Abstract
Objective
Sporadic Jakob-Creutzfeldt disease (sCJD) and dementia with Lewy bodies (DLB) have overlapping clinical symptoms that can lead to their misdiagnosis. We delineated the clinical overlap between sCJD and DLB, and assessed the value of MRI to differentiate between them.
Methods
Medical records, MRI, EEG and CSF were reviewed from 56 sCJD and 30 DLB subjects.
Results
46% of sCJD subjects met probable DLB criteria and 40% of DLB subjects met probable CJD criteria. A greater proportion of sCJD subjects had cerebellar signs (66% vs. 10%, p<0.001), myoclonus (64% vs. 30%, p=0.002), and visual symptoms (other than hallucinations) (61% vs. 7%, p<0.001), whereas more DLB subjects had hallucinations (70% vs. 39%, p=0.007) and fluctuations (57% vs. 23%, p=0.002). Cortical and/or basal ganglia MRI DWI hyperintensities consistent with sCJD were seen in 96% of sCJD subjects but in none with DLB. Logistic regression in sCJD revealed that those meeting probable DLB criteria were more likely to have occipital lobe involvement on MRI (OR 1.4, p=0.058, model p=0.022). Parietal lobe involvement on MRI was a predictor of “Other Focal Cortical signs” (OR 1.9, p=0.021) in sCJD. EEG and CSF assessments lacked sensitivity for sCJD as 48% of sCJD patients had a negative EEG and 67% of the 36 sCJD patents with a CSF evaluation, had a negative or inconclusive result. Too few DLB patients had EEG or CSF to assess their utility.
Conclusion
Sporadic CJD and DLB have significant symptom overlap. MRI helps differentiate these diseases and is related to the signs/symptoms observed in sCJD.
Keywords: Creutzfeldt-Jakob disease, Lewy body disease, Lewy body dementia, diffusion-weighted imaging, DWI
Introduction
Jakob-Creutzfeldt disease (CJD) and dementia with Lewy bodies (DLB) are progressive neurodegenerative diseases characterized by abnormalities in cognition, movement and behavior. Although there are significant differences between these illnesses, there also is considerable clinical overlap, particularly early on, often causing these conditions to be confused with each other.1–3 Most patients with sporadic CJD (sCJD) have a short disease course with a survival of less than one year from first symptom to death, but some patients have longer survival with slower progression.4 Patients with DLB usually have a slower course with life expectancy varying from 3 to 7 years,5 but many present with precipitous decline, especially in the context of a delirium.2, 6 Several subjects referred to our center as suspected sCJD actually had DLB;3 conversely, we also found that some of our sCJD patients were initially suspected to have DLB.7
Brain MRI, and to a lesser extent electroencephalogram (EEG), can help differentiate these two diseases. MRI fluid attenuated inversion recovery (FLAIR) and particularly diffusion weighed imaging (DWI) sequences have high sensitivity and specificity for CJD.8–10 EEG shows periodic sharp wave complexes in only around two-thirds of sCJD subjects,11 which is relatively specific, but rarely even DLB can show these abnormalities in late stages.2, 12
We examined our sCJD and DLB cohorts to determine the clinical overlap between these two conditions, whether subjects with one diagnosis met diagnostic clinical criteria for the other diagnosis and whether the pattern of radiological abnormalities on the DWI in the sCJD patients were associated with specific signs and symptoms in the DLB diagnostic criteria.
Methods
Subjects
The records of all serial sCJD and DLB subjects evaluated at the University of California San Francisco (UCSF) Memory and Aging Center between January 2001 and July 2006 were reviewed (unblinded). All subjects (or their surrogates) provided written informed consent for this study and the study was approved by our Institutional Review Board. Only subjects with adequate records, including detailed neurological examinations and histories, were included. Six patients with a presumed diagnosis of DLB and 52 with sCJD were excluded from the analysis because of inadequate records. DLB subjects met possible or probable DLB criteria,13 and we required that they had a good quality FLAIR and/or DWI brain MRI at time of assessment (to further rule out other conditions). For inclusion in the analysis, sCJD subjects had to meet definite14 or probable UCSF15 or WHO16 sCJD criteria. Fifty-six subjects with UCSF and/or WHO probable (14%) or definite (86%) sCJD14, 15 and 30 DLB subjects meeting possible (13%) or probable (87%) McKeith criteria13 were identified. Although all sCJD subjects had MRI available at time of diagnosis, for only 45 sCJD subjects did we have an adequate DWI MRI at the time of the “MRI-symptom analysis” portion of this study. All but one DLB subjects had both DWI and FLAIR MRI (one only had FLAIR). Forty-eight subjects (86%) in the sCJD group had autopsy; all had definite sCJD.
