Abstract
Objectives
Although the relative risk of lung cancer due to smoking is reported to be lower in Japan than in other countries, few studies have examined the characteristics of Japanese cigarettes or potential differences in smoking patterns among Japanese smokers.
Methods
To examine tar, nicotine and carbon monoxide (TNCO) emissions from ten leading cigarettes in Japan, machine smoking tests were conducted using the International Organization for Standardization (ISO) protocol and the Health Canada Intense (HCI) protocol. Smoking topography and tobacco-related biomarkers were collected from 101 Japanese smokers to examine measures of exposure.
Results
The findings indicate considerable variability in the smoking behavior of Japanese smokers. On average, puffing behaviors observed among smokers were more similar to the parameters of the HCI protocol, and brands with greater ventilation that yielded lower machine values using the ISO protocol were smoked more intensely than brands with lower levels of ventilation. The smokers of “ultra-low/low” nicotine-yield cigarettes smoked 2.7-fold more intensively than those of “medium/high” nicotine-yield cigarette smokers to achieve the same level of salivary cotinine (p = 0.024). CO levels in expiratory breath samples were associated with puff volume and self-reported smoking intensity, but not with nominal values of nicotine-yield reported on cigarette packages.
Conclusions
Japanese smokers engaged in “compensatory smoking” to achieve their desired nicotine intake, and levels of exposure were greater than those suggested by the nominal value of nicotine and tar yields reported on cigarette packages.
Keywords: CReSSmicro device, Cotinine, Carbon monoxide, HCI protocol, Japanese smokers
Introduction
Tobacco use is responsible for one in ten global deaths and remains the leading cause of preventable death worldwide[1]. The health burden from tobacco reflects the wide range of smoking-related diseases, including cardiovascular disease, respiratory disease, and ten different forms of cancer [2].
In an effort to reduce the health effects of smoking, many smokers have reported switching to cigarette brands that yield lower tar levels under machine smoking test conditions [3]. Indeed, the average tar rating of brands has steadily decreased in many markets throughout the world, including the USA, where machine-measured tar levels decreased by more than 44 % from 1968 to 1998 [4]. However, it is well known that the use of low-tar-yield cigarettes has not reduced lung cancer deaths [5, 6]. In machine testing, the yields of nicotine and tar are measured in main stream smoke (MSS) generated by a smoking machine operated under the International Organization for Standardization (ISO) protocol: 35-mL puff volume, 60-s puff interval, 2-s puff duration, and no blocking of ventilation holes. However, it has been demonstrated that smokers smoke their cigarettes more intensely than is simulated by smoking machines operated under the ISO protocol [7–9]. Moreover, smokers tend to adapt smoking behaviors upon changing from high- to low-nicotine-yield cigarettes in an attempt to compensate for the lower emission of nicotine in the MSS [7]. In order to reflect a broader range of smoking behaviors, some governmental agencies have adopted more intensive machine smoking protocols on which to base their regulations, such as the Health Canada Intense (HCI) protocol: 55-mL puff volume, 30-s puff interval, 2-s puff duration, and 100 % blocking of ventilation holes [10]. The World Health Organization (WHO) recommends that national health authorities release their respective data on tar, nicotine, and carbon monoxide (TNCO) yields per cigarette according to both the ISO and HCI protocols [11].
The cigarette market in Japan is notable for the very low levels of machine-measured tar in cigarettes, as well as the popularity of charcoal filter cigarettes. Japan is also exceptional because of its lower relative risks for lung cancer compared to many other countries: the relative risk for lung cancer due to smoking among Japanese was recently estimated to be 4.5, and the attributable risk of smoking was about 70 % [12]. Potential explanations for the lower rates of lung cancer include genetic differences, life-style factors other than smoking, and differences in either the patterns of smoking or the types of tobacco products used in Japan. In the study reported here, we sought to characterize patterns of smoking among Japanese smokers using their “usual” brand, as well as the association between biomarkers of exposure and machine-measured TNCO yields.
Materials and methods
Subjects
In 2007, we recruited 101 individuals from in and around the Wako city region, Saitama prefecture, Japan, to our study. The inclusion criteria were daily smokers, age of between 20 and 65 years, no history of heart or lung disease, and reported smoking of one of ten leading Japanese cigarette brands for at least the past 3 months. Table 1 shows a list of the top ten best selling Japanese brands tested in our study. Table 2 shows the demographic characteristics of the participants.
Table 1.
Top ten best selling cigarette brands in Japan in 2006
| Brand name | Categorization according to nicotine yield | Tar (mg/cigarette) | Nicotine (mg/cigarette) | Filter typea | Ventilation holeb | Market share (%) |
|---|---|---|---|---|---|---|
| Pianissimo One | Ultra-low | 1 | 0.1 | P | 40 | 1.5 |
| Mild Seven One | Ultra-low | 1 | 0.1 | DC | 200 | 4.3c |
| Mild Seven Extra Lights | Low | 3 | 0.3 | DC | 100 | 3 |
| Caster Mild | Low | 5 | 0.4 | NC | 90 | 2.7 |
| Mild Seven Super Lights | Low | 6 | 0.5 | DC | 100 | 6.8 |
| Cabin Mild | Medium | 8 | 0.6 | NC | 50 | 1.9 |
| Mild Seven Lights | Medium | 8 | 0.7 | DC | 50 | 6.2 |
| Mild Seven Original | Medium | 10 | 0.8 | DC | 50 | 4.9 |
| Hope | High | 14 | 1.1 | P | 0 | 1.3 |
| Seven Stars | High | 14 | 1.2 | DC | 0 | 6.8c |
aP Plain, DC dual charcoal, NC neo charcoal
bNumber of ventilation holes
cShares include box-type packaging
Table 2.
