Abstract
Cognitive deficits in behavioral-variant frontotemporal dementia (bvFTD) and AD are linked to frontal and temporal lobe gray matter (GM) pathology. The aim of this study was to assess the relative contribution of white (WM) and GM abnormalities to cognitive dysfunction in bvFTD and AD. Fractional anisotropy (FA) for the corpus callosum, cingulum (Cg), and uncinate fasciculus (Unc) was determined in 17 bvFTD and 10 AD patients who underwent neuropsychological testing. Regressions were performed to assess the relative contribution of WM and GM abnormalities to cognitive deficits. Multiple regression analysis revealed that in bvFTD, the left anterior Cg FA was related to executive function, the right anterior Cg FA to visual-spatial attention and working memory, the right posterior Cg to visual-constructional abilities and the left Unc FA to Modified Trails Errors. After adding corresponding GM volumes, the left anterior Cg FA, the right anterior cingulate FA, the right posterior cingulate FA and the left uncinate FA remained significant predictors of the cognitive tasks. In the AD group, the left posterior Cg FA and right descending Cg FA were related to visual recall performance but did not remain significant predictors when GM volumes were added to the regression. These results suggest that reduced integrity of specific WM tracts contribute to cognitive deficits observed in bvFTD after accounting for GM atrophy. In AD, memory impairment was related to WM tract injury but this relationship was no longer observed when GM volumes were included.
Keywords: Cingulum, Executive function, Frontotemporal dementia, Alzheimer’s disease, White matter tracts
Introduction
Neurodegenerative diseases are associated with specific cognitive deficits. While typical Alzheimer’s disease (AD) patients display primarily memory and visual-spatial impairments, behavioral-variant frontotemporal dementia (bvFTD) patients have greater executive dysfunction and behavioral deficits [16]. Executive function encompasses planning, judgment, reasoning, problem solving, organization, attention, abstraction, and mental flexibility [42]. The cognitive deficits observed in AD and FTD are traditionally attributed to gray matter (GM) pathology [33]. The memory and visual-spatial deficits observed in AD have been associated with abnormalities in the medial temporal, parietal and lateral frontal regions [34] and atrophy in medial frontal, orbitofrontal, insular and dorsolateral prefrontal cortex has been associated with the behavioral and executive deficits seen in bvFTD patients [34].
There is accumulating evidence that some neurodegenerative diseases target large-scale networks [37]. The distinct spatial disease patterns observed in the different neurodegenerative diseases reflect the healthy brain’s intrinsic functional network architecture. These networks are made up of spatially distinct, functionally related groups of cortical and subcortical regions whose spontaneous activity is correlated [5, 38]. A number of different networks have been demonstrated including an executive network comprising several medial–frontal areas, including the anterior cingulate and paracingulate area [40]; a default mode network made up of medial-parietal (precuneus and posterior cingulate), bilateral inferior–lateral–parietal and ventromedial frontal cortex [9]; a salience network comprising orbital frontoinsular cortices and dorsal anterior cingulate with robust connectivity to subcortical and limbic structures [36, 38]. AD-related atrophy has been noted in the default mode network, [9, 18]. In bvFTD, the salience and executive networks are targeted with the salience network being an early target of disease [38].
Focal GM pathology in default mode structures in AD, and executive and salience network structures in bvFTD has been established but both illnesses also exhibit white matter (WM) pathology [8, 9, 28, 38, 47]. Tau and amyloid deposits, the abnormal proteins in AD, appear in GM as well as WM. The three underlying molecular pathologies in bvFTD, i.e., Tau, TAR DNA-binding protein 43 and fused in sarcoma appear in both WM and GM [28, 29, 47]. Volumetric analysis has demonstrated that FTD patients have reduced left frontal WM compared to controls [11]. A recent diffusion tensor imaging (DTI) study that allows in vivo reconstruction of white matter fiber tracts and provides a measure of WM integrity demonstrated reduced FA in anterior corpus callosum (CC), bilateral anterior and descending cingulum (Cg) tracts, and uncinate fasciculus (Unc) in bvFTD compared to normal age-matched controls and in anterior CC and bilateral Unc compared to AD patients [46]. Patients with AD showed reduced FA in left anterior and posterior Cg, bilateral descending Cg and left Unc compared to controls. The AD patients did not show reduced FA compared to bvFTD [46].
There is evidence that WM injury contributes to cognitive deficits. In older adults, executive dysfunction correlated with frontal white matter FA [30]. Cortical thickness plus FA correlated with MMSE score in AD patients, whereas in FTD patients there was a correlation between thickness plus FA and verbal fluency [2]. Specific WM tracts located within areas of maximum damage such as the frontal lobes in bvFTD and parietal and medial temporal in AD may be preferentially affected and so contributing to the cognitive and/or behavioral deficits observed in these illnesses. The Cg, running within the cingulate cortex connects medial regions including the anterior cingulate to the hippocampus, is part of the limbic system and is involved in attention, memory and emotion. The Unc connects the mediolateral orbitofrontal region with the anterior temporal lobe including the amygdala. Also part of the limbic system, it functions in emotion processing, inhibition, memory and language. The corpus callosum is important for interhemispheric connection and different parts subserve the crossing of fibers from different cortical areas [32]. The anterior half of the corpus callosum including genu and half of the body would comprise fibers from the ventro-prefrontal cortex and dorsal prefrontal lobes, areas involved in executive function as well as social cognition. The posterior half of the CC includes fibers from the superior frontal lobe, sensory-motor areas, and temporal, parietal and visual cortices. The extent to which WM tract abnormalities in specific tracts located within injured areas contribute to cognitive dysfunction in bvFTD and AD beyond GM abnormalities is unknown and may provide another biomarker for neurodegenerative pathology. Our aim was to assess the relative contribution of WM pathology as measured with DTI to cognitive performance in bvFTD and AD, taking into account GM atrophy.
