallels between TagF and Sec22b can be drawn. Nonetheless, the structures of Sec22b and TagF contain homology to the actin regulatory protein profilin (Krishnan & Moens, 2009). In particular, they share the surface used by profilin to bind poly-proline sequences. It is conceivable that TagF interacts with proline-rich proteins via this motif. Interestingly, Fha1 has an extensive proline-rich domain of unknown function (amino acids 173-294; 36% proline) located between its N-terminal FHA domain and its C-terminal domain that is phosphorylated by PpkA (Mougous et al., 2007). So far we have not detected an interaction between these proteins. An important future direction will be to elucidate components of the TagF-mediated activation pathway by identifying TagF-interacting proteins.
Our finding that the H1-T6SS is activated by the TPP when P. aeruginosa is grown on a surface fits well with our current understanding of the environmental conditions physiologically relevant to T6SS function. It first became apparent that the system might be important to the sessile lifestyle of P. aeruginosa through circumstantial evidence, including the discovery that HSI-I is stringently co-regulated with exopolysaccharides involved in cellular aggregation and adhesion to surfaces (Goodman et al., 2004, Mougous et al., 2006a). Later, interactions between bacteria mediated by the T6SS were shown to be exquisitely dependent on intimate cell contact – ameliorated entirely in liquid medium (Hood et al., 2010). Perhaps the strongest evidence linking sessile physiology and T6S function is our recent finding that in the absence of T6SS-1, B. thailandensis is rapidly displaced from a flow-cell chamber by P. putida (Schwarz et al., 2010b). One candidate signaling molecule that may directly or indirectly be responsible for TPP activation during surface growth is cyclic-di-GMP. This molecule is a secondary messenger that modulates the expression of traits important for the transition between sessile and planktonic lifestyles of certain bacteria, including P. aeruginosa (Hengge, 2009). Direct regulation by c-di-GMP has been observed at both transcriptional and posttranslational levels (Boehm et al., 2010, Fang & Gomelsky, 2010, Hickman & Harwood, 2008, Paul et al., 2010). Given the requirement for sessile growth in T6S function and the precedent for c-di-GMP acting as a posttranslational regulator, it is reasonable to speculate that this molecule might directly or indirectly be responsible for TPP activation during surface growth. Indeed, c-di-GMP-dependent regulation of T6S in P. aeruginosa was recently reported by Filloux and colleagues (Moscoso et al., 2011). This study showed that c-di-GMP levels in P. aeruginosa ΔretS correlate to cellular Hcp1 levels, however the point(s) of regulation at which the signaling molecule acts was not determined, raising the possibility that c-di-GMP regulates the H1-T6SS on the posttranslational level as well.
Highly regulated triggering of effector export is likely to be a critical factor in the potency and efficiency of bacterial intercellular protein delivery machines. While the great majority of evidence for this claim derives from studies of the type III secretion system (Francis et al., 2002, Deane et al., 2010), our work on posttranslational activation of the H1-T6SS suggests that it may be equally true in this system. Our data indicate that the products of at least eight HSI-I genes are involved in regulating the activation state of the H1-T6S apparatus. Seven of these are wholly dispensable for the function of the system, suggesting that they are dedicated regulatory proteins. The complexity of T6S regulation rivaling that of type III is not surprising when one considers the consequences of either system releasing effectors out of step with target cell interaction. Effectors from both systems lack the ability to reach their ultimate sites of action without target cell engagement; therefore, a failure to precisely spatially and temporally coordinate effector release is a complete loss of the metabolic investment in their synthesis.
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The sequenced P. aeruginosa strain, PAO1, was used for this study (Stover et al., 2000). P. aeruginosa strains were grown in Luria-Bertani (LB) medium at 37°C supplemented with 30 μg mL−1 gentamicin, 300 μg mL−1 carbenicillin, 25 μg mL−1 carbenicillin, 100 μg mL−1 tetracyclin, 25 μg mL−1 irgasan, 5% w/v sucrose, 0.2% w/v arabinose or 0.5 mM IPTG as required. Plasmids and strains used in this study are shown in Table S1 and primers used to for cloning are listed in Table S2. Plasmids used for inducible expression in P. aeruginosa included pPSV35 (Fha1 and Fha1T362A complementation) and pPSV35-CV (complementation with TagF fused to C-terminal VSV-G tag) (Hsu et al., 2009, Rietsch et al., 2005), pSW196 (ClpV1 complementation) (Baynham et al., 2006), and pUC18-mini-Tn7 (lacZ20::PA0081, lacZ20::PA0082 yfp, cfp reporters) (Brencic & Lory, 2009, Lambertsen et al., 2004). pPSV18 (Rietsch et al., 2004) was used for constitutive expression of Tse2 and Tsi2. tse2 and tsi2 were cloned into pPSV35 using primers 591 and 743 and were subcloned into pPSV18 using restriction sites SacI and XbaI. Escherichia coli strains, DH5α and SM10, were used for plasmid maintenance and conjugal transfer and were grown at 37°C. P. aeruginosa strains were transformed with plasmids by electroporation or conjugation with E. coli SM10.