Sample characteristics and smoking topography of Japanese smokers
| Characteristic | Total (n = 101)a | Ultra-low (n = 14) | Low (n = 38)a | Medium (n = 27) | High (n = 22) |
|---|---|---|---|---|---|
| Male/female | 88/13 | 10/4 | 33/5 | 24/3 | 21/1 |
| Age (years) | 40.0 ± 11.0 | 39.2 ± 10.2 | 40.1 ± 12.6 | 41.9 ± 11.2 | 38.0 ± 8.4 |
| Cigarette consumption (cigarette/day) | 18.4 ± 7.5 | 18.7 ± 8.3 | 17.8 ± 7.2 | 18.2 ± 6.6 | 19.3 ± 8.8 |
| Body mass index | 23.2 ± 3.8 | 22.2 ± 2.6 | 22.8 ± 3.0 | 23.9 ± 4.6 | 23.7 ± 4.5 |
| Self-reported smoking intensity | 57.8 ± 17.0 | 56.1 ± 17.3 | 57.0 ± 15.5 | 53.6 ± 15.6 | 65.5 ± 19.5 |
| Time to first smoking cigarette (%) | |||||
| 0–5 min | 23.8 % | 21.4 % | 23.7 % | 29.6 % | 18.2 % |
| 6–30 min | 44.6 % | 50.0 % | 36.8 % | 40.7 % | 59.1 % |
| 31–60 min | 18.8 % | 0.0 % | 26.3 % | 22.2 % | 13.6 % |
| ≥61 min | 12.9 % | 28.6 % | 13.2 % | 7.4 % | 9.1 % |
| Puff volume (mL) | 54.3 ± 14.1 | 64.6 ± 12.3 | 56.0 ± 12.2 | 46.9 ± 12.3 | 53.8 ± 15.8 |
| Puff volume/cigarette (mL) | 767.2 ± 259.5 | 1160.1 ± 302.7 | 810.0 ± 179.6 | 609.4 ± 158.7 | 638.7 ± 136.9 |
| Puff number/cigarette | 14.5 ± 3.6 | 17.9 ± 3.2 | 15.0 ± 3.1 | 13.4 ± 3.4 | 12.6 ± 3.1 |
| Puff volume/day (mL) | 14,456.2 ± 8,769.0 | 22,579.9 ± 15,557.4 | 14,423.2 ± 7,249.8 | 11,435.1 ± 5,232.0 | 13,049.7 ± 5,556.8 |
Data are presented as the mean ± standard deviation (SD), unless indicated otherwise
aTopographic data were not available for one smoker who smoked low nicotine-yield cigarettes, and there were therefore 100 and 37 smokers in the “Total” and “Low” experimental smoking groups, respectively
Participants were asked to visit the study laboratory, provide informed consent and to complete a questionnaire on their smoking habits. Based on the information supplied on the completed questionnaires, the maximum smoking history was 528 months, in a 64-year-old individual. Since most participants stated that they had started smoking at 20 years of age, we concluded that the age reflects the smoking history. Furthermore, salivary cotinine level and CO level in the expiratory breath, which we focused on in this study, are biomarkers that decay immediately after smoking; therefore, smoking history was not counted as a statistical variable.
Questionnaire
Participants were asked to indicate their smoking intensity on a scale between 0 and 100 depending on the depth of inhalation and on the number of puffs they took. Participants were also asked to report the number of cigarettes they smoked the day before and the time to their first cigarette of the day, which we used as a proxy measure of nicotine dependence [13].
Salivary cotinine
At the first visit, participants were asked to wear latex gloves in order to collect salivary samples using sterile cotton swabs (Salivette; Sarstedt, Nümbrecht, Germany). Participants kept the Salivettes in their mouth for 2 min. Samples were frozen at −30 °C until analysis. The levels of cotinine in the saliva were not affected by the use of cotton swab collection methods [14].
Cotinine assay
After thawing, the saliva samples were recovered from the cotton swabs by centrifugation (1,000 g, 4 °C, 2 min) and arbitrarily diluted tenfold with phosphate buffered saline (Wako Pure Chemical, Osaka, Japan). Duplicate samples (10 μL) were assayed by a Saliva Cotinine Microplate EIA kit (Cozart, Milton Park, UK). Absorbance at 450 nm was measured on an Ultrospec Visible Plate Reader 96 (GE Healthcare Bio-Sciences, Little Chalfont, UK). The determination range of a Microplate EIA kit was from 5 to 50 ng/mL cotinine, and the mean and 95 % confidence interval (CI) of the absorbance value at concentrations of 5, 10, and 50 ng/mL were 0.99 ± 0.04, 0.72 ± 0.05, and 0.29 ± 0.02, respectively (n = 16). Standard solutions were assayed in 16 replicates. Samples were assayed in duplicate, and the average of two results was taken as the final salivary cotinine concentration. In the case that the absorbance of the sample was out of range of the standard curve, other dilutions of saliva were re-analyzed. Salivary cotinine data were available for 94 subjects.