Records were reviewed for the following: (1) Signs and symptoms in revised WHO 1998 sCJD criteria16 and (2) UCSF probable sCJD criteria.15 (Table 1) (3) central and core features of McKeith criteria for DLB13: dementia, visual hallucinations, fluctuations, and parkinsonism. (4) EEG findings and (5) FLAIR and/or DWI MRI findings at time of assessment. EEGs reports, when available, were scored using an ordinal scale from 0 to 3 based on a progression to the presence of periodic sharp wave complexes (PSWC). (Table 2) A normal EEG was assigned a score of 0, the presence of generalized slowing scored 1, presence of focal or intermittent PSWCs scored 2, diffuse PSWCs scored 3; scores of 2 or 3 were considered consistent with sCJD. MRIs were read by one of us (MDG) at time of patient assessment and evaluated for abnormalities consistent with sCJD.9
Table 1.
Summary of Criteria for Probable sCJD
| UCSF Require dementia w/2 of 6 following: | WHO Require dementia w/2 of 4 following: |
| AND | AND |
| Typical EEG or MRI8, 9 | Typical EEG OR positive CSF 14-3-3 (if total disease duration less than 2 years) |
| AND | AND |
| no other condition exists to explain the disorder | no other condition to exists to explain the disorder |
such as limb rigidity, parkinsonism, and dystonic, choreatic, athetoid or ballistic movements.
including but not limited to: deteriorated or blurred vision, visual field restriction, and disturbed perception of structures and colors. Visual hallucinations were rated separately.
Other such as neglect, aphasia, apraxia, acalculia, etc….
Table 2.
UCSF EEG scale.
| UCSF EEG Scale | |
|---|---|
| EEG Findings | EEG Scale Score |
| Normal | 0 |
| Focal or diffuse slowing | 1 |
| Diffuse intermittent or continuous focal PSWCs* | 2 |
| Diffuse continuous PSWCs | 3 |
Periodic sharp wave complexes (PSWCs) or periodic epileptiform discharges (PEDs) per Steinhoff et al. 10
Using the results from the above review, sCJD subjects were assessed according to whether they met DLB diagnostic criteria and DLB subjects were classified as to whether they met complete (i.e., including ancillary tests), or at least symptom, criteria for sCJD.
MRI regional assessment analysis
MRIs were re-evaluated for regional involvement to determine if sCJD or DLB criteria symptoms correlated with the abnormal hyperintensities on FLAIR/DWI MRI. All sCJD, but only 17 (57%) of DLB, subjects had a brain MRI available at their evaluation (reviewed by MDG). Forty-five (80%) of the 56 sCJD subjects had a DWI and ADC of adequate quality available for analysis. DWI and ADC sequences were consensus reviewed by at least two of three neurologists (MCT, MDG, TC) with extensive experience reading MRIs of subjects with sCJD. Eight regions (frontal, temporal, parietal, occipital lobes, thalami, caudate, putamen and globus pallidi) in each MRI were scored according to subjective 4-point ordinal scales of intensity (0–3; none, mild, moderate, severe) and percent area involved (0–3; none, ≤25%, >25-<50%, ≥50-<75%, ≥100%). These two values were multiplied for a total “MRI severity score” for each region, and the right and left sided scores for each region were combined.
Statistics
The two groups were assessed for differences in age, gender, Mini Mental State Examination (MMSE) and duration of the disease using Student’s T-test. Disease duration was calculated from first symptom until the date of death, or if the subject was alive or survival status unknown, the date of last contact. The sCJD and DLB groups were compared using a Chi square for differences in frequency of probable sCJD and DLB criteria signs/symptoms: myoclonus, pyramidal and/or extrapyramidal signs, visual symptoms, cerebellar signs, akinetic mutism, “other higher focal cortical signs,” parkinsonism, visual hallucinations, fluctuations and dementia.13, 15, 16
The sCJD subjects were divided into two groups based on whether or not they met probable or possible DLB criteria and were compared using Chi squares for the frequency of sCJD and DLB criteria signs/symptoms.