Methods
Subjects
Patients diagnosed with bvFTD according to Neary criteria [27] who had undergone DTI and a full neuropsychological exam as part of a larger DTI study were included in this study [46]. The patients were recruited from the Memory and Aging Center of the University of California, San Francisco. The subjects were aged 30- to 80-years and had no history of other illnesses that could cause dementia. Two patients had concurrent motor neuron-related symptoms. AD patients were diagnosed according to the NINCDS/ADRDA criteria [25]. All subjects or their guardians gave written informed consent, which was approved by the Committees of Human Research at the University of California at San Francisco.
Neuropsychology
bvFTD and AD patients underwent neuropsychological evaluation within 3 months of MRI as previously described [23]: memory (CVLT-II learning and delay trials [14], Modified Rey—10 min recall), executive function (a simple set-shifting task known as Modified Trail (MT) Making Test [MT Correct lines, Time (MT time), MT Errors], DKEFS Design Fluency [14], Trails Composite score of DKEFS Trails Shifting (Trails Composite) [14], WMS-III Spatial Span [45], letter fluency (D words), WAIS-III Digit Span backwards [45], visual-spatial perception [Modified Rey copy, Visual Object and Space Perception (VOSP)], Number Location [44], written calculations, WAIS-III Block Design [45], language (animal fluency, Boston Naming Test [21], single word comprehension, and sentence repetition). An error summary score (Total Executive Function Errors) was calculated by summing all errors made during the executive function tasks. An MMSE score was obtained and global functional impairment was assessed using Clinical Dementia Rating (CDR) Box Score.
Data acquisition and processing
MRI was performed on a 4 Tesla (Bruker/Siemens) MRI system. T1-weighted images were obtained with a 3D volumetric magnetization prepared rapid gradient echo (MPRAGE) sequence, TR/TE/TI = 2300/3/950 ms, timing; 7° flip angle; 1.0 × 1.0 × 1.0 mm3 resolution; 157 continuous sagittal slices; acquisition time of 5 min.
DTI was acquired using a spin-echo echo-planar sequence, with a factor 2 GRAPPA acceleration, TR/TE = 6000/77 ms; 6 directions, b = 0, 800 s/mm2, 2 × 2 mm2 in-plane resolution; 40 continuous slices, each 3 mm thick; 4 averages.
FA maps were calculated for tractography-based regions of interest analysis.
Tractography-based regions of interest measurements
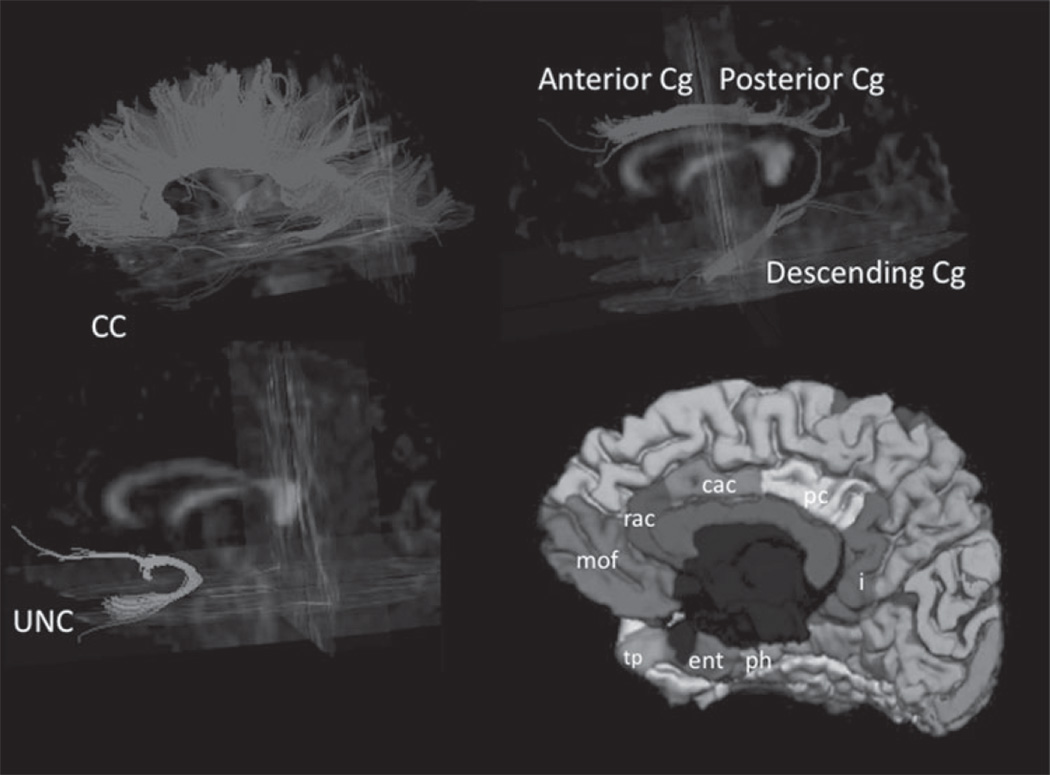
Fiber tracking was performed with the Volume-one and dTV software packages, which are based on the Fiber Assignment by Continuous Tracking (FACT) approach as previously described [26, 46]. Binary maps of fiber tracts including the anterior and posterior corpus callosum (CC), bilateral anterior, posterior Cg, and descending Cg to the parahippocampus, and Unc were obtained and served as TOIs (Fig. 1). Mean FA values in each TOI were measured.