CO in expiratory breath
The level of CO in the expiratory breath was measured by the Micro Smokerlyzer (Bendfont Scientific, Rochester, UK) according to the manufacturer’s instruction. Repeat measurements of CO were made immediately after the participants had smoked one cigarette. The average CO value of each participant was used for the data analysis, and data were available for all 101 subjects.
Smoking topography
The smoking topography of each participant was measured for a 24-h period using a CReSSmicro device (Plowshare Technologies, Baltimore, MD). CReSSmicro is a battery-operated portable device (2.5 × 2.2 × 1.2 inch, 3.1 oz) that measures a full complement of smoking topography variables, including puff volume, puff count, puff duration, average flow, peak flow, inter-puff interval, time, and date [7]. Participants were instructed to use the device for all cigarettes smoked during a 24-h period and to keep a diary of their smoking behavior. Puff volume/day was calculated by multiplying the mean puff volume/cigarette by the number of cigarettes smoked in a 24-h period. Smoking topography data were available for 100 subjects.
TNCO yields by machine smoking
Tar and nicotine in the MSS were collected by a smoking machine (Borgwaldt single-channel linear smoking machine model LM1; Borgwaldt KC, Hamburg, Germany) operated under the conditions specified by either the ISO 4387 protocol or the HCI T-115 protocol. Cigarettes were conditioned prior to machine smoking according to the ISO 3402 protocol. MSS was collected on a 44-mm Cambridge filter pad (Borgwaldt KC) and was immediately extracted with 20 mL of 2-propanol (Wako Pure Chemical) by gentle shaking for 20 min using an electric shaker (Personal-11; Taitec, Saitama, Japan). For the nicotine analysis, 50 μL of extracted solution was dissolved in 5 mL of 2-propanol containing 1 μg/mL isoquinoline (Tokyo Chemical Industry, Tokyo, Japan) as an internal standard, and 1 μL (model 7683B autosampler; Agilent Technologies, Santa Clara, CA) was separated by gas chromatographically on a 30-m DB-17 column (J&W Scientific, Cordova, CA; internal diameter 0.25 mm; film thickness 0.25 μm) in a gas chromatography system (HP 6890; Hewlett-Packard, Palo Alto, CA) under the following conditions: (1) temperature program: 2 min at 50 °C, then to 200 °C at the rate of 15 °C/min, then to 280 °C at a rate of 5 °C/min, and hold at 280 °C for 5 min; (2) flow rate: 1 mL/min. Nicotine was quantified using m/z 84 amu after electron impact ionization at 70 eV (internal standard: m/z 129 amu; mass selective detector model 5975, Agilent Technologies). Determination of water in the 2-propanol extraction solution was carried out in accordance with the ISO 10362-1 protocol using a gas chromatograph equipped with a thermal conductivity detector. Tar was calculated by deducting the nicotine and water content from the total particulate matter of crude smoke condensates according to the ISO 4387 protocol. CO was determined used a modified ISO 8454 protocol.
Statistical analysis
We used one-way analysis of variance (ANOVA) to test differences in the CO concentrations of the expiratory breath, salivary cotinine concentrations, and puff volume/day, across categories of nicotine yield. The Bonferroni method was carried out to reduce the chance of false positives in multiple comparisons. The association between cotinine in saliva and nominal brand tar yield, nominal brand nicotine yield, number of cigarettes’ filter ventilation holes, cigarette consumption, number of cigarettes smoked the day before, number of cigarettes smoked after getting up in the morning, self-reported smoking intensity, puff volume/day, time to first smoking cigarette, height, and weight was evaluated by a simple linear regression analysis. Variables at either significant (p < 0.05) or suggestive levels (p < 0.1) were re-analyzed for their association with salivary cotinine by a multivariable regression analysis. The same statistical analyses were conducted using CO in the expiratory breath as the outcome variable. All analyses were conducted using SPSS software ver. 16.0 (SPSS, Chicago, IL).