For the MRI regional assessment analysis, logistic regressions were performed with Possible or Probable DLB as the independent variable and a severity score based on the multiplication of area and intensity on DWI in each region as independent variables with sex, age of onset and duration of disease as covariates. SPSS was used for statistical analysis (Version 17).
Results
Demographics
Demographics of the subjects are presented in Table 3. Both groups had more men than women; 54% male in the sCJD group and 60% male in the DLB group. There was no significant difference in gender between the two groups (p=0.651). Subjects with sCJD were younger at onset (median 61.5 years; range of 26 to 80 years) than DLB (median 69 years; range 48 to 86 years, p= 0.008). Disease duration was shorter in sCJD with a median of 10.5 months (range 2–36 months) versus 66 months in DLB (range 12- 192 months), p=0.000, and only 10 of the DLB cases were deceased at the time of the analysis. Only 14% of the sCJD subjects (n=8) had disease duration of greater than two years compared to 90% in the DLB group. The mean MMSE was significantly lower in subjects with sCJD compared with DLB, (17.9 +/− 8 and 23 +/− 6, respectively, p<0.05).
Table 3.
Demographics for sCJD and DLB patients.
| sCJD | DLB | P- value | ||
|---|---|---|---|---|
| Age at onset (years) | Mean | 60.75+/−12.3 | 67.73 +/− 9.2 | 0.008 |
| Median | 61.5 | 69 | ||
| Range | 26 to 80 | 48 to 86 | ||
| Gender | M = 30 F = 26 |
M = 18 F = 12 |
0.651 | |
| Disease Duration months) | 12.63 +/− 9.4 | 73.2 +/− 45.5 | 0.000 |
Comparison of sCJD and DLB subjects
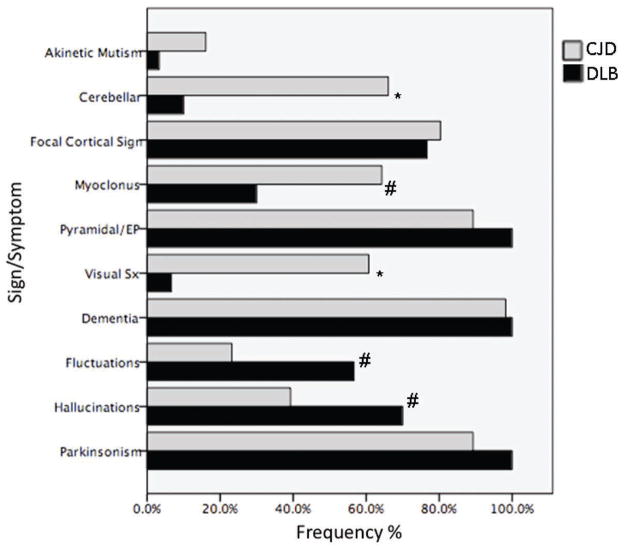
Using the McKeith criteria for DLB, 32% (n=18) of the sCJD cohort met clinical criteria for probable, and 68% (n=38) did not. In comparing the frequency of all signs/symptoms in the sCJD and DLB criteria (Fig. 1), sCJD subjects had significantly more cerebellar signs (66% vs. 10%, p<0.001), myoclonus (64% vs. 30%, p=0.002), visual symptoms (61% vs. 7%, p<0.001; N.B. not hallucinations). Akinetic mutism was more frequent in sCJD subjects but not statistically significant (16% vs. 3%, p=0.155). DLB subjects had significantly more hallucinations (70% vs. 39%, p=0.007) and fluctuations (57% vs. 23%, p=0.002). There was no significant difference between sCJD and DLB subjects in frequency of focal cortical signs (80% vs. 77%, p=0.688), and pyramidal or extrapyramidal (89 vs. 100%, 83 vs. 100%, respectively, p=0.087) signs. Dementia was present in all DLB (n=30) subjects and all but one sCJD (n=55), who had mild cognitive impairment at initial evaluation.
Fig. 1.