Fig. 1.
Illustration of fibre tracts of the corpus callosum (CC), cingulum (Cg), and uncinate (Unc) from a single subject superimposed on a 3-dimensional FA image. Freesurfer segmented areas of interest used to calculate GM volumes that corresponded to the tracts: anterior cingulate GM rostral anterior cingulate (rac) GM + caudal anterior cingulate (cac) GM; uncinate GM medial orbital frontal (mof) GM + temporal pole (tp) GM + entorhinal (ent) GM; Descending cingulate GM parahippocampal gyrus (ph) GM + isthmus (i) GM; Posterior cingulate GM.
The identification of fiber tracts was initiated by placing a ‘Seed’ and a ‘Target’ area in anatomical regions through which the particular fibers are expected to propagate [43]. The propagation of tracts was constrained by a lower threshold of FA = 0.18 and limits of angular deviations from the path trajectory of <45 degrees. Trajectories that passed through the Seed and Target were assigned to specific fiber tracts based on anatomical knowledge. Several major fiber pathways were selected due to their presumed relationship to higher brain functions. The tracts included: (i) the cingulum tract, which is associated with memory function, connects the anterior and posterior cingulate gyrus and descends into the medial temporal lobe; (ii) the uncinate fasciculus, which connects the inferior frontal lobe with the anterior medial temporal lobe and is associated with memory and behavioural functions; and (iii) the corpus callosum, which connects the two hemispheres and is associated with various functions depending on the location along the corpus callosum. See Zhang et al. [46] for a detailed description. The fiber tracts were then transformed into binary maps to guide tractography-based regions of interest measurements.
Gray matter volumetric analysis
Gray matter volumes adjacent to the white matter tracts under study were obtained using Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/), which performs cortical reconstruction and volumetric segmentation. The technical details of these procedures are described in prior publications [15].
All subjects’ segmentations were visually inspected for accuracy. Errors in white matter segmentation, including those due to atrophy, were corrected using control points. Visual inspection was repeated and if the results were inadequate, the subject was excluded from the second regression analysis.
GM volumes were calculated using individual automated registration of a set of regions from a standardized atlas [15]. GM volumes adjacent to the tracts of interest were obtained directly from Freesurfer output if available i.e., posterior cingulate GM volume or were calculated by combining Freesurfer volumes that corresponded to each WM tract as follows: anterior cingulate GM = rostral anterior cingulate GM + caudal anterior cingulate GM; uncinate GM = medial orbital frontal GM + temporal pole GM + entorhinal GM; descending cingulate GM = parahippocampal gyrus GM + isthmus GM. Left and right GM volumes were generated separately (Fig. 1).
Statistics
Nonparametric tests were used to compare demographic, neuropsychological, structural and diffusion tensor data between bvFTD and AD. A multiple linear regression with age and gender as covariates was used to evaluate the relationship between neuropsychological tests and FA in specific tracts.
Results from the first regression analyses were used to test whether addition of a tract’s corresponding GM volume altered its predictive power.
Results
Seventeen bvFTD patients [12M:5F, mean age 61.2 ± 10.7 years (range 32–76 years)] and ten AD patients [7M:3F, mean age 59.3 ± 6 years (range 51–69 years)] underwent neuropsychological testing and DTI. There was no significant difference in their ages, education or sex distribution (Table 1). The mean disease duration was 9.0 ± 7 years in the bvFTD group and significantly shorter in the AD group, 4.4 ± 3 years (p = 0.03). The mean MMSE was 24.2 ± 4.9 in bvFTD and 21.7 ± 5.3 in AD (p = 0.09). The mean CDR box score was significantly higher in bvFTD (6.44 ± 2.3) than AD (4.40 ± 1.8) (p = 0.027) indicating worse functional deficits.
Table 1.