Results
Analysis of MSS by smoking machine
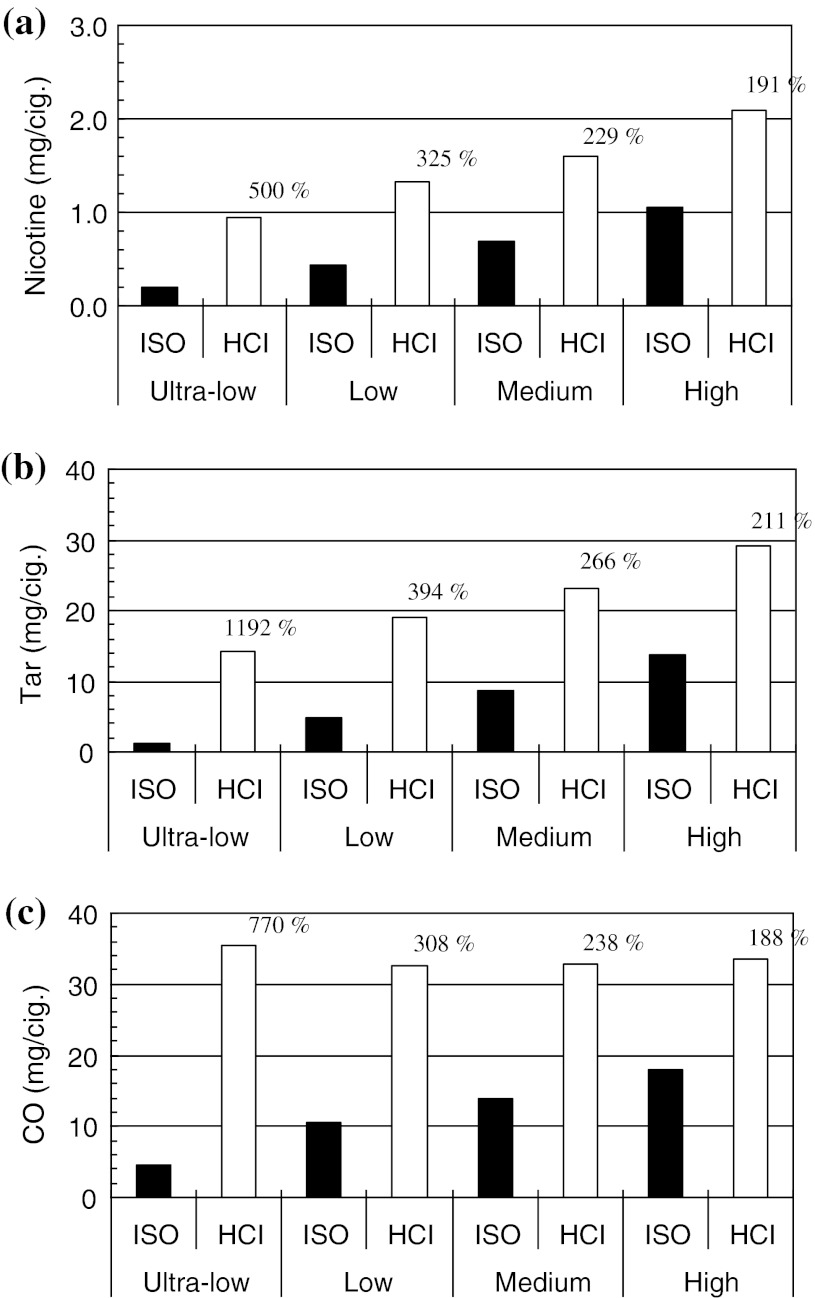
Figure 1 shows the tar, nicotine, and CO yields in MSS generated by the smoking machine operated under the ISO and HCI protocols. The yields were categorized into four groups according to the nominal brand nicotine yield expressed on the cigarette packages: “ultra-low”- (0–0.1 mg nicotine/cigarette), “low”- (>0.1, <0.6 mg nicotine/cigarette), “medium”- (≥0.6, <1.0 mg nicotine/cigarette), and “high”-yield brands (≥1.0 mg nicotine/cigarette) [15, 16]. Under the ISO protocol, the experimentally determined yields were the same as those appearing on the packages. Under the HCI protocol, the nicotine yields determined in the “ultra-low”-, “low”-, “medium”-, and “high”-yield groups were 5.0-, 3.3-, 2.3-, and 1.9-fold higher, respectively, than those obtained under the ISO protocol (Fig. 1a). Similarly, tar yields under the ISO protocol were the same as the values appearing on the cigarette packages, while under HCI protocol, the yields increased 11.9-, 3.9-, 2.7-, and 2.1-fold for the “ultra-low”-, “low”-, “medium”- and “high”-yield brands, respectively (Fig. 1b). CO concentrations under the ISO protocol were 4.6, 10.6, 13.8, and 17.9 mg/cigarette for the “ultra-low”-, “low”- “medium”-, and “high”-yield brands, respectively (Fig. 1c). In contrast to tar and nicotine yields, CO concentrations under the HCI protocol were very similar across groups, with a mean of 33.6 ± 1.3 mg/cigarette (Fig. 1c).
Fig. 1.
Tar, nicotine, and carbon monoxide (TNCO) yields by machine smoking tests. Ten cigarette brands were categorized into four groups according to the nicotine yields expressed on the cigarette packages: “ultra-low”- (0–0.1 mg nicotine/cigarette), “low”- (>0.1, <0.6 mg nicotine/cigarette), “medium”- (≥0.6, <1.0 mg nicotine/cigarette), and “high”-yield brands (≥1.0 mg nicotine/cigarette). Numbers over the columns of Health Canada Intense (HCI) measurements indicate the increase (in percentage) compared with the corresponding International Organization for Standardization (ISO) measurements
Smoking topography data of Japanese smokers
Table 2 shows the smoking topography data of 100 Japanese smokers. Overall, the mean puff volume and mean puff volume per cigarette were closer to the puffing parameters of the HCI protocol (55 mL per puff) than the ISO protocol (35 mL per puff). In addition, “ultra-low/low” nicotine yield brands were smoked significantly more intensely than “medium”- and “high”-yield brands: for example, the puff volume per day was 37 % greater for “ultra-low/low” brands.