Chromosomal fusions and mutations in P. aeruginosa
Chromosomal fusions and in-frame deletions were generated by allelic replacement using the pEXG2 suicide plasmid (Mougous et al., 2006b, Rietsch et al., 2005). The sequences used for these constructs were made by splicing by overlap extension PCR and cloned into the 5′ XbaI and 3′ HindIII sites of pEXG2. The primers used for amplification were designed such that the first several codons were fused to the last several codons with an intervening sequence of 5′-TTCAGCATGCTTGCGGCTCGAGTT-3′. For tagQ, tagS, tagT, tagF, pppA tagF deletion constructs, upstream DNA flanking sequences were amplified using primer pairs 871/872, 471/472, 774/775, 291/292 and 291/292, respectively. Downstream flanking DNA sequences were amplified with primer pairs 873/874, 473/474, 776/777, 293/294 and 564/565, respectively. For the deletion of the accessory gene cluster (PA0070-PA0075 and PA0070-PA0076), flanking and overlapping primers were made for the 5′ and 3′ genes (Primers 873/874 and 60/1395 or 291/292) (Table S2). Chromosomal VSV-G epitope tagged hcp1, tse1 and fha1 were also generated by splicing by overlap extension PCR and cloned into pEXG2 (Mougous et al., 2006a, Mougous et al., 2007). The VSV-G sequence 5-TATACAGATATTGAAATGAATAGATTAGGAAAATGA-3′ was used to replace the stop codon of each gene. Upstream and downstream primer pairs used to generate tse1-VSV-G were 596/597 and 598/599. Strains harboring clpV1-gfp chromosomal fusions were generated as described previously (Mougous et al., 2007). The pUC18-mini-Tn7 and pSW196 derivatives were transformed into P. aeruginosa by conjugation. All chromosomal mutations were confirmed through PCR analysis.
β-Galactosidase assay
A chromosomal insertion vector carrying a fusion of lacZ to the fha1 or tssA1 promoters, pUC18-mini-Tn7-lacZ20-Gm::PA0081 and pUC18-mini-Tn7-lacZ20-Gm::PA0082, respectively, were used to generate all strains for analysis. The sequences used for these fusions contained the promoter of each indicated strain, the 5′ leader sequence and the first several coding nucleotides (Brencic & Lory, 2009). The plasmids were introduced into P. aeruginosa by four parental mating conjugation or electroporation (Choi and Schweizer, 2006). The vector backbones were not removed, except for strains MLS 1526 and MLS 2715. Overnight cultures were diluted 1:1000 in LB with 50 mM MOPS pH 7 and grown to mid-log phase. Cells were permeabilized with chloroform (15% chloroform) and assayed using the Tropix Galacon-Plus® reagents (Tropix, Bedford, MA). Luminescence was measured by using a GENios Pro-Basic microplate reader (Tecan Group Ltd. Mannedorf, Switzerland). Values were normalized to the optical density at 600 nm for each strain. All β-galactosidase assays were performed in triplicate. Statistical significance was determined using a one-way analysis of variance (ANOVA) and Tukey’s post-hoc test.