Demographic and Neuropsychological results for bvFTD and AD patients
| Test | Diagnosis | Mean ± SD | p |
|---|---|---|---|
| Gender | bvFTD | 12M:5F | 1 |
| AD | 7M:3F | ||
| Age (years) | bvFTD | 61.29 ± 10.2 | 0.338 |
| AD | 59.30 ± 6.0 | ||
| MMSE | bvFTD | 24.24 ± 4.9 | 0.090 |
| AD | 21.70 ± 5.3 | ||
| Education (years) | bvFTD | 16.94 ± 2.6 | 0.148 |
| AD | 15.00 ± 3.5 | ||
| Box score | bvFTD | 6.44 ± 2.3 | 0.027 |
| AD | 4.40 ± 1.8 | ||
| Duration (years) | bvFTD | 9.00 ± 7.0 | 0.041 |
| AD | 4.40 ± 3.0 | ||
| CVLT-total | bvFTD | 18.12 ± 9.2 | 0.820 |
| AD | 16.40 ± 4.4 | ||
| Delayed CVLT recall | bvFTD | 2.65 ± 3.4 | 0.441 |
| AD | 1.40 ± 1.6 | ||
| Delayed Rey recall | bvFTD | 7.12 ± 5.3 | 0.013 |
| AD | 2.10 ± 3.0 | ||
| MT time (sec) | bvFTD | 65.00 ± 37.2 | 0.149 |
| AD | 93.10 ± 42.5 | ||
| MT correct lines | bvFTD | 11.88 ± 4.6 | 0.178 |
| AD | 9.60 ± 5.7 | ||
| MT errors | bvFTD | 1.88 ± 2.6 | 0.342 |
| AD | 0.90 ± 1.6 | ||
| DKEFS Design Fluency | bvFTD | 5.24 ± 2.7 | 0.892 |
| AD | 5.22 ± 3.1 | ||
| Trails Composite score of | bvFTD | 12.83 ± 9.5 | 0.465 |
| DKEFS Trails Shifting | AD | 10.14 ± 8.5 | |
| Spatial Span | bvFTD | 11.55 ± 3.8 | 0.361 |
| AD | 9.57 ± 3.8 | ||
| D Words | bvFTD | 7.76 ± 5.1 | 0.519 |
| AD | 9.60 ± 6.5 | ||
| Total Executive Function Errors | bvFTD | 16.35 ± 13.8 | 0.497 |
| AD | 11.10 ± 6.6 | ||
| WAIS-Digit span backward | bvFTD | 4.67 ± 1.3 | 0.053 |
| AD | 3.38 ± 1.6 | ||
| Rey copy | bvFTD | 14.18 ± 2.5 | 0.010 |
| AD | 8.50 ± 6.1 | ||
| VOSP | bvFTD | 7.56 ± 2.8 | 0.727 |
| AD | 7.33 ± 2.8 | ||
| Written calculations | bvFTD | 3.71 ± 1.5 | 0.324 |
| AD | 3.30 ± 1.3 | ||
| WAIS III Block design | bvFTD | 26.42 ± 11.0 | 0.008 |
| AD | 13.14 ± 6.4 | ||
| Category fluency–animals | bvFTD | 10.76 ± 5.7 | 0.940 |
| AD | 10.00 ± 3.5 | ||
| Naming | bvFTD | 11.38 ± 3.3 | 0.872 |
| AD | 11.50 ± 3.4 | ||
| Comprehension | bvFTD | 56.78 ± 7.2 | 0.378 |
| AD | 59.33 ± 0.58 | ||
| Repetition | bvFTD | 4.29 ± 0.8 | 0.025 |
| AD | 3.00 ± 1.6 |
DTI
Comparing bvFTD with AD (Table 2), the bvFTD group had a significantly reduced FA in the anterior CC (p = 0.006) and a trend for a lower FA in the right descending Cg in AD (p = 0.089).
Table 2.
Group differences of regional fractional anisotropy (FA) in the tracts of interest and gray matter (GM) volumes
| Tract FA | FA mean ± SD |
p | |
|---|---|---|---|
| bvFTD (n = 15) | AD (n = 9) | ||
| Ant CC | 0.463 ± 0.07 | 0.522 ± 0.05 | 0.009 |
| Post CC | 0.612 ± 0.05 | 0.619 ± 0.04 | 0.788 |
| L Ant Cg | 0.428 ± 0.06 | 0.455 ± 0.05 | 0.297 |
| L Post Cg | 0.452 ± 0.04 | 0.450 ± 0.03 | 0.788 |
| R Ant Cg | 0.375 ± 0.04 | 0.394 ± 0.06 | 0.325 |
| R Post Cg | 0.414 ± 0.04 | 0.425 ± 0.03 | 0.493 |
| L Desc Cg | 0.322 ± 0.03 | 0.320 ± 0.03 | 0.976 |
| R Desc Cg | 0.350 ± 0.05 | 0.327 ± 0.04 | 0.089 |
| L Unc | 0.333 ± 0.03 | 0.358 ± 0.03 | 0.200 |
| R Unc | 0.329 ± 0.05 | 0.370 ± 0.04 | 0.114 |
| GM region | GM vol. mean ± SD |
p | |
| bvFTD (n = 15) | AD (n = 9) | ||
| L Ant Cg GM | 2.581 ± 0.44 | 2.882 ± 0.40 | 0.103 |
| R Ant Cg GM | 2.517 ± 0.44 | 2.385 ± 0.42 | 0.471 |
| L Post Cg GM | 1.955 ± 0.34 | 2.017 ± 0.30 | 0.650 |
| R Post Cg GM | 2.214 ± 0.31 | 2.002 ± 0.34 | 0.151 |
| L Desc Cg GM | 2.705 ± 0.37 | 2.654 ± 0.32 | 0.736 |
| R Desc Cg GM | 2.672 ± 0.29 | 2.529 ± 0.33 | 0.303 |
| L Unc GM | 4.261 ± 0.92 | 4.555 ± 0.52 | 0.325 |
| R Unc GM | 3.912 ± 0.88 | 4.738 ± 0.56 | 0.010 |
Gray matter volumes
GM volumes were available for 15 bvFTD and nine AD patients as Freesurfer failed in three patients even after multiple control points and reanalysis. Only the GM volume adjacent to right uncinate was smaller in the bvFTD group than AD. No other significant differences were observed (Table 2).