Biomarker levels in smokers
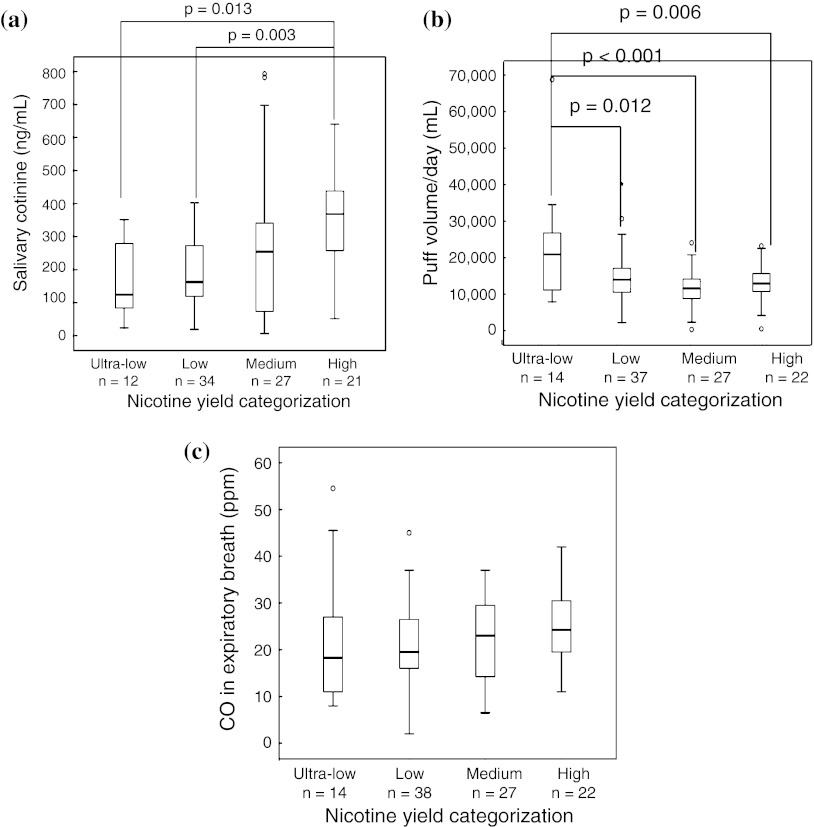
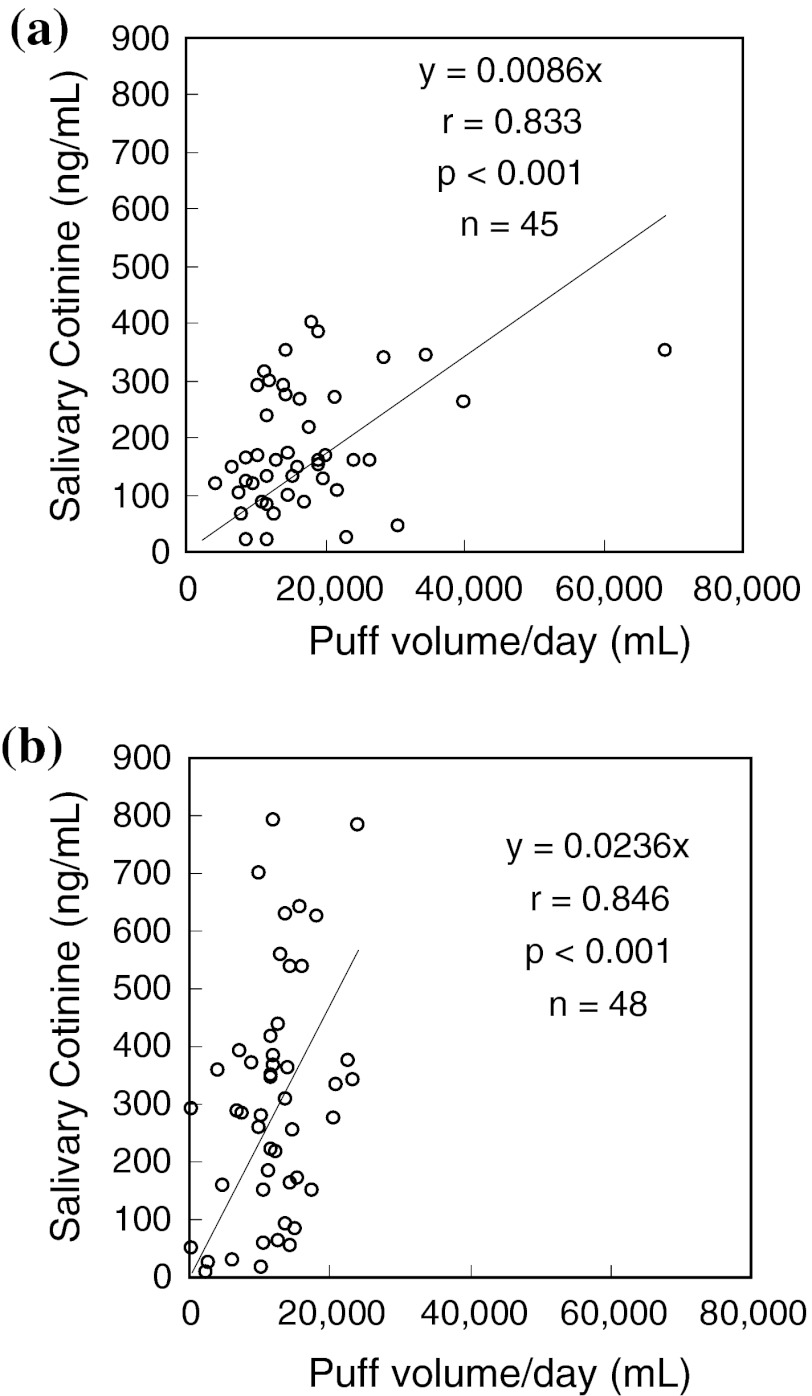
Levels of salivary cotinine were correlated with the nominal nicotine levels of brands smoked by each participant according to ANOVA analysis (p = 0.002) and multiple comparison (Fig. 2a). Puff volume/day was negatively correlated with the nominal nicotine levels of brands smoked by the respective smoker by ANOVA analysis (p = 0.001) and multiple comparison (Fig. 2b) and by simple linear regression analysis (r = −0.295, p = 0.003, n = 100). In order to examine whether the association between salivary cotinine levels and puff volume/day was constant across different nominal yields, we tested “utra-low/low” yield brands separately from “medium/high” nominal nicotine brands (Fig. 3). From the linear slope of each figure, we determined that smokers of the former smoked 2.7-fold more intensely than those of the latter in terms of puff volume/day to achieve the same levels of salivary cotinine (p = 0.024). Thus, the topographical data in Table 2 and biomarker results in Fig. 3 demonstrated that Japanese smokers also practiced so-called compensatory smoking. In contrast, the levels of CO in the expiratory breath were not associated with the nominal nicotine levels of the brands they smoked. The pattern of CO levels in the expiratory breath more closely mimicked the pattern of CO emissions by HCI than that by ISO in Fig. 1c.
Fig. 2.
a Salivary cotinine concentrations, b puff volume/day, c CO concentrations in expiratory breath by cigarette nicotine yield categorization. The number of participants was 94 for the saliva samples, 100 for the puff volume/day measurements, and 101 for the CO in expiratory breath samples. The correlation between puff volume/day and nominal nicotine yield was r = −0.295, p = 0.003 (n = 100). p values in the figures are results of multiple comparison applying the Bonferroni method. Horizontal lines Median salivary cotinine concentrations, puff volume/day, and CO concentrations. Open symbol Outliers
Fig. 3.
Correlation between puff volume/day and salivary cotinine for “ultra-low/low” nicotine yield smokers (a) and for “medium/high nicotine” yield smokers (b). Salivary cotinine concentrations were normally distributed whether or not they were log-transformed. When a subject [(x, y) = (68764.8, 351.4)] was excluded in a, regression parameters changed only slightly: y = 0.0097x, r = 0.845, p < 0.001, n = 44