Graph of sCJD vs. DLB for frequency of signs and symptoms in UCSF and WHO criteria for sCJD and McKeith criteria for DLB. * p<0.001; # p<0.01
The hallucinations seen in the DLB cohort were primarily visual and all non-disturbing, often insects, small animals, people, and sometimes extracampine, very typical of what has been widely published in the literature. In the sCJD cohort, 80% had a single specific hallucination described, 20% described multiple specific hallucinations. Thirty-five percent of these sCJD cases with hallucinations had disturbing hallucinations. Eighty-two percent of hallucinations in the sCJD cohort were visual and 18% were auditory, with the following breakdown: 21% objects, 18% unspecified visual, 18% people, 14% animals, 14% auditory (person’s voices), 11% insects, and 4% unspecified auditory.
Clinical symptom criteria contributing to a probable sCJD diagnosis
Regarding clinical symptoms in diagnostic criteria, 77% (n= 23) of DLB subjects met the symptom (although not EEG or MRI) criteria for a UCSF probable diagnosis of sCJD, whereas 40% (n= 12) had enough symptoms to meet possible WHO criteria. Only 10% (n=3) of DLB subjects had a total disease duration of two years or less, versus 86% (n=48) of sCJD subjects. Some subjects in both the DLB and sCJD groups met all the signs and symptoms comprising the criteria for WHO and UCSF probable sCJD.
Results of technical investigations
Of the 56 sCJD cases, 96% (n=54) had cortical and/or basal ganglia hyperintensities consistent with probable or definite MRI findings for sCJD.9 For the two cases with negative MRIs, one had moderate motion artifact and for the second, the first MRI was considered possibly consistent sCJD, whereas the second MRI was read as definitely consistent with sCJD. In the DLB group, all subjects had no evidence of DWI or FLAIR abnormalities consistent with sCJD.
Periodic sharp wave complexes were present on EEG in 52% of subjects with sCJD. Only eight DLB subjects had an EEG and none met EEG criteria for sCJD 11, 17; three had a normal EEG and five had generalized slowing.
Evaluation of CSF 14-3-3 in the sCJD group was only available for 36 (64%) of subjects. Only 33% were positive, 39 % were negative and 28% were inconclusive; most consider inconclusive as negative.18 There were too few DLB subjects with CSF 14-3-3 testing to evaluate this test.
CJD subjects’ clinical symptoms
In comparing the sCJD subjects who met criteria for probable DLB (N=18) from those who did not (N=38), the only sign/symptom that was significantly different between the two groups was hallucinations. Eighty-five percent sCJD patients who met probable DLB criteria had hallucinations, while none of the sCJD patients who did not met probable criteria experienced hallucinations (p< 0.001; Table 4)..
TABLE 4.
Demographics and frequencies of signs/symptoms in sCJD subjects that met DLB criteria compared to those that did not.
| Did not meet Probable DLB criteria (N=38) | Met Probable DLB criteria (N=18) | P value^ | |
|---|---|---|---|
| Age onset (years) | 61.16 +/−11.8 | 59.94 +/− 13.7 | 0.74 |
| Duration (years) | 12.11 +/− 9.5 | 13.72 +/− 9.4 | 0.554 |
| Time to dementia (months) | 2.61 +/− 6.9 | 1.44 +/− 2.8 | 0.378 |
| Sex | 24M (63%) | 6M (30%) | 0.048 |
| Pyramidal Signs | 33 (87%) | 17 (94%) | 0.652 |
| Cerebellar | 24 (63%) | 13 (72%) | 0.56 |
| Myoclonus | 24 (63%) | 12 (67%) | 1 |
| Visual Symptoms | 20 (53%) | 14 (78%) | 0.087 |
| Akinetic mutism | 8 (21%) | 1 (18%) | 0.245 |
| Focal Cortical signs | 31(82%) | 14 (78%) | 0.732 |
| Hallucinations | 9 (24%) | 13 (72%) | 0.001 |
| Fluctuations | 7 (18%) | 6 (30%) | 0.310 |
| Parkinsonism | 33(87%) | 17 (94%) | 0.652 |
| Dementia | 37(97%) | 18 (100%) | 1 |
| 14-3-3 | 7 Pos (32%); 15 Neg or inconclusive (68%)* | 5 Pos (36%); 9 Neg (64%) * | 0.668 |
Inconclusive considered as negative for percentages;
bold indicates statistically significant difference between groups
In the group of 45 sCJD patients with MRI available for review, again the only sign/symptom that was significantly different between those that met probable DLB criteria and those that did not was hallucinations, which had a significantly higher incidence in the sCJD subjects who met criteria for probable DLB compared with those that did not (69 vs. 28%, p= 0.012).