Preparation of proteins and Western blotting
Protein samples from liquid grow cultures were prepared as previously described (Hsu et al., 2009). Specifically, overnight cultures of P. aeruginosa were used to inoculate 2 mL of LB (1:1000) supplemented with appropriate additives. Cultures were grown at 37°C with shaking at 250 r.p.m. Samples were harvested at mid-log phase by centrifugation at 9,000 r.p.m. for 3 minutes and 1.3 mL of the supernatant were centrifuged a second time to remove contaminating bacterial cells. After this second step, 1 mL of the supernatant fractions was treated with 110 μL 100% w/v Trichloroacetic acid (final concentration of 10%). To collect precipitated proteins, the samples were centrifuged at for 30 minutes at 13,500 r.p.m. Supernatants were removed and the protein pellets were washed with 1 mL of 100% acetone and centrifuged at 4°C for 15 minutes at 13,500 r.p.m. The protein pellets were resuspended in 20 μL of SDS-PAGE sample loading buffer. To isolate cell-associated protein samples, the cell pellets were resuspended in 100 μL of Buffer 1 (0.5 M NaCl, 50 mM Tris pH 7.5 and 10% glycerol) and mixed 1:2 with SDS-PAGE sample loading buffer. The secreted and cell-associated protein samples were analyzed by Western blotting as previously described (Mougous et al., 2006) using rabbit α-VSV-G (1:5000, Sigma) or rabbit α-Fha1 (diluted 1:5000) and detected with α-rabbit horseradish peroxidase-conjugated secondary antibodies (Sigma). Mouse α-GFP (1:1000, Roche) or mouse α-RNA polymerase β-subunit antibody (1:2000, Neoclone) and α-mouse horseradish peroxidase-conjugated antibodies (Sigma) were used to detect ClpV1-GFP or RNAP. Western blots were developed using chemiluminescent substrate (SuperSignal West Pico Substrate, Thermo Scientific) and imaged with a FluorChemQ (ProteinSimple).
For surface grown strains, overnight cultures were diluted 1:100 in LB and 3 mL of this dilution was spotted onto a 0.2 mM polycarbonate membrane placed on onto LB agar. After approximately 4 hours of growth at 37°C the cells were resuspended by placing the filter in 2 mL of LB and incubating at 37°C with shaking (250 r.p.m.) for 5 minutes. Cell and supernatant samples were then collected as described above
Densitometry was performed using AlphaView®Q software (ProteinSimple). The percentage of phosphorylated Fha1 was determined by measure band intensity of phosphorylated and total Fha1 from three independent experiments. The values were normalized to ΔppkA, which was set at 0% p-Fha1.
Preparation of supernatant protein samples for quantitative analysis
Supernatant samples analyzed for Tse1 and Hcp1 in the ΔpppA ΔtagF backgrounds were prepared using a filter based method. A total of 4 mL were grown for each strain (2 mL/tube) and were harvested by centrifugation in a 15 mL Falcon tube (Becton Dickinson) at 4,000 r.p.m. in a swing bucket rotor for 5 minutes. The supernatants were filtered over through a 0.2 μM Supor® Membrane (Pall Life Sciences) into two 2 mL microcentrifuge tubes containing 250 μL of 100% w/v Trichloroacetic acid. The precipitation and acetone wash were carried out as described in the previous section. Washed samples were then resuspended in 50 μL of SDS-PAGE sample loading buffer. Densitometry was performed using AlphaView®Q software (ProteinSimple). Statistical significance was determined using ANOVA and Tukey’s post-hoc test.
Fluorescence microscopy
P. aeruginosa strains were cultured in an identical manner as described for protein sample preparations. All samples were harvested at mid-log phase by centrifuging at 7,000 r.p.m and resuspending the pellet to an optical density (600 nm) of 5 with PBS supplemented with 0.5mM TMA-DPH, for membrane staining. Three microliters of the mixture were spotted onto a 1% PBS agarose pad. All images were acquired with the same exposure settings on a Nicon 80i microscope with a 100X PlanAprochromat objective (numerical aperature 1.4) and Chroma Technology Corp. filter sets. Images were recorded using a CoolSnap HQ camera (Photometerics). DAPI and GFP were used for imaging TMA-DPH and ClpV1-GFP, respectively. MetaMorph 6.3r2 software was used (Hsu et al., 2009). Three randomly selected frames containing 200 ≤ N ≤ 700 cell were chosen for the analysis of GFP foci. Statistical significance was determined using ANOVA and Tukey’s post-hoc test.
Interbacterial growth competition assays and quantification
Overnight cultures were mixed at a 1:1 ratio to a total density of approximately 1.0×108 CFU/mL in 1 mL LB medium. In each experiment the donor and recipient strain contained constitutively expressing yellow fluorescent protein (YFP) or cyan fluorescent protein (CFP), respectively. To construct these strains the mini-Tn7 system, pUCP18-mini-Tn7 containing yfp or cfp, was inserted on the PAO1 chromosome at the neutral phage attachment site, attB, downstream of the glmS (Lambersten et al., 2004). The plasmids were introduced into P. aeruginosa via four-parental mating conjugation or electroporation (Choi and Schweizer, 2006). The vector backbones were not removed. For our assays, over-expression of Tse2 and Tsi2 in the donor strain was required for the H1-T6S-dependent fitness advantage. Donor strains harbored pPSV18::PA2702 PA2703 for constitutive expression of Tse2 and Tsi2. Recipient strains harbored the empty vector, pPSV18 (Rietsch et al., 2004). Competitions were grown on 0.2 mM polycarbonate membranes on LB agar for 18 hours at 37°C, or in 2 mL of LB with shaking. Cells were resuspended in LB medium and spotted onto 1.0% agarose PBS pads and imaged as described above for FM. YFP and CFP filters were used to image the two cell populations. Assays were performed in triplicate. Three fields containing 100 to 200 cells were imaged for each competition. To determine the competitive index (the number of YFP positive cells to CFP positive cells) MetaMorph® computer-assisted morphometry based on area was used. Thresholds were set identically for each image generated using YFP and CFP filters. The areas of the thresholded regions were calculated using MetaMorph® software and the ratios of YFP and CFP labeled cells were calculated. Statistical significance was determined using ANOVA and Tukey’s post-hoc test. Grubbs’ test was used to detect outlier data points.