Multivariable regression analysis
From the results of simple linear regression analysis, salivary cotinine was associated with nominal brand tar yield (r = 0.362, p = 0.001), nominal brand nicotine yield (r = 0.371, p < 0.001), number of cigarettes, filter ventilation holes (r = −0.341, p = 0.001), cigarette consumption (r = 0.336, p = 0.001), number of cigarettes smoked the day before (r = 0.314, p = 0.003), number of cigarettes smoked upon waking up (r = 0.194, p = 0.072), self-reported smoking intensity (r = 0.228, p = 0.034), and time to first smoking cigarette (r = −0.210, p = 0.050). CO in the expiratory breath was associated with number of filter ventilation holes in a cigarette (r = −0.168, p = 0.092), cigarette consumption (r = 0.299, p = 0.002), number of cigarettes smoked the day before (r = 0.306, p = 0.002), number of cigarettes smoked after waking up (r = 0.230, p = 0.020), self-reported smoking intensity (r = 0.367, p < 0.001), puff volume/day (r = 0.312, p = 0.002) and time to first smoking cigarette (r = −0.183, p = 0.067).
A multivariable regression analysis was performed to examine predictors of salivary cotinine and CO in the expiratory breath. Variables that were correlated at p < 0.1 in the simple regression analysis and which had no multicollinearity were selected and included in the model as independent variables (Table 3). As shown in Table 3, salivary cotinine levels were positively associated with puff volume/day (β = 0.27, p < 0.01) and nominal brand nicotine yield (β = 0.44, p < 0.01), and negatively associated with time to first smoking of a cigarette (β = −0.18, p = 0.05). Thus, even after the nominal nicotine levels were adjusted, smokers smoked more intensely in terms of puff volume/day to increase plasma nicotine levels as judged by salivary cotinine. We also performed multivariable regression analysis between the levels of CO in the expiratory breath and other independent variables. CO levels in expiratory breath was positively associated with puff volume/day (β = 0.27, p = 0.01) and self-reported smoking intensity (β = 0.30, p < 0.01), and negatively associated with time to first smoking of a cigarette (β = −0.19, p = 0.03), but not with nominal nicotine levels of the different brands.
