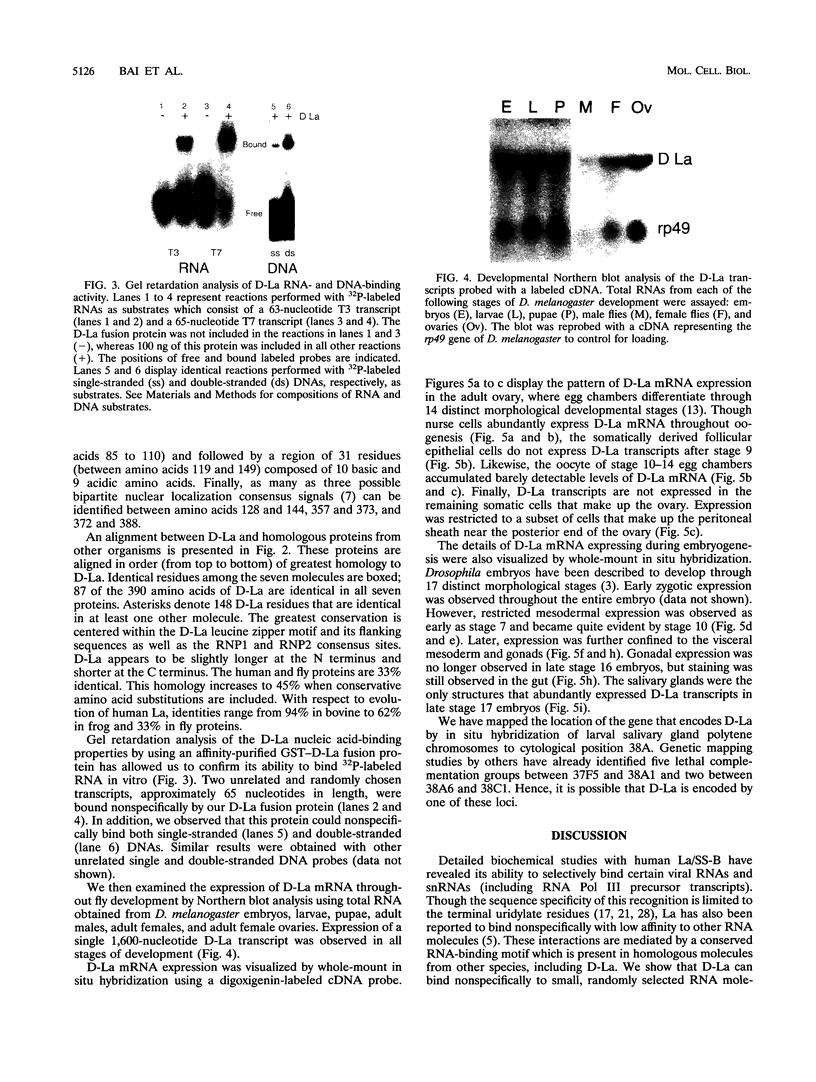
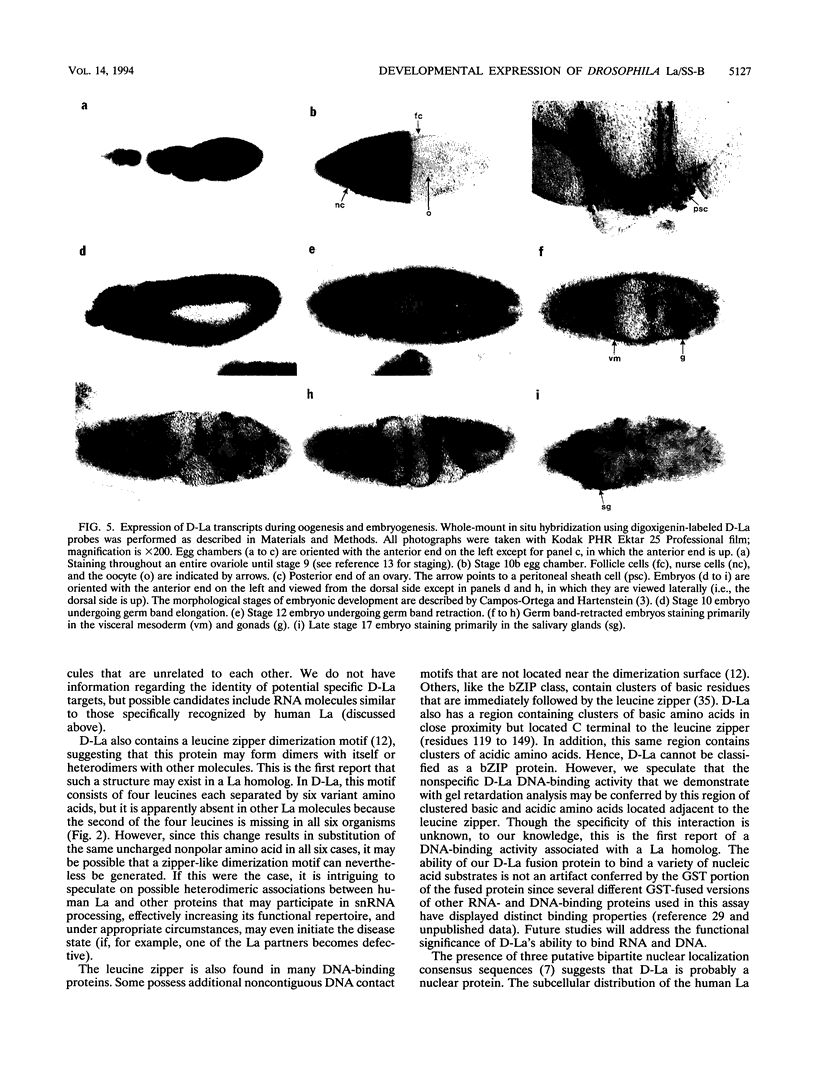
Abstract
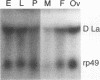
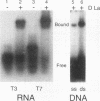
Patients with humoral autoimmune diseases such as systemic lupus erythematosus and Sjögren's syndrome contain antibodies in their sera directed against certain normal cellular components such as the La/SS-B autoantigen, an RNA-binding protein believed to function as a putative processor of RNA polymerase III precursor transcripts. We have identified cDNA clones from the fruit fly Drosophila melanogaster that encode a protein displaying significant sequence homology with human La/SS-B. The fly protein (which we refer to as D-La) contains a putative ribonucleoprotein 1 (RNP1) and RNP2 RNA-binding domain. D-La also possesses a leucine zipper motif, suggesting that it may interact with itself or other proteins. Using gel retardation analysis, we show that D-La can bind RNA; in addition, we demonstrate the first reported DNA-binding activity associated with a La protein. Northern (RNA) blot analysis revealed a single 1,600-nucleotide transcript expressed throughout embryonic, larval, pupal, and adult development. Surprisingly, whole-mount in situ hybridization experiments revealed that D-La transcripts are not present in all ovarian tissues. In addition, early expression throughout the embryo is followed by a restricted pattern of mesodermal expression that is later confined to the visceral mesoderm, gonads, gut, and salivary glands. These results suggest that D-La may play a more specialized role during fly development as opposed to a rather general role inferred by its homology to La proteins from other organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Falke D., Schröder H. C., Müller W. E. Intracellular distribution of the La antigen in CV-1 cells after herpes simplex virus type 1 infection compared with the localization of U small nuclear ribonucleoprotein particles. J Gen Virol. 1989 Apr;70(Pt 4):881–891. doi: 10.1099/0022-1317-70-4-881. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Pfeifer K., Schröder H. C., Müller W. E. The La antigen shuttles between the nucleus and the cytoplasm in CV-1 cells. Mol Cell Biochem. 1989 Feb 21;85(2):103–114. doi: 10.1007/BF00577106. [DOI] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Mariani B. D., Lingappa J. R., Kafatos F. C. Temporal regulation in development: negative and positive cis regulators dictate the precise timing of expression of a Drosophila chorion gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3029–3033. doi: 10.1073/pnas.85.9.