Neuropsychology
Neuropsychology scores were compared in AD and bvFTD patients (Table 1). bvFTD had significantly better scores than AD in Rey copy, Delayed Rey recall, sentence repetition, Digits backwards, and Block design. Conversely, bvFTD patients had a significantly worse CDR Box score, indicating more severe illness.
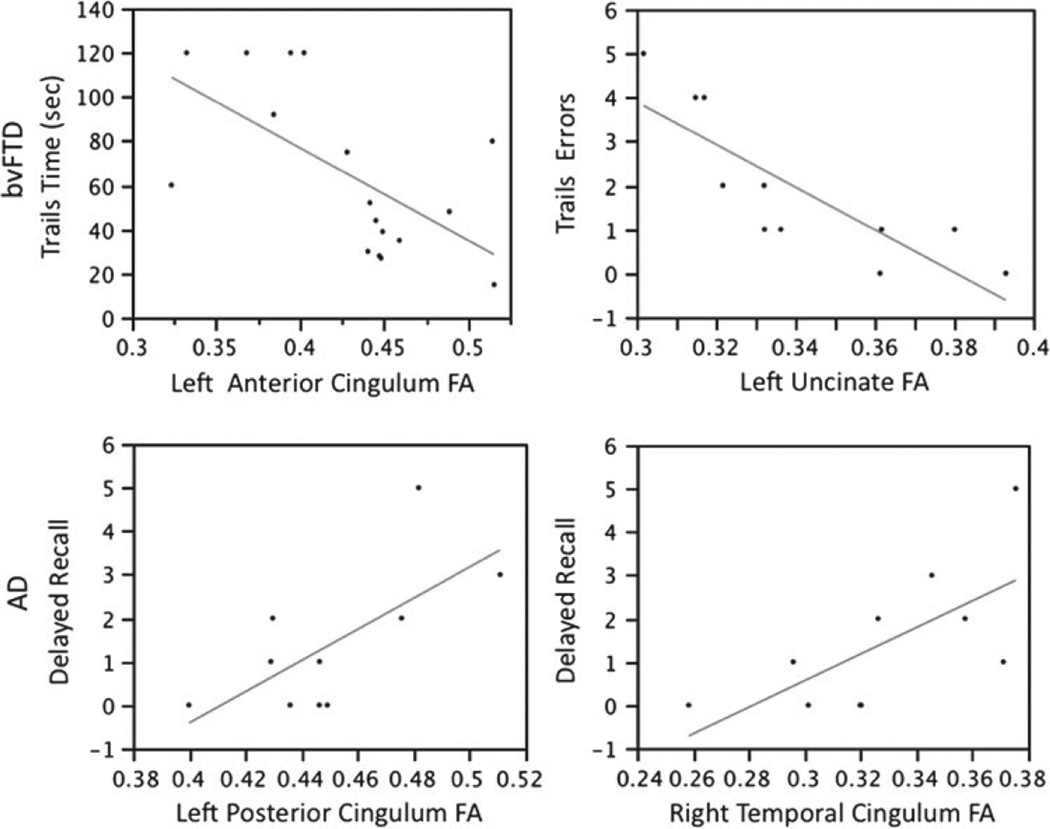
The results of the multiple regression analysis are presented in Table 3. Three executive function tasks were related to the left anterior Cg FA in bvFTD: left anterior Cg FA was related to both accuracy, Trails Composite and time required to complete a speeded set-shifting task (MT Time) (Fig. 2), and a sum of Total Executive Function Errors. The right anterior Cg FA was related to visualspatial attention and working memory (WMS-III Spatial Span) and the right posterior Cg to visual-constructional abilities (WAIS-III Block Design:). The left Unc FA was related to MT Errors (Fig. 2). The right descending Cg FA was related to visual-spatial working memory (Delayed recall Modified Rey).
Table 3.
Multiple regression results for neuropsychological tests and tract FA that were corrected for age and gender in bvFTD and AD
| Disease | Area | Test | r2 | p |
|---|---|---|---|---|
| bvFTD | Left anterior Cg FA | *Trails Composite | 0.777 | 0.003 |
| *MT time | 0.39 | 0.008 | ||
| Sum of Total Executive Function Errors | 0.26 | 0.037 | ||
| Right anterior Cg FA | *Visual-spatial attention and working memory (WMS-III Spatial Span) | 0.63 | 0.004 | |
| Right posterior Cg | *Visual-constructional abilities (WAIS-III Block Design) | 0.46 | 0.015 | |
| Left Unc FA | *MT errors | 0.69 | 0.002 | |
| Right descending Cg FA | Visual-spatial working memory (Delayed recall Modified Rey Figure) | 0.227 | 0.05 | |
| AD | Left posterior Cg FA | Visual-spatial working memory (Delayed recall Modified Rey Figure) | 0.47 | 0.03 |
| Right descending Cg FA | 0.46 | 0.03 | ||
| Right posterior Cg FA | 0.41 | 0.05 |
Indicates regression remained significant (p < 0.05) after inclusion of GM volume associated with tract
Fig. 2.