Table 3.
Predictors of biomarkers of exposure
| Predictors | Partial regression coefficient B | Standardized partial regression coefficient β | Significance probability p | 95 % confidence interval for B | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Salivary cotinine (ng/mL) | |||||
| Puff volume/day (mL) | 0.01 | 0.27 | <0.01 | <0.01 | 0.01 |
| Nominal brand nicotine yield (mg/cigarette) | 214.32 | 0.44 | <0.01 | 118.25 | 310.38 |
| Self-reported smoking intensity | 1.25 | 0.11 | 0.22 | −0.74 | 3.23 |
| Time to first smoking cigarette | −0.24 | −0.18 | 0.05 | −0.47 | <0.01 |
| CO in expiratory breath (ppm) | |||||
| Puff volume/day (mL) | <0.01 | 0.27 | 0.01 | <0.01 | <0.01 |
| Nominal brand nicotine yield (mg/cigarette) | 2.35 | 0.09 | 0.40 | −3.05 | 7.76 |
| Self-reported smoking intensity | 0.17 | 0.30 | <0.01 | 0.06 | 0.29 |
| Time to first smoking cigarette | −0.02 | −0.19 | 0.03 | −0.03 | <0.01 |
Multiple correlation coefficient is r2 = 0.275 (upper model) and r2 = 0.239 (lower model), respectively
Discussion
This study is among the first to characterize the TNCO emissions of the best-selling cigarettes in Japan, a critically important tobacco market. We observed significant increases in emissions under the more intensive smoking protocol of the Health Canada. The association between the ISO and HCI values varied depending upon the emission parameter measured: whereas tar and nicotine yields under the ISO and HCI yields appeared to be highly correlated, CO yields under the HCI protocol showed very little difference across cigarette brands, in contrast to the ISO values.
This study is also the first to report measures of puffing behavior among Japanese smokers and to examine the behavioral measures of smoking compensation. Topographical data on the smoking behavior of each participant were collected by a CReSSmicro device for one whole day. Puff volume and puff volume per cigarette, as measured by machine smoking under the ISO or HCI protocols, were 35 or 55 mL, and 203–245 or 385–534 mL, respectively (our unpublished data). These findings demonstrate that Japanese smokers engage in compensatory smoking: brands with lower nominal tar and nicotine yields and higher levels of ventilation are smoked systematically more intensely than higher yield brands. The average puff volume per cigarette recorded among our Japanese smokers (767.2 mL) is somewhat higher than previous estimates from the UK [17] and the USA [8], but similar to estimates from Canadian smokers who switched to cigarettes with 4 mg of ISO tar [7]. The differences between our values and those of the UK and USA studies may reflect the higher levels of filter ventilation and lower tar and nicotine machine yields included in our study. The average puff volume in our study for “ultra-low/low” brands (58 mL) was similar to that reported in previous studies conducted with similar brand categories in the USA (57 mL) [8] and in Canada (58 mL) [7]. Together, these data suggest that behavioral compensation in response to more heavily ventilated, “lower tar” cigarettes may be a universal phenomenon among smokers. The findings also underscore the fact that the Health Canada “Intense” protocol does not represent an “upper limit” in terms of its smoking parameters. Indeed, the puff volume used in the HCI protocol was lower than the average puff volume observed among the smokers of “ultra-low” and “low” brands in our study. Although the HCI protocol also blocks filter ventilation holes, the Our findings nevertheless suggest that a considerable number of smokers may be exposed to greater levels of chemical emissions than the “intensive” method would suggest.
There was positive association with the levels of salivary cotinine levels and the nominal nicotine levels of brands being smoked. The same trend has also been reported between nominal nicotine levels and urinary cotinine levels [15], serum cotinine levels [16], and salivary cotinine levels [18]. It is noteworthy, however, that the median value of salivary cotinine concentration, 124 ng/mL, in smokers who smoked “ultra-low” nicotine yield brands and the concentration, 368 ng/mL, in smokers who smoked “high” nicotine yield brand are much closer than differences in the nominal nicotine value would suggest. Although nominal nicotine levels on packages differed more than tenfold under the ISO protocol, the difference in salivary cotinine levels was only threefold. Nakazawa et al. also reported that there was only a twofold difference in urinary cotinine concentration between Japanese smokers who smoked “ultra-low” nominal nicotine brands and those who smokes “high” nominal nicotine brands [19]. Although the association between filter ventilation levels, puffing behaviors, and nominal yields under the ISO protocol were not explicitly tested in the current study due to missing data for some “ultra-low/low” brands, the data depict a positive association of salivary cotinine with puffing behavior and a strong negative association of puffing behavior with nominal nicotine yields under the ISO protocol. This pattern is consistent with previous research indicating that the primary design strategy for lower tar and nicotine yields under the ISO protocol is to increase filter ventilation as opposed to “genuine” reductions in the nicotine content [20]. CO in the expiratory breath showed very modest differences across brands regardless of the nominal nicotine yields listed on the cigarette package or nominal CO yield under ISO protocol, similar to previous findings [15, 16]. This finding is also consistent with the notion that CO is primarily a measure of smoking intensity and that it is less dependent on differences in tobacco blend or product design.