There was a trend for sCJD subjects who met criteria for probable DLB to have a higher incidence of visual symptoms compared to those that did not meet criteria (78 vs. 53, p=0.087). There were significantly more men in the sCJD group who did not meet criteria for probable DLB compared to those who did (63 vs. 30%, p= 0.048). Similar results were seen in the subgroup of sCJD patients with MRI for review, there were significantly more men in the sCJD group who did not meet criteria for probable DLB compared to those who did (69 vs. 25%, p= 0.006; Table e1).
MRI Regional Analysis in sCJD group
For the 45 sCJD subjects with an available, sufficient quality DWI MRI for our MRI regional analysis, this group was divided into those who met criteria for probable DLB (n=16) and those that did not (n=29). The demographics and pattern of symptoms was the same in this subgroup of 45 sCJD subjects with MRIs available for regional analysis as compared to the complete sCJD cohort of 56 subjects (Supplementary Table 1).
Logistic Regression in sCJD group with MRI
Logistic regressions in the sCJD group, using those who met DLB criteria or not as the dependent variable, and each brain region individually entered in the model along with sex, age of onset and duration of disease as covariates revealed occipital involvement as a predictor of meeting DLB criteria (OR 1.4, p=0.058, overall model p=0.022). Evaluation of individual criteria signs and symptoms of sCJD (UCSF and WHO) and DLB criteria as the dependent variable and each brain region individually entered in the model along with duration of disease as a covariate resulted in “Other Focal Cortical signs” predicted by parietal lobe involvement (OR 1.9, p=0.021), and a trend for frontal lobe involvement (OR 1.7, p=0.096). No other sign/symptom was predicted by DWI abnormalities.
Discussion
In this retrospective study of a well-characterized cohort of sCJD and DLB subjects, we found significant clinical overlap between the two conditions as has been reported by others2. Dementia and parkinsonism were found in nearly all of our subjects in both groups and therefore the presence of these symptoms did not facilitate separation between sCJD and DLB. Similarly, other focal cortical signs were common in both sCJD and DLB. Myoclonus was present in 65% of sCJD subjects, but importantly 35% of DLB subjects also had myoclonus; this finding, a diagnostic feature of sCJD, could lead to diagnostic confusion. Akinetic mutism, another diagnostic feature of sCJD, was found in only one DLB patient; akinetic mutism, however, occurs so late in the course of sCJD that is rarely helps in the differential diagnosis. Hallucinations occur in the majority (70%) of DLB, but many (39%) sCJD subjects also had hallucinations, so this symptom, considered by some to be pathognomonic of DLB, probably lacks specificity in differentiating these two conditions. Furthermore, in both disorders the majority of hallucinations were complex visual hallucinations and so unhelpful for differentiating between DLB and sCJD. Similarly, fluctuation, a core feature of DLB, is also present in sCJD and so it too lacks specificity in differentiating these diseases from each other.
Using the clinical symptom criteria for sCJD and DLB, we found the sensitivity of the UCSF and WHO clinical symptom criteria for probable sCJD to be very high (98% and 93%, respectively). These sCJD symptom criteria alone, however, had low specificity (23% for UCSF and 60% for WHO criteria). As an insufficient number of DLB subjects had EEG or CSF testing, we could not determine in this analysis if these would improve the specificity of sCJD criteria. MRI, however, was very useful in separating sCJD from DLB cases. No DLB subjects had DWI/FLAIR MRI findings consistent with sCJD, whereas 96% of sCJD cases had positive MRIs consistent with sCJD and for the two that did not, one had motion artifact and the other was equivocal, and a second scan in that subject was positive.