Graphs showing relationship between FA in tracts and the neuropsychological variable of interest. In bvFTD, trails time was most related to the left anterior Cg and total trails errors was most related to the left uncinate. In AD, delayed visual recall was most related to the left posterior and right descending Cg.
In the bvFTD group, there was no relationship between WM tracts’ FA and language, verbal memory measures, certain visual-spatial perception tasks (Modified Rey copy, VOSP, written calculations), and some executive function tasks (letter fluency-D words, WAIS-III Digit Span, and DKEFS Design Fluency).
In the AD group, the left posterior Cg FA, right descending Cg FA and right posterior Cg FA were related to visual recall performance (Modified Rey Figure Delayed Recall) (Fig. 2). There was no relationship between WM tracts’ FA and any of the other cognitive tests in the AD group.
Volumetric analysis was only available in 15 bvFTD patients and nine AD patients as Freesurfer analysis failed to accurately segment three patients (two bvFTD; one AD), even after repeated attempts in conjunction with manual insertion of control points. Based on tract analysis, linear regressions including the tract’s FA and corresponding GM volume were run. In bvFTD, the left anterior Cg FA remained a significant predictor of Trails Composite (r2 = 0.8, beta = 0.908, p = 0.002) and MT Time (r2 = 0.305, beta = −0.648, p = 0.015) when left anterior cingulate GM was added to regression. Right anterior Cg FA remained a significant predictor of visual-spatial attention and working memory (Spatial Span) when the right anterior cingulate GM was included in regression, r2 = 0.676, beta = −0.883, p = 0.017). Right posterior Cg FA remained a significant predictor of visual-constructional abilities (WAIS-III Block Design) when the right posterior cingulate GM was included in regression (r2 = 0.596, beta = −0.764, p = 0.024). The left Unc FA remained a significant predictor of MT Errors when the left uncinate GM was included (r2 = 0.856, beta = −0.797, p = 0.002).
The MT correct lines, Total Executive Function Errors and visual-spatial working memory measure were no longer significantly related to WM tract FA when GM volumes were added to the regression.
In the AD group, visual recall was no longer predicted by either the left or right posterior Cg or right descending Cg FA when GM volumes were added to the regression.
Discussion
Overall, these results show correlation between cognitive impairments and WM tract pathology as measured with DTI in bvFTD and AD. Furthermore, even after GM volumes adjacent to the WM tracts were included in the analysis, specific tracts’ FA remained a significant predictor of some tests of cognitive deficits in bvFTD.
The left anterior Cg tract FA remained a significant predictor of a number of executive tasks in the bvFTD group even after addition of GM volumes. Patients with bvFTD have significant degeneration in anterior cingulate regions [37]. There are distinct differences in cytoarchitectonics, connections and function of the anterior and posterior cingulate gyrus. Anterior motor-related cortex is strongly interconnected with amygdala, nucleus accumbens, medial dorsal thalamus, and dorsolateral prefrontal cortex, whereas the posterior, sensory-related cortex is interconnected with temporal association cortex, medial temporal cortex, and parietal and orbitofrontal cortex. The anterior division is believed to be integral to the processing of error detection and decision monitoring [17].
Spatial Span, an executive task of visual-spatial attention and working-memory was significantly related to the right anterior Cg even after addition of the right anterior cingulate GM volume. This task has a significant working memory component and so relies on intact executive function. Visual-spatial processing is usually attributed to the right hemisphere [12]. A PET study revealed the right dorsolateral prefrontal cortex is important for Spatial Span function [6].
The various executive tasks in this study are not necessarily taxing the same neuroanatomical regions. The Trails Composite score of DKEFS Trails Shifting is the only task that requires coordination between set-shifting as well as visual search and motor performance [24] and in one study was the only test that showed correlation between frontal FA and cognitive task in AD patients [39]. The anterior cingulate is implicated in error detection and performance monitoring [10]. Total Executive Function Errors was no longer significantly related to the left ant Cg when the left anterior cingulate GM volume was added to the model indicating that it did not make any independent contribution. However, MT time, a measure of reaction time on an executive task, was related to the left ant Cg FA even with addition of GM volume.
A number of the executive tasks in this study were not predicted by any of the WM tracts. The reason may be that the tasks do not make the same demands on frontal lobe function. Design Fluency has been correlated with more widespread gray matter atrophy [31].
Interestingly, Block design, a task thought to test visualspatial function, was significantly predicted by the right posterior Cg tract FA even after addition of the posterior cingulate GM volume. This test relies heavily on parietal function and patients with bvFTD usually demonstrate relative preservation of visual-spatial function. Kaplan [20] proposed four explanations for difficulty with task: (i) loss of overall configuration due to distortion of gestalt (right hemisphere) [20]; (ii) incorrect block placement within a grossly correct gestalt (left hemisphere) [19]; (iii) impulsive, disorganized approach (frontal lobe); (iv) perseverative responding and cognitive inflexibility (frontal lobe). It may be that executive dysfunction and difficulty with actual block placement may be responsible for the relationship observed between the right posterior Cg and this task while visual-spatial function and gestalt perception are relatively intact.
