Abstract
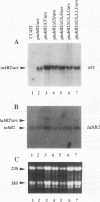
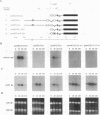
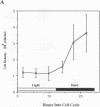
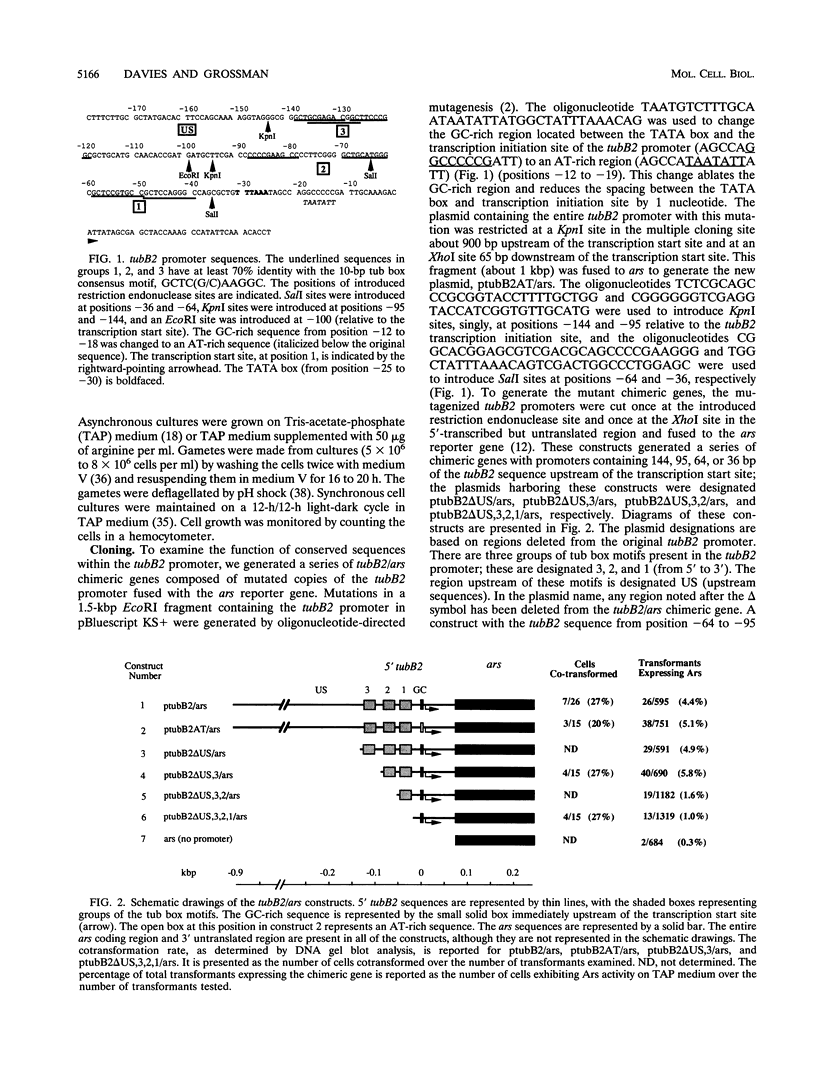
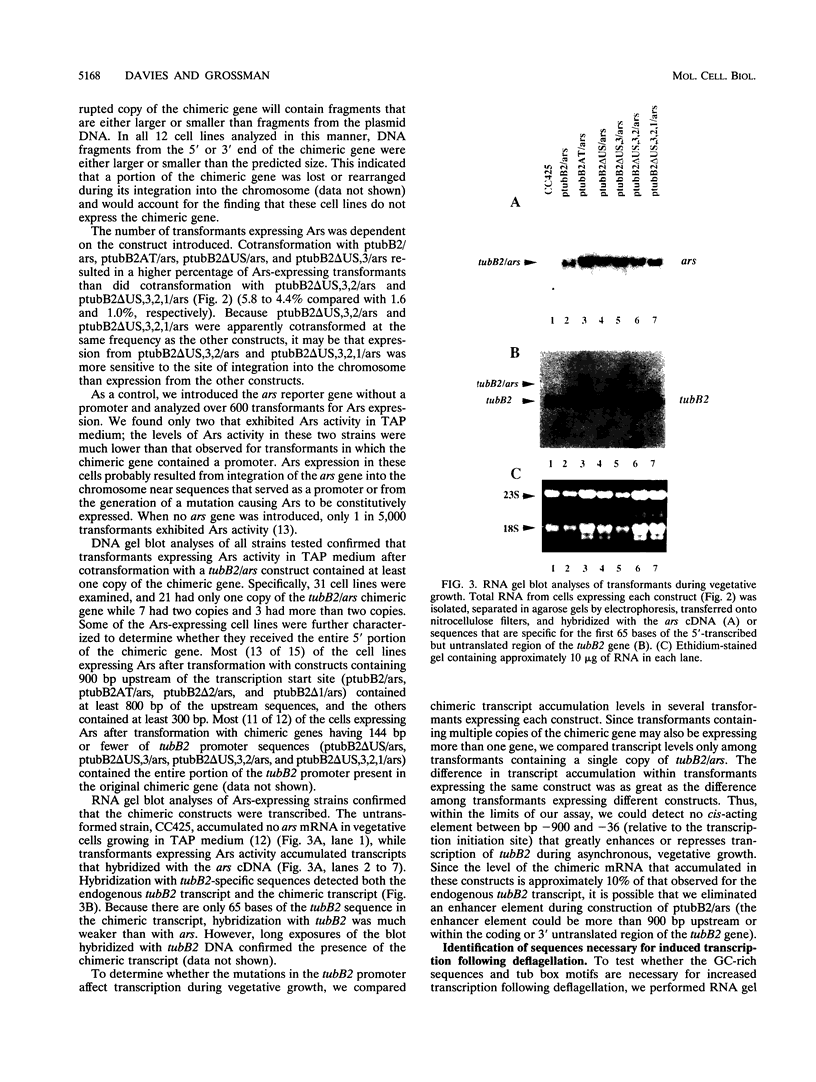
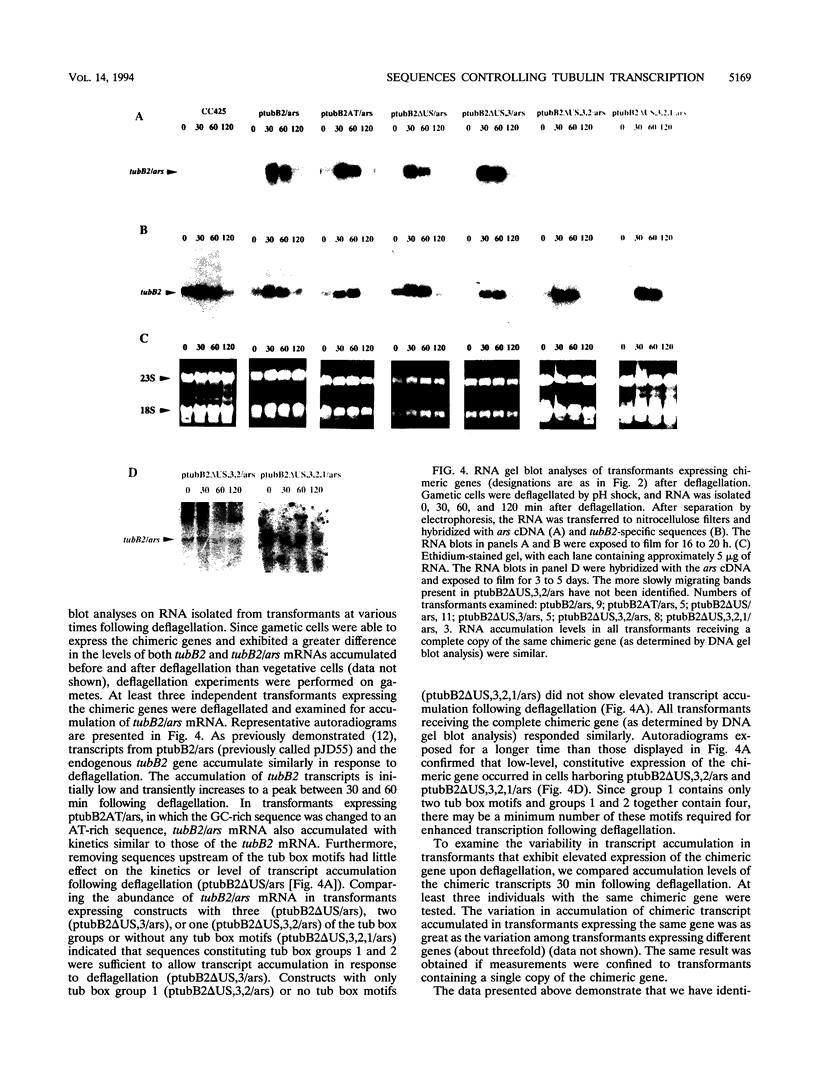