The McKeith DLB criteria also performed relatively poorly because of the clinical overlap between sCJD and DLB, resulting in a sensitivity of 87% for probable DLB but a specificity of only 54%. Although generally the rapidity of progression typically separates these two groups, some DLB patients present with a fairly short duration of illness or have periods of rapid decline, making it difficult for clinicians to determine whether the illness is DLB or sCJD.19
Laboratory studies are used to diagnose sCJD and thereby differentiate it from DLB and other conditions, however the EEG and CSF 14-3-3 were of rather limited utility. The periodic EEG changes characteristic of sCJD11 and rare in DLB were present in only 52% of sCJD subjects and usually occurred at late or end-stages of the illness. This sensitivity of EEG in this sCJD cohort is close to that found in the literature of about 65%.11, 17 Whereas there is some disagreement in the Neurology community about the clinical utility of the CSF 14-3-3 protein for sCJD diagnosis, in our experience it lacks both sensitivity and specificity.20 In this cohort of sCJD, it fared poorly, positive in only 33% (negative or inconclusive in 67%) of sCJD subjects.20 By contrast, DWI and FLAIR MRI abnormalities of cortical ribboning and basal ganglia or thalamic hyperintensities were present in 96% of sCJD and none of the DLB cohort. FLAIR and DWI MRI, especially diffusion-weighted imaging, is particularly useful in the diagnosis of sCJD with a sensitivity of 91–96% and a specificity of 93–95% for sCJD.8–10 MRI hyperintensities in sCJD are usually observed in the cerebral cortex (i.e., cortical ribboning), less commonly in the striatum, and least commonly in the thalamus.9, 10 Abnormal hyperintensities often appear early on in the disease course, cortical signal abnormalities often precede basal ganglia changes.8, 9 The DWI signal hyperintensity can increase through the course of disease,21 but may disappear in the last stages of disease.10, 22, 23 The hyperintense cortex and basal ganglia observed on DWI and ADC map in sCJD are thought to reflect pathological involvement of that area, probably due to vacuolation,24–26 and less likely astrocytic gliosis23 or PrPSc deposition.26, 27
In trying to determine which symptom criteria or pattern of symptoms could lead to misdiagnosis of sCJD patients as DLB, we found that among sCJD subjects, those who met probable DLB criteria were more likely to have hallucinations, and there was a trend for them to have more visual symptoms. Interestingly, the radiological analysis portion of this study revealed that hyperintensity on DWI in the occipital lobe was most predictive of a sCJD patient meeting criteria for DLB. Thus this finding could be related to the associated visual findings and possibly hallucinations that can be seen with occipital lobe involvement. Given that hallucinations are part of the McKeith criteria, sCJD patients presenting with these may be labeled as possible or probable DLB. In fact, in the Heidenhain variant of sCJD, visual symptoms are early and predominant.28, 29 Evaluation of the signs/symptoms that are part of the criteria for sCJD and DLB in this sCJD cohort, resulted in only “Other focal cortical signs” (e.g., aphasia, apraxia, acalculia, agnosia, etc…) being associated with involvement of a particular DWI MRI region, in this case the parietal lobe. Lack of an association between other signs/symptoms and brain area involvement might be explained by diffuse pathology as the disease progresses and due to subjects being at different disease stages in this study.
Clinically diagnosed probable DLB is often found to be a pathologically heterogenous disorder; often a combination of DLB mixed with vascular disease or AD.30, 31 This makes accurate diagnosis of DLB even more problematic.
sCJD and DLB are dementing conditions that are associated with degeneration of cortical and basal ganglia structures. This anatomic overlap can lead to overlapping clinical syndromes. The current symptoms/signs in research criteria for both conditions demonstrate a significant overlap, a challenging problem for clinicians, particularly in the early stages of the illnesses. The 14-3-3- protein and periodic EEG both lack sensitivity for sCJD. EEG might only help differentiate sCJD from DLB in the later stages of the illness. This study emphasizes the importance of obtaining a brain MRI with DWI when sCJD is suspected. As we’ve shown previously, an ADC map is also important.10 As the prognosis for these illnesses is very different, it is important to distinguish the two conditions early on to avoid misdiagnosis.
Supplementary Material
Supplementary Table 1 Demographics and frequencies of signs/symptoms in sCJD subjects with DWI available that met DLB criteria compared to those that did not.
Acknowledgments
The authors would like to thank the National Prion Disease Pathology Surveillance Center (NPDPSC), Cleveland, OH for assistance in pathological diagnosis of several sCJD cases and for prion gene analysis, John Neuhaus, PhD for advice on statistical analysis as well as our patients and their families.