Generation of TagF structure images
PyMOL was used to generate molecular graphic images (http:://www.pymol.org) (Schrodinger, 2010). The TagF (PA0076) X-ray crystal structure (Protein Data Bank, http://www.rcsb.org accession code 2QNU) was solved by the Midwest center for structural genomics (Filippova et al.). Structural superpositions were determined by DALI (Holm & Rosenstrom, 2010).
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Stephen Lory for the kind gift of the pUC18-mini-Tn7-lacZ20-Gm::PA0081/82 vectors, Ina Attree, Guillermina Casabona, Sylvie Elsen, and members of the Mougous laboratory for review of the manuscript, and Carrie Harwood and members of the Harwood laboratory for the use of their fluorescence microscope. J.M.S. was supported in part by Public Health Service, National Research Service Award, T32 GM07270, from the National Institute of General Medical Sciences. This work was funded by grants to J.D.M from the NIH (AI080609) and the Burroughs Wellcome Foundation Investigator in the Pathogenesis of Infectious Disease Program (BWF####).
References
- Aschtgen MS, Bernard CS, De Bentzmann S, Lloubes R, Cascales E. SciN is an outer membrane lipoprotein required for Type VI secretion in enteroaggregative Escherichia coli. J Bacteriol. 2008;190:7523–7531. doi: 10.1128/JB.00945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschtgen MS, Thomas MS, Cascales E. Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP… what else? Virulence. 2010;1:535–540. doi: 10.4161/viru.1.6.13732. [DOI] [PubMed] [Google Scholar]
- Baynham PJ, Ramsey DM, Gvozdyev BV, Cordonnier EM, Wozniak DJ. The Pseudomonas aeruginosa ribbon-helix-helix DNA-binding protein AlgZ (AmrZ) controls twitching motility and biogenesis of type IV pili. J Bacteriol. 2006;188:132–140. doi: 10.1128/JB.188.1.132-140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard CS, Brunet YR, Gueguen E, Cascales E. Nooks and Crannies in type VI secretion regulation. J Bacteriol. 2010;192:3850–3860. doi: 10.1128/JB.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Current opinion in microbiology. 2008;11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. Second messenger-mediated adjustment of bacterial swimming velocity. Cell. 2010;141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Bonemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. The EMBO journal. 2009;28:315–325. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol. 2009;72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol. 2009;73:434–445. doi: 10.1111/j.1365-2958.2009.06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrowes E, Baysse C, Adams C, O’Gara F. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology (Reading, England) 2006;152:405–418. doi: 10.1099/mic.0.28324-0. [DOI] [PubMed] [Google Scholar]
- Cascales E. The type VI secretion toolkit. EMBO reports. 2008;9:735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, Parsek MR. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JE, Abrusci P, Johnson S, Lea SM. Timing is everything: the regulation of type III secretion. Cell Mol Life Sci. 2010;67:1065–1075. doi: 10.1007/s00018-009-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A, Christie PJ, C R, Fernandez T, Palmer G, Plano V, Pugsley AP. Secretion by numbers: protein traffic in prokaryotes. Mol Microbiol. 2006;62:308–319. doi: 10.1111/j.1365-2958.2006.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright AJ, Iliopoulos I, Kyrpides NC, Ouzounis CA. Protein interaction maps for complete genomes based on gene fusion events. Nature. 1999;402:86–90. doi: 10.1038/47056. [DOI] [PubMed] [Google Scholar]
- Fang X, Gomelsky M. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol. 2010;76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- Filippova EV, Chruszcz M, Skarina T, Kagan O, Cymborowski M, Savchenko A, Edwards AM, Joachimiak A, Minor W, G. (MCSG) M. C. f. S. Crystal structure of PA0076 from Pseudomonas aeruginosa PAO1 at 2.05 A resolution. 2007 [Google Scholar]