The Modified Rey drawing, a simplified relative to the standard Rey-Osterreith Figure, the VOSP and calculations were not related to any tract. Parietal lobe damage is implicated in deficits on the VOSP and so the superior longitudinal fasciculus (SLF) may play a more important role. Mental calculations in a PET study activated a widespread anatomical network, which includes aspects of attention, auditory, and motor processing and working memory [13]. One could presume that tract injury involving the SLF might have more of an impact on calculation. The working memory component is eliminated in our study because they are written calculations.
The number of MT errors was most related to the left Unc, which is part of the salience network and postulated as important for attributing value to rewards. Injury to this tract may lead to disinterest or lack of motivation to correct mistakes. The Trails task is the only executive task with feedback and the inability to attribute value to performing well may be brought out by this task and thus explain its unique relationship to the left Unc FA.
Recall of the Modified Rey diagram tests visual memory and was related to the right descending Cg. These fibers that cross through the parahippocampal gyrus, could be implicated in recognition of scenes as this area is important for topographical learning [1]. Visual processing of Rey figure has been ascribed to right hemisphere [7].
Adding the GM volumes adjacent to the WM tracts reduced significance of Trails Composite score of DKEFS Trails Shifting, Total Executive Function Errors and Delayed recall in the bvFTD. One explanation is that GM contributes more to certain cognitive tasks. MT time, a measure of speed, might be more sensitive to WM injury than cortical damage while the inverse might be true for MT number of correct lines, a measure of accuracy, which may require a more significant cortical component. Delayed visual recall may rely more heavily on the hippocampus and parahippocampal gyrus [22] than any WM tract.
In the AD group, visual-spatial recall was related to FA in bilateral posterior Cg tracts and in the right descending Cg. When the GM volumes were added to the regression, none of the tracts remained significant predictors of visualspatial recall. The AD group was small and so there was less power to detect a relationship between WM tracts’ FA and tasks of cognitive function.
None of the language measures evaluated related to the WM tracts studied. This is likely because the left SLF, the most important language tract, was not tracked in this study. Since these patients have bvFTD, and usually face dysexecutive deficits and less often language, that tract was not included in this study. Verbal memory was also not related to the tracts and could be due to the strong hippocampal influence on verbal memory.
In regards to structural differences between the two groups, only the anterior corpus callosum FA and right uncinate GM were significantly different with both these measures lower in the bvFTD group. There were, however, a number of areas that showed trends that may have been significant with larger numbers including: right descending Cg FA lower in AD, right Unc FA lower in FTD, left anterior Cg GM lower in bvFTD, right Post Cg lower in AD. These differences although not statistically significant likely contribute to the cognitive differences seen in these two groups. These results are similar to previous pathological and radiological findings of gray matter and WM pathology in bvFTD and AD [8, 28, 46, 47]. Focal degeneration in bvFTD has been demonstrated in the rostral and subgenual anterior cingulate cortex, frontoinsular region, and the frontal pole [35]. In the case of AD, the posterior cingulate region and medial temporal regions are early targets in the disease [3]. Classically, decrease in FA has been used as a marker of myelin injury with axonal loss. Alternatively, fiber reorganization, glial alterations, increased membrane permeability and diffusivity, destruction of intracellular structures, alterations in the cytoskeleton and axonal transport, could also occur in neurodegenerative disease, and could influence DTI [4, 41]. At this time it is unknown whether WM pathology in neurodegenerative disease is a primary process, independent of GM pathology, or there is secondary reduction of WM integrity because of wallerian degeneration. As we learn more about the pathology that underlies the abnormal FA signal seen with DTI, this measure becomes another potential biomarker for detecting neurodegenerative disease. With a greater understanding of DTI’s pathological correlation, one can envision using a tract’s FA to look for concomitant WM and GM abnormalities that could be contributing to the cognitive/behavioral deficits seen in these illnesses or to stage a disease if it turns out that WM pathology occurs before or after GM injury.
In summary, this study demonstrates preliminary evidence within a small sample that the reduced integrity of specific WM tracts contributes to cognitive deficits observed in bvFTD after accounting for GM atrophy. Further study is required to assess whether WM pathology is a primary process in the neurodegenerative process or secondary to GM pathology.
Acknowledgments
We thank the participants and their families for their participation in this study. We thank Will Irwin for assistance with the figures. This publication was made possible by Grant numbers P01 AG019724 and P50 AG023501 from NIH National Institute on Aging. MCT is supported by Fonds de la recherche en santédu Québec.
Footnotes
Conflict of interest None.
Contributor Information
M. C. Tartaglia, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA Neurology Clinic/Memory Clinic, Toronto Western Hospital, University of Toronto, West Wing 5-449, 399 Bathurst St., Toronto, ON M5T 2S8, Canada, carmela.tartaglia@uhn.ca.
