Abstract
A 60-year-old female presented with abdominal pain and tenderness of five-day duration. Contrast enhanced CT showed a mass of 9 × 6 × 5.5 cm in size with almost complete obliteration of the inferior vena cava and massive extension to the extravascular space. CT-guided biopsy demonstrated a low-grade leiomyosarcoma. The patient underwent 125Iodine seeds implantation in two sessions, and another balloon cavoplasty. Abdominal pain and tenderness gradually improved and the patient continues to remain as disease free state for three years after the procedures.
Keywords: Leiomyosarcoma, Inferior vena cava, Brachytherapy, Cavoplasty
INTRODUCTION
Vascular smooth muscle tumors are rare and include a variety of neoplastic lesions characterized by their histological similarity to adult smooth muscle tissue. These tumors are usually benign as with diseases such as leiomyoma, intravascular leiomyomatosis and angioleiomyoma. There is also a very rare malignant form-leiomyosarcoma.
Vascular leiomyosarcomas arise predominantly in the inferior vena cava (IVC), and less commonly in the pulmonary arteries or veins or other peripheral vessels (1). Surgical resection is considered to be the main treatment for this disease.
We present a rare case of leiomyosarcoma in the IVC with massive extension to extravascular space which was treated successfully with iodine 125 (125I) seeds implantation rather than surgery. This was considered as the best option taking account of the risk and the high rate of recurrence. Brachytherapy was planned because of consecutive radioactive effects on the tumor, lower damage to normal tissue and the definite curative effects.
Despite our extensive review of English literature, we could not find any similar case of successfully treated IVC leiomyosarcoma.
CASE REPORT
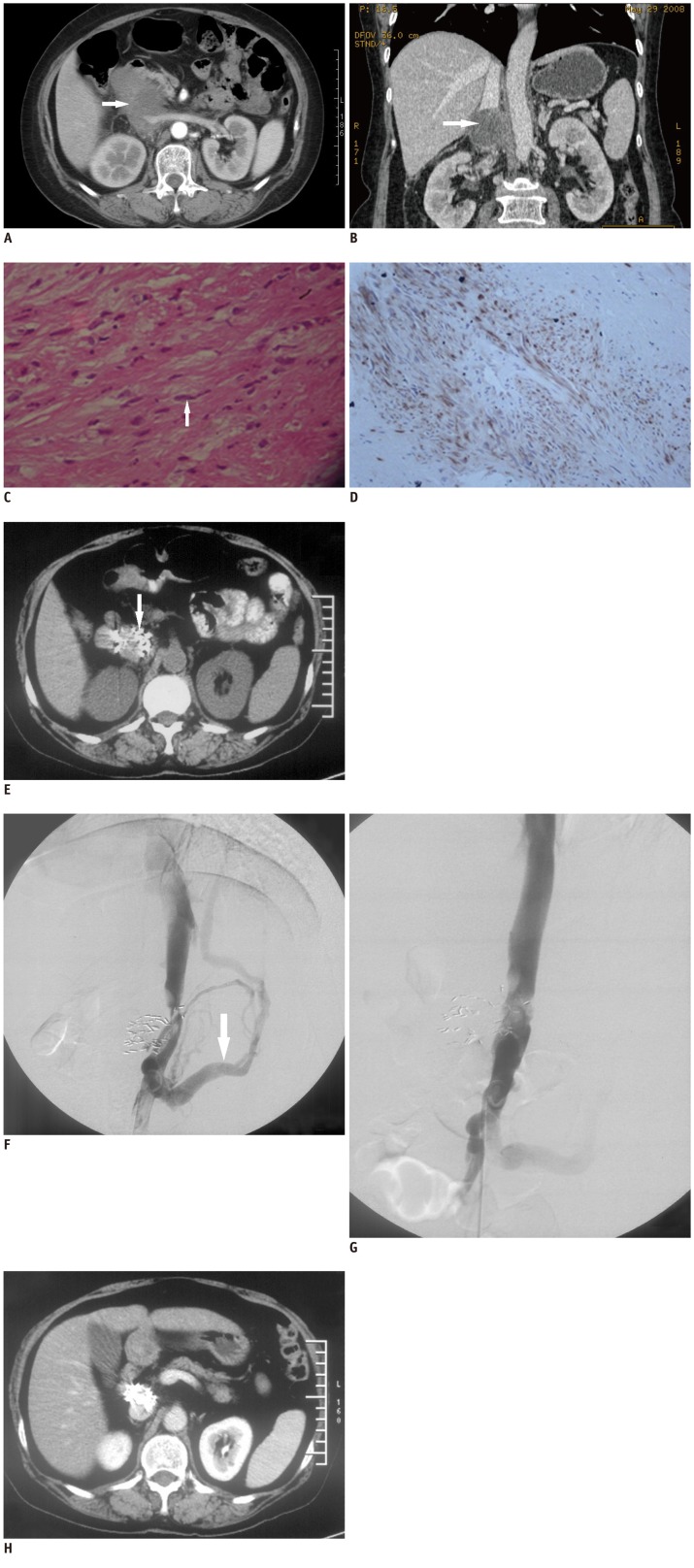
A 60-year-old woman was admitted to our hospital with abdominal pain over the course ofthe last five days. Tenderness in the right upper abdomen was noted on physical examination. Intravenous contrast enhanced CT scan showed a mass 9 × 6 × 5.5 cm in size with almost complete obliteration of the IVC with a large extravascular component (Fig. 1A, B). The mass was located in the suprarenal IVC with its epicenter outside of the IVC, and presented no extension to the renal vein. Exact diagnosis in this case was uncertain thus biopsy of the mass under CT guidance was performed. Histology revealed diffuse distribution of spindle cells with mild atypia in fibrous tissue. Immunohistochemical stains for smooth muscle desmin were positive and those for interstitialoma, endothelium and neurogenic tumors, including CD34, CD117, S100, nestin and Alkaline were negative, which were all consistent with the diagnosis of low-grade malignant leiomyosarcoma (Fig. 1C, D).
Fig. 1.
Tumor, pathology, treatments and follow-up CT.