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M., Huang Z. M. Interleukin-1-mediated enhancement of mouse factor B gene expression via NF kappa B-like hepatoma nuclear factor. Mol Cell Biol. 1990 Dec;10(12):6283–6289. doi: 10.1128/mcb.10.12.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., Van Venrooij W. J. Structure and function of La and Ro RNPs. Mol Biol Rep. 1990;14(2-3):43–48. doi: 10.1007/BF00360410. [DOI] [PubMed] [Google Scholar]
- Reddy R., Henning D., Tan E., Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 1985 Apr 11;13(7):2617–2629. doi: 10.1093/nar/13.7.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Saunders R. D., Glover D. M., Ashburner M., Siden-Kiamos I., Louis C., Monastirioti M., Savakis C., Kafatos F. PCR amplification of DNA microdissected from a single polytene chromosome band: a comparison with conventional microcloning. Nucleic Acids Res. 1989 Nov 25;17(22):9027–9037. doi: 10.1093/nar/17.22.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Stutz F., Lin-Marq N., Clarkson S. G. La proteins from Xenopus laevis. cDNA cloning and developmental expression. J Mol Biol. 1993 May 20;231(2):196–204. doi: 10.1006/jmbi.1993.1275. [DOI] [PubMed] [Google Scholar]
- Semsei I., Tröster H., Bartsch H., Schwemmle M., Igloi G. L., Bachmann M. Isolation of rat cDNA clones coding for the autoantigen SS-B/La: detection of species-specific variations. Gene. 1993 Apr 30;126(2):265–268. doi: 10.1016/0378-1119(93)90378-g. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Suter B., Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991 Nov 29;67(5):917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tolias P. P., Kafatos F. C. Functional dissection of an early Drosophila chorion gene promoter: expression throughout the follicular epithelium is under spatially composite regulation. EMBO J. 1990 May;9(5):1457–1464. doi: 10.1002/j.1460-2075.1990.tb08262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topfer F., Gordon T., McCluskey J. Characterization of the mouse autoantigen La (SS-B). Identification of conserved RNA-binding motifs, a putative ATP binding site and reactivity of recombinant protein with poly(U) and human autoantibodies. J Immunol. 1993 Apr 1;150(7):3091–3100. [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]