- Francis MS, Wolf-Watz H, Forsberg A. Regulation of type III secretion systems. Current opinion in microbiology. 2002;5:166–172. doi: 10.1016/s1369-5274(02)00301-6. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell. 2004;7:745–754. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Rosenstrom P. Dali server: conservation mapping in 3D. Nucleic acids research. 2010;38:W545–549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell host & microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F, Schwarz S, Mougous JD. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol Microbiol. 2009;72:1111–1125. doi: 10.1111/j.1365-2958.2009.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani AJ, Cotter PA. Type VI secretion: not just for pathogenesis anymore. Cell host & microbe. 2010;8:2–6. doi: 10.1016/j.chom.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru S. Structural similarity of tailed phages and pathogenic bacterial secretion systems. Proc Natl Acad Sci U S A. 2009;106:4067–4068. doi: 10.1073/pnas.0901205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Moens PD. Structure and functions of profilins. Biophys Rev. 2009;1:71–81. doi: 10.1007/s12551-009-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasekara HD, Miller SI. Threonine phosphorylation times bacterial secretion. Nature cell biology. 2007;9:734–736. doi: 10.1038/ncb0707-734. [DOI] [PubMed] [Google Scholar]
- Lambertsen L, Sternberg C, Molin S. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environmental microbiology. 2004;6:726–732. doi: 10.1111/j.1462-2920.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesic B, Starkey M, He J, Hazan R, Rahme LG. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology (Reading, England) 2009;155:2845–2855. doi: 10.1099/mic.0.029082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell host & microbe. 2009;5:234–243. doi: 10.1016/j.chom.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. The Pseudomonas aeruginosa sensor RetS switches Type III and Type VI secretion via c-di-GMP signalling. Environmental microbiology. 2011 doi: 10.1111/j.1462-2920.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006a;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nature cell biology. 2007;9:797–803. doi: 10.1038/ncb1605. [DOI] [PubMed] [Google Scholar]
- Mougous JD, Senaratne RH, Petzold CJ, Jain M, Lee DH, Schelle MW, Leavell MD, Cox JS, Leary JA, Riley LW, Bertozzi CR. A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006b;103:4258–4263. doi: 10.1073/pnas.0510861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ. The opportunistic pathogen Serratia marcescens utilises Type VI Secretion to target bacterial competitors. J Bacteriol. 2011 doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita S, Tokuda H. An ABC transporter mediating the membrane detachment of bacterial lipoproteins depending on their sorting signals. FEBS letters. 2006;580:1164–1170. doi: 10.1016/j.febslet.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell. 2010;38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR. The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc Natl Acad Sci U S A. 2009;106:4160–4165. doi: 10.1073/pnas.0900044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2005;102:8006–8011. doi: 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Wolfgang MC, Mekalanos JJ. Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infection and immunity. 2004;72:1383–1390. doi: 10.1128/IAI.72.3.1383-1390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi V, Banfield DK, Vacca M, Dietrich LE, Ungermann C, D’Esposito M, Galli T, Filippini F. Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends in biochemical sciences. 2004;29:682–688. doi: 10.1016/j.tibs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder C, Byrd M, Wozniak DJ. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Current opinion in microbiology. 2007;10:644–648. doi: 10.1016/j.mib.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger LLC. The PyMOL Molecular Graphics System. 2010 Version 1.3r1. In. [Google Scholar]
- Schwarz S, Hood RD, Mougous JD. What is type VI secretion doing in all those bugs? Trends Microbiol. 2010a doi: 10.1016/j.tim.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 2010b;6 doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology (Reading, England) 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- Skrabanek L, Saini HK, Bader GD, Enright AJ. Computational prediction of protein-protein interactions. Molecular biotechnology. 2008;38:1–17. doi: 10.1007/s12033-007-0069-2. [DOI] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A. 2006;103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Hasic M, Chen C, Wai SN, Milton DL. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environmental microbiology. 2009;11:3018–3028. doi: 10.1111/j.1462-2920.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Greenberg EP. The genetic basis for the commitment to chronic versus acute infection in Pseudomonas aeruginosa. Mol Cell. 2004;16:497–498. doi: 10.1016/j.molcel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hinz AJ, Nadeau JP, Mah TF. Pseudomonas aeruginosa tssC1 Links Type VI Secretion and Biofilm-Specific Antibiotic Resistance. J Bacteriol. 2011;193:5510–5513. doi: 10.1128/JB.00268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