Intravenous contrast enhanced CT of mass. Axial view (A, arterial phase) at level of renal vein demonstrates huge mass (white arrow) involving inferior vena cava and partially surrounding left renal vein. Coronal reconstruction (B, venous phase) shows normal corticomedullary enhancement bilaterally, confirming normal function of both kidneys (white arrow). Histology and immunohistochemistry of leiomyo-sarcoma in inferior vena cava. H&E (C, × 200) stain reveals spindle cells (white arrow). Tumor is positive for desmin (D, × 400, brown) indicating leiomyosarcoma deriving from smooth muscle. E. Precontrast follow-up CT three months after two sessions of 125I implantation. Tumor size has markedly decreased and disappeared almost completely. Note high density spots of 125I seeds (white arrow).
Balloon angioplasty for inferior vena cava (IVC) stenosis eight months after 2nd session of 125I seeds implantation. Inferior vena cavography before angioplasty (F) demonstrates stenosis and partial IVC obstruction, with retroperitoneal collateral circulation (white arrow) in keeping with IVC thrombosis. View after cavoplasty (G) shows almost normal lumen of IVC segment. H. Enhanced follow-up CT of 33 months after 2nd session of 125I seeds implantation. CT scan reveals disappearance of tumor without significant caval stenosis or thrombosis.
After informed consent from the patient and approval of the Ethics Committee of our hospital, 125I seeds implantation of the mass by posterior approach under CT guidance was performed. Three days before 125I seeds implantation, the patient underwent a detailed tumor volume study using CT scans with slice thickness of 5 mm. A radiation oncologist outlined the gross tumor volume (GTV) and marked areas at risk for subclinical disease on each transverse image. The planning target volume (PTV) included the entire GTV and 0.5-1.0 cm margins. The dose was prescribed as the minimal peripheral dose (MPD) encompassing the PTV. The distribution, MPD of 125I seeds and dose volume histogram (DVH) were calculated using computerized treatment planning system (RT-RSI, Beijing Atom and High Technique Industries Inc., Beijing, China).
Lead protective bucket, vest, apron, spectacles and gloves were used by the operator and the assistants. Puncture region was marked based on the CT image. Following local anesthesia with 0.5% lidocaine and sedation with pethidine hydrochloride and promethazine hydrochloride, eighteen-gauge needles were placed via posterior paraspinal approach or through the space between right kidney and the vertebraes. The procedure included 2-3 steps under CT guidance in a parallel array of 1.0 to 1.5 cm apart, and extended at least 0.5-1.0 cm beyond the GTV. 125I seeds (Jaco pharmaceuticals CO. LTD, Ningbo, China, t1/2: 59.6 days, energy level: 27.4-31.4. keV) were implanted using a Jaco applicator and Radiation-proof implanting needles (Hakko trading CO. LTD., Shanghai, China). The space between seeds (center to center) was kept at 1.0 cm. 30 seeds were implanted with an activity of 0.8 mCi. The distribution and uniformity of the 125Iodine seeds in the lesion and drifting seeds analysis was done under the CT scan. The total procedure lasted 60 minutes, and the dose burden of radiation was 10 mSv under CT guidance.