Study supported by: NIA/NIH grant R01 AG031189; K23 AG021989, grant P50 AG023501 from NIH National Institute on Aging; NIH/NIA AG021601, NIH-NINDS N01 NS02328, the California Alzheimer’s Disease Centers (06 –55318 DHS/ADP/ARCC); NIH/NCRR UCSF-CTSI grant number UL1 RR024131; the Michael J. Homer Family Fund (M.D.G.); the John Douglas French Foundation for Alzheimer’s Research (M.D.G.); the McBean Foundation (M.D.G.); Fonds de la Recherche en Sante du Quebec (M.C.T.)
Dr. Tartaglia receives funding from Fonds de la Recherche en Sante du Quebec. Dr. D.Y. Johnson and J.N. Thai report no disclosures. Dr. DeArmond has received research support from the NIH (AG023501 [Co-I]). Dr. Miller serves on a scientific advisory board for the Alzheimer’s Disease Clinical Study, serves as an Editor for Neurocase, and served as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Allon Therapeutics, Inc., Novartis, and TauRx Pharmaceuticals; serves on the Board of Directors for the John Douglas French Foundation for Alzheimer’s Research and for The Larry L. Hillblom Foundation; and receives research support from Novartis, the NIH/NIA, and the State of California Alzheimer’s Center. Dr. Geschwind has served on a scientific advisory board for Lundbeck Inc.; serves on the editorial board of Dementia and Behavior; has served as a consultant for MedaCorp Inc, Gerson-Lehman Group, and The Council of Advisors; has served on speakers’ bureaus for Forest Laboratories, Inc., Pfizer Inc, Novartis, and Lundbeck Inc.; and receives research support from the NIH/NIA, the Michael J. Homer Family Fund.
References
- 1.Haik S, Brandel JP, Sazdovitch V, et al. Dementia with Lewy bodies in a neuropathologic series of suspected Creutzfeldt-Jakob disease. Neurology. 2000 Nov 14;55(9):1401– 4. doi: 10.1212/wnl.55.9.1401. [DOI] [PubMed] [Google Scholar]
- 2.Tschampa HJ, Neumann M, Zerr I, et al. Patients with Alzheimer’s disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001 Jul;71(1):33–9. doi: 10.1136/jnnp.71.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL. Rapidly progressive dementia. Ann Neurol. 2008 Jul;64(1):97–108. doi: 10.1002/ana.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parchi P, Giese A, Capellari S, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999 Aug;46(2):224–33. [PubMed] [Google Scholar]
- 5.Walker Z, Allen RL, Shergill S, Mullan E, Katona CL. Three years survival in patients with a clinical diagnosis of dementia with Lewy bodies. Int J Geriatr Psychiatry. 2000 Mar;15(3):267–273. doi: 10.1002/(sici)1099-1166(200003)15:3<267::aid-gps107>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Gaig C, Valldeoriola F, Gelpi E, et al. Rapidly progressive diffuse Lewy body disease. Mov Disord. Jun;26(7):1316–23. doi: 10.1002/mds.23506. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Chae C, Nguyen E, Ando T, Haman A, Miller BL, MDG The differential of sCJD. Neurology. 2008;70:A149. [Google Scholar]
- 8.Shiga Y, Miyazawa K, Sato S, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology. 2004 Aug 10;63(3):443–9. doi: 10.1212/01.wnl.0000134555.59460.5d. [DOI] [PubMed] [Google Scholar]
- 9.Young GS, Geschwind MD, Fischbein NJ, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol. 2005 Jun-Jul;26(6):1551–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Vitali P, Maccagnano E, Caverzasi E, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011 Apr 6; doi: 10.1212/WNL.0b013e31821a4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhoff BJ, Zerr I, Glatting M, Schulz-Schaeffer W, Poser S, Kretzschmar HA. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol. 2004 Nov;56(5):702–8. doi: 10.1002/ana.20261. [DOI] [PubMed] [Google Scholar]