Y. Zhang, Department of Radiology, Center for Imaging of Neurodegenerative Diseases, VA Medical Center, University of California San Francisco, San Francisco, CA, USA
C. Racine, Departments of Neurological Surgery and Radiation Oncology, University of California, San Francisco, CA, USA
V. Laluz, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA
J. Neuhaus, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA
L. Chao, Department of Radiology, Center for Imaging of Neurodegenerative Diseases, VA Medical Center, University of California San Francisco, San Francisco, CA, USA
J. Kramer, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA
H. Rosen, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA
B. Miller, Department of Neurology, Memory and Aging Center, University of California, San Francisco, CA, USA
M. Weiner, Department of Radiology, Center for Imaging of Neurodegenerative Diseases, VA Medical Center, University of California San Francisco, San Francisco, CA, USA
References
- 1.Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 2.Avants BB, Cook PA, Ungar L, Gee JC, Grossman M. Dementia induces correlated reductions in white matter integrity and cortical thickness: a multivariate neuroimaging study with sparse canonical correlation analysis. Neuroimage. 2010;50:1004–1016. doi: 10.1016/j.neuroimage.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bor D, Duncan J, Lee AC, Parr A, Owen AM. Frontal lobe involvement in spatial span: converging studies of normal and impaired function. Neuropsychologia. 2006;44:229–237. doi: 10.1016/j.neuropsychologia.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Boxer AL, Kramer JH, Du AT, Schuff N, Weiner MW, Miller BL, Rosen HJ. Focal right inferotemporal atrophy in AD with disproportionate visual constructive impairment. Neurology. 2003;61:1485–1491. doi: 10.1212/01.wnl.0000090568.34810.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E, Kalus P. Alzheimer’s disease: areal and laminar pathology in the occipital isocortex. Acta Neuropathol. 1989;77:494–506. doi: 10.1007/BF00687251. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 11.Chao LL, Schuff N, Clevenger EM, Mueller SG, Rosen HJ, Gorno-Tempini ML, Kramer JH, Miller BL, Weiner MW. Patterns of white matter atrophy in frontotemporal lobar degeneration. Arch Neurol. 2007;64:1619–1624. doi: 10.1001/archneur.64.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corballis PM, Funnell MG, Gazzaniga MS. An evolutionary perspective on hemispheric asymmetries. Brain Cogn. 2000;43:112–117. [PubMed] [Google Scholar]
- 13.Cowell SF, Egan GF, Code C, Harasty J, Watson JD. The functional neuroanatomy of simple calculation and number repetition: a parametric PET activation study. Neuroimage. 2000;12:565–573. doi: 10.1006/nimg.2000.0640. [DOI] [PubMed] [Google Scholar]
- 14.Delis D, Kaplan E, Kramer J. The Delis-Kaplan executive function system. San Antonio: The Psychological Corporation: 2001. [Google Scholar]
- 15.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Elderkin-Thompson V, Boone KB, Hwang S, Kumar A. Neurocognitive profiles in elderly patients with frontotemporal degeneration or major depressive disorder. J Int Neuropsychol Soc. 2004;10:753–771. doi: 10.1017/S1355617704105067. [DOI] [PubMed] [Google Scholar]
- 17.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 18.Greicius MD, Srivastava G, Reiss AL, Menon V. Defaultmode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan E. Process versus achievement revisited. In: Wapner S, Kaplan B, editors. Toward a holistic developmental psychology. Hillsdale: Lawrence Erlbaum; 1983. [Google Scholar]
- 20.Kaplan E, Fein D, Morris R, Delis D. The WAIS-R as a neuropsychological instrument. Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 21.Kaplan E, Goodglass H, Wintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 22.Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, Bronskill MJ. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer’s disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lezak MD. Executive functions and motor performance. In: Lezak MD, editor. Neuropsychological assessment. 4th edn. Oxford: Oxford University Press; 2004. pp. 611–646. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Crain BJ, Chacko VP, van Zijl PC. Threedimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 28.Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, Clark CM, Grossman M, Miller BL, Trojanowski JQ, Lee VM. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75:441–447. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pa J, Possin KL, Wilson SM, Quitania LC, Kramer JH, Boxer AL, Weiner MW, Johnson JK. Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J Int Neuropsychol Soc. 2010;16:640–650. doi: 10.1017/S1355617710000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinovici GD, Rascovsky K, Miller BL. Frontotemporal lobar degeneration: clinical and pathologic overview. Handb Clin Neurol. 2008;89:343–364. doi: 10.1016/S0072-9752(07)01233-X. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Allison SC, Halabi C, Kramer JH, Johnson JK, Weiner MW, Forman MS, Trojanowski JQ, Dearmond SJ, Miller BL, Rosen HJ. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia—a meta-analysis. Neurobiol Aging. 2008;29:418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Seeley WW. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjobeck M, Elfgren C, Larsson EM, Brockstedt S, Latt J, Englund E, Passant U. Alzheimer’s disease (AD) and executive dysfunction. A case-control study on the significance of frontal white matter changes detected by diffusion tensor imaging (DTI) Arch Gerontol Geriatr. 2010;50:260–266. doi: 10.1016/j.archger.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 42.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 44.Warrington EK, James M. A new test of object decision: 2D silhouettes featuring a minimal view. Cortex. 1991;27:370–383. [PubMed] [Google Scholar]
- 45.Wechsler D. WAISIII and WMSIII—Wechsler Adult Intelligence Scale and Wechsler Adult Memory Scale. 3rd edn. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 46.Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, Trojanowski JQ, Lee VM. Sporadic Pick’s disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol. 2002;51:730–739. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]