Abdominal pain of the patient was relieved in one month after the procedure was performed. Three months later, a follow-up CT examination showed that the size of tumor was decreased, although a cold area was noted at the posterior margin. A second 125I seeds implantation by latero-posterior approach through the lateral space of right kidney under CT guidance was then performed and 30 0.8 mci 125I seeds were introduced into the cold area. After the 2nd session of brachytherapy the course was uneventful and the patient remained in the hospital for 5 days for observation of any unexpected complications. No procedure-related complications were observed. Three months later, CT examination showed high density 125I seeds surrounding the IVC and the previous space-occupying mass disappeared (Fig. 1E).
Five months after the last CT examination the patient was readmitted with lower abdominal pain and presence of lower leg edema. Inferior vena cavography showed narrowing and partial IVC obstruction at L2-3 level, with retroperitoneal collateral circulation in keeping with IVC thrombosis (Fig. 1F). After anticoagulant and thrombolytic therapy for one week, cavoplasty was performed with balloons of 5 × 40 mm and 20 × 40 mm consecutively. After angioplasty, the diameter of IVC segment was about 20 mm (Fig. 1G). The patient was free of symptoms five days after the procedure. 2.5 mg warfarin per day was used as anticoagulant therapy for the next 3 months.
125I seeds implantation and balloon cavoplasty worked very well in this patient. At follow-up, the patient was free of symptoms and CT scan showed disappearance of the tumor and no recurrence for 33 months after the 2nd session of brachytherapy, with no thrombosis in the IVC (Fig. 1H).
DISCUSSION
Vascular smooth muscle tumors are rare, including benign and malignant forms (1). Leiomyosarcomas of vascular origin, the malignant form, arise most frequently in the IVC. It is most commonly detected in women in their sixth decade, and presents with 3 main growth patterns: 62% of the cases demonstrate extraluminal, 5% intraluminal, and 33% extra- and intraluminal growth patterns (2).
Leiomyosarcomas of the IVC are classified into 3 groups according to the localization in the IVC. Upper segment involvement extends to the level of the renal veins and lower segment involvesthe infrarenal portion. The percentages of upper, middle, and lower section involvement are 24%, 42%, and 34%, respectively (2).
Modern imaging modalities using computed tomography, magnetic resonance imaging, cavography, ultrasound, and positron emission tomography allow for an accurate preoperative diagnosis of tumor extension and resectability. Despite advanced imaging technologies, the final diagnosis is still requires pathological assessment. Histopathology of leiomyosarcoma reveals spindle tumor cells, which are positive for markers of smooth muscle activity including vimentin, muscle actin, alpha-smooth muscle actin, and desmin. Malignancy is indicated by variable cell size and shapes and increased nuclear/cytoplasmic ratio.
Since the experiene with this disease is very limited, the optimal management of IVC leiomyosarcoma is unestablished (3). At present aggressive surgical therapy is recommended by most authors. En-bloc resection demands primary tumor extirpation and resection of involved adjacent structures, with or without caval reconstruction (4). Major disadvantage of surgery is the high rate of recurrence. Cho and colleagues reported a mortality rate of approximately 11.11%, and recurrence rate of 44.45% after surgery (5).
As for adjuvant therapy, radiation treatment is generally considered beneficial. Preoperative external-beam radiation therapy (EBRT) could facilitate marginally negative resection (6), and postoperative radiation treatment can diminish local recurrence and may improve survival (7). However, EBRT can not avoid normal tissue effectively, and there are difficulties regarding the dose of radiation. In three-dimensional conformal radiotherapy (3D-CRT) high concentration of dose radiation conforms to PTV and sparing normal adjacent tissue is delivered compared with EBRT. This in turn leans to minimal radiation to the surrounding normal tissues is minimal. However, 3D-CRT is a sophisticated technique compared with brachytherapy which is a relative simple technique.
The use of postoperative chemotherapy has long been recommended for these types of patients, although evidence for the efficacy of this treatment is sparse (7). Preoperative chemotherapy has rarely allowed significant improvement permitting surgical resection, and definitive role of postoperative chemotherapy showed no positive impact in Hines et al. (7). No study or report has demonstrated a consistent advantage of chemotherapy for these patients.