More generally, the findings highlight the elastic nature of cigarette design and the fact that all conventional cigarette brands are capable of delivering a wide range of emission levels. For example, salivary cotinine levels varied by more than tenfold for the same brand. In other words, the consumer controls his/her level of chemical exposure from each brand by changing his/her smoking behavior to a much greater extent than does the design of different cigarette brands. Our data on biomarkers of Japanese smokers resembled the smoking pattern of the HCI protocol.
Overall, our results suggest that smokers who smoke “ultra-low/low” nominal nicotine brands tend to draw smoke more deeply into the lung and take more puffs. The findings underscore the fact that tar and nicotine levels from machine smoking protocols should not be used as indicators of risk. They also highlight the importance of using measures of human exposure to understand cigarette delivery and potential differences between brands. Finally, our findings provide additional evidence on the misleading nature of tar and nicotine values from machine smoking protocols as a source of consumer information on packs. Cigarette properties, smoking patterns, lifestyles (including dietary habits), and genetic difference are considered to be reasons why lung cancer risk is lower in Japan than in other countries. In our study, we have found that smoking pattern was not the main cause of this difference. Exposure to tobacco-specific nitorosamines and aldehydes may also differ according to the charcoal filter. Future research should therefore focus on cigarette properties.
Acknowledgments
We would like to acknowledge the assistance of Carla Parkinson, Alex Lee, Yasuko Hara and Minako Segawa. The study was funded by the NIH/NCI TTURC Developmental Research project grant, USA, the Canadian Tobacco Control Research Initiative, Canada, and a Grant-in-Aid from the Third Term Comprehensive Control Research for Cancer, MHLW, Japan. The research protocol was reviewed and approved by the Ethical Committee University of Waterloo (ORF file #13284; 11/14/06 Asia Pacific Smoking Study”) and the Research Ethical Review Board of the NIPH (NIPH-IBRA #06012).
Conflict of Interest
All authors declare that we have no financial relationship with a biotechnology manufacturer, a pharmaceutical company, or other commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
References
- 1.World Health Organization. Report on the global tobacco epidemic. WHO: Geneva, Switzerland; 2008.
- 2.US Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The Health consequence of smoking: a report of the surgeon general. Atlanta: US Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- 3.US Department of Health and Human Services PHS, National Institute of Health; National Cancer Institute. Risk associated with smoking cigarettes with low machine measured yields of tar and nicotine. Bethesda, MD: US department of Health and Human Services PHS, National Institute of Health; National Cancer Institute; 2001.
- 4.Federal Trade Commission. “Tar,” nicotine, and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998. Washington D.C.: Federal Trade Commission; 2000.
- 5.Marugame T, Sobue T, Nakayama T, Suzuki T, Kuniyoshi H, Sunagawa K, et al. Filter cigarette smoking and lung cancer risk; a hospital-based case–control study in Japan. Br J Cancer. 2004;90:646–651. doi: 10.1038/sj.bjc.6601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thun MJ, Burns DM. Health impact of “reduced yield” cigarettes: a critical assessment of the epidemiological evidence. Tob Control. 2001;10(Suppl 1):i4–i11. doi: 10.1136/tc.10.suppl_1.i4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14:1370–1375. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- 8.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9:511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–111. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Health Canada. Determination of “tar”, nicotine and carbon monoxide in mainstream tobacco smoke. Ottawa, Canada: Health Canada; 1999.
- 11.World Health Organization (TobReg) SGoTPR, Recommendation T. Guiding principles for the development of tobacco product reseach and testing capacity and proposed protocols for the initiation of tobacco product testing. Geneva, Switzerland: World Health Organization; 2004.
- 12.Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: the JPHC study. Int J Cancer. 2002;99:245–251. doi: 10.1002/ijc.10308. [DOI] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/S0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Kawachi I, Nakamura M, Nogami H, Shirokawa N, Masui S, et al. Cigarette nicotine yields and nicotine intake among Japanese male workers. Tob Control. 2002;11:55–60. doi: 10.1136/tc.11.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald N, Froggatt P. Nicotine, smoking, and the low tar program. Oxford: Oxford Medical Publications; 1989.
- 17.Shahab L, Hammond D, O’Connor RJ, Cummings KM, Borland R, King B, et al. The reliability and validity of self-reported puffing behavior: evidence from a cross-national study. Nicotine Tob Res. 2008;10:867–874. doi: 10.1080/14622200802027156. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: evidence from a representative population survey. J Natl Cancer Inst. 2001;93:134–138. doi: 10.1093/jnci/93.2.134. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa A, Shigeta M, Ozasa K. Smoking cigarettes of low nicotine yield does not reduce nicotine intake as expected: a study of nicotine dependency in Japanese males. BMC Public Health. 2004;4:28. doi: 10.1186/1471-2458-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor RJ, Kozlowski LT, Borland R, Hammond D, McNeill A. Relationship between constituent labelling and reporting of tar yields among smokers in four countries. J Public Health (Oxf) 2006;28:324–329. doi: 10.1093/pubmed/fdl056. [DOI] [PubMed] [Google Scholar]