- 12.Zerr I, Brandel JP, Masullo C, et al. European surveillance on Creutzfeldt-Jakob disease: a case-control study for medical risk factors. J Clin Epidemiol. 2000 Jul;53(7):747–54. doi: 10.1016/s0895-4356(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 13.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005 Dec 27;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 14.Budka H, Aguzzi A, Brown P, et al. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases) Brain Pathol. 1995 Oct;5(4):459–66. doi: 10.1111/j.1750-3639.1995.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 15.Geschwind MD, Josephs KA, Parisi JE, Keegan BM. A 54-year-old man with slowness of movement and confusion. Neurology. 2007 Nov 6;69(19):1881–87. doi: 10.1212/01.wnl.0000290370.14036.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies: report of a WHO consultation; Paper Presented at the World Health Organization: Emerging and other communicable diseases, surveillance and control; Geneva (Switzerland). 1998. [Google Scholar]
- 17.Zerr I, Pocchiari M, Collins S, et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology. 2000 Sep 26;55(6):811–5. doi: 10.1212/wnl.55.6.811. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Valle R, Saiz A, Graus F. 14-3-3 Protein isoforms and atypical patterns of the 14-3-3 assay in the diagnosis of Creutzfeldt-Jakob disease. Neurosci Lett. 2002 Mar 1;320(1–2):69–72. doi: 10.1016/s0304-3940(02)00045-9. [DOI] [PubMed] [Google Scholar]
- 19.Gaig C, Valldeoriola F, Gelpi E, et al. Rapidly progressive diffuse lewy body disease. Mov Disord. 2011 doi: 10.1002/mds.23506. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003 Jun;60(6):813–6. doi: 10.1001/archneur.60.6.813. [DOI] [PubMed] [Google Scholar]
- 21.Russmann H, Vingerhoets F, Miklossy J, et al. Sporadic Creutzfeldt-Jakob disease: a comparison of pathological findings and diffusion weighted imaging. J Neurol. 2005 Mar;252(3):338–42. doi: 10.1007/s00415-005-0648-8. [DOI] [PubMed] [Google Scholar]
- 22.Ukisu R, Kushihashi T, Kitanosono T, et al. Serial diffusion-weighted MRI of Creutzfeldt-Jakob disease. AJR Am J Roentgenol. 2005 Feb;184(2):560–6. doi: 10.2214/ajr.184.2.01840560. [DOI] [PubMed] [Google Scholar]
- 23.Demaerel P, Heiner L, Robberecht W, Sciot R, Wilms G. Diffusion-weighted MRI in sporadic Creutzfeldt-Jakob disease. Neurology. 1999 Jan 1;52(1):205–8. doi: 10.1212/wnl.52.1.205. [DOI] [PubMed] [Google Scholar]
- 24.Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol. 1999 May;56(5):577–83. doi: 10.1001/archneur.56.5.577. [DOI] [PubMed] [Google Scholar]
- 25.Mittal S, Farmer P, Kalina P, Kingsley PB, Halperin J. Correlation of diffusion-weighted magnetic resonance imaging with neuropathology in Creutzfeldt-Jakob disease. Arch Neurol. 2002 Jan;59(1):128–34. doi: 10.1001/archneur.59.1.128. [DOI] [PubMed] [Google Scholar]
- 26.Geschwind MD, Potter CA, Sattavat M, et al. Correlating DWI MRI with pathologic and other features of Jakob-Creutzfeldt disease. Alzheimer Dis Assoc Disord. 2009 Jan-Mar;23(1):82–7. doi: 10.1097/wad.0b013e31818323ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haik S, Brandel JP, Oppenheim C, et al. Sporadic CJD clinically mimicking variant CJD with bilateral increased signal in the pulvinar. Neurology. 2002 Jan 8;58(1):148–149. doi: 10.1212/wnl.58.1.148-a. [DOI] [PubMed] [Google Scholar]
- 28.Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999 Jan;56(1):55–61. doi: 10.1001/archneur.56.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Cooper SA, Murray KL, Heath CA, Will RG, Knight RS. Isolated visual symptoms at onset in sporadic Creutzfeldt-Jakob disease: the clinical phenotype of the “Heidenhain variant”. Br J Ophthalmol. 2005 Oct;89(10):1341–2. doi: 10.1136/bjo.2005.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel W, Alford M, Hofstetter CR, Hansen L. Dementia with Lewy bodies versus pure Alzheimer disease: differences in cognition, neuropathology, cholinergic dysfunction, and synapse density. J Neuropathol Exp Neurol. 1997 May;56(5):499–508. doi: 10.1097/00005072-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer’s disease. J Mol Neurosci. 2001 Oct;17(2):225–232. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Demographics and frequencies of signs/symptoms in sCJD subjects with DWI available that met DLB criteria compared to those that did not.