Brachytherapy is an established method of safely providing adjuvant local radiotherapy used alone or in combination with resection for prostate cancer, breast cancer, cervical cancer and soft tissue sarcomas (8). Compared with external beam irradiation, it spares more normal tissue and permits irradiation of the tumor bed in the immediate postoperative period (9). Brachytherapy options include low dose rate techniques with iridium 192 or iodine 125, fractionated high dose rate brachytherapy, and intraoperative high dose rate therapy. 125I seeds are characterized by a long half life period and low quantity of radiant energy (10). For certain tumors, consecutive radiotherapy can be performed by implanting 125I seeds, and the curative effects are better than external radiotherapy. As a kind of brachytherapy, 125I seeds implantation shows simplicity, safety and effectiveness in the treatment of primary, metastatic and recurrent malignant tumors (10).
Brachytheraphy was used to treat primary leiomyosarcoma of the lungs, uterus, rectum and prostate. To the best of our knowledge, no brachytherapy in the treatment of vascular leiomyosarcoma has been reported.
We present a rare case of IVC leiomyosarcoma, which protruded mainly to the extravascular space and adjoined aorta, liver, duodenum and kidney. In this case, surgical procedure may be hazardous and life-threatening, which is associated with a high rate of recurrence. EBRT was excluded as it might cause skin injury and radiation enterocolitis. Based on our previous experience with 125I seeds implantation in malignant soft tissue tumors, brachytherapy and balloon dilatation combined with anticoagulant therapy were applied in this case. Considering the immediate positive results of the procedure, it was decided not to implement an IVC stent. Pre-procedure administration of anticoagulant agent and thrombolytics remarkably decreased the risks of thrombus migration, therefore implantation of retrievable IVC filter was ignored. Chemotherapy was not applied because the patient refused adjuvant treatment.
A rare case of primary leiomyosarcoma of the IVC with large intra and extravascular component is presented in this case report. Brachytheraphy is a relatively simple procedure and was applied in this case with excellent results. This success may possibly be attributed in part to the low grade type of the tumor. However, more experience is needed to establish the value of brachytherapy in the treatment of IVC leiomyosarcoma.
References
- 1.Dalainas I. Vascular smooth muscle tumors: review of the literature. Int J Surg. 2008;6:157–163. doi: 10.1016/j.ijsu.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ceyhan M, Danaci M, Elmali M, Ozmen Z. Leiomyosarcoma of the inferior vena cava. Diagn Interv Radiol. 2007;13:140–143. [PubMed] [Google Scholar]
- 3.Reddy VP, Vanveldhuizen PJ, Muehlebach GF, Dusing RW, Birkbeck JP, Williamson SK, et al. Leiomyosarcoma of the inferior vena cava: a case report and review of the literature. Cases J. 2010;3:71. doi: 10.1186/1757-1626-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mingoli A, Sapienza P, Brachini G, Tarantino B, Cirillo B. Surgical treatment of inferior vena cava leiomyosarcoma. J Am Coll Surg. 2010;211:145–146. doi: 10.1016/j.jamcollsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Cho SW, Marsh JW, Geller DA, Holtzman M, Zeh H, 3rd, Bartlett DL, et al. Surgical management of leiomyosarcoma of the inferior vena cava. J Gastrointest Surg. 2008;12:2141–2148. doi: 10.1007/s11605-008-0700-y. [DOI] [PubMed] [Google Scholar]
- 6.Daylami R, Amiri A, Goldsmith B, Troppmann C, Schneider PD, Khatri VP. Inferior vena cava leiomyosarcoma: is reconstruction necessary after resection? J Am Coll Surg. 2010;210:185–190. doi: 10.1016/j.jamcollsurg.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Hines OJ, Nelson S, Quinones-Baldrich WJ, Eilber FR. Leiomyosarcoma of the inferior vena cava: prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999;85:1077–1083. [PubMed] [Google Scholar]
- 8.Aflatoon K, Manoso MW, Deune EG, Frassica DA, Frassica FJ. Brachytherapy tubes and free tissue transfer after soft tissue sarcoma resection. Clin Orthop Relat Res. 2003:248–253. doi: 10.1097/01.blo.0000093886.12372.74. [DOI] [PubMed] [Google Scholar]
- 9.Laskar S, Bahl G, Puri A, Agarwal MG, Muckaden M, Patil N, et al. Perioperative interstitial brachytherapy for soft tissue sarcomas: prognostic factors and long-term results of 155 patients. Ann Surg Oncol. 2007;14:560–567. doi: 10.1245/s10434-006-9137-2. [DOI] [PubMed] [Google Scholar]
- 10.Ware ML, Larson DA, Sneed PK, Wara WW, McDermott MW. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54:55–63. doi: 10.1227/01.neu.0000097199.26412.2a. discussion 63-64. [DOI] [PubMed] [Google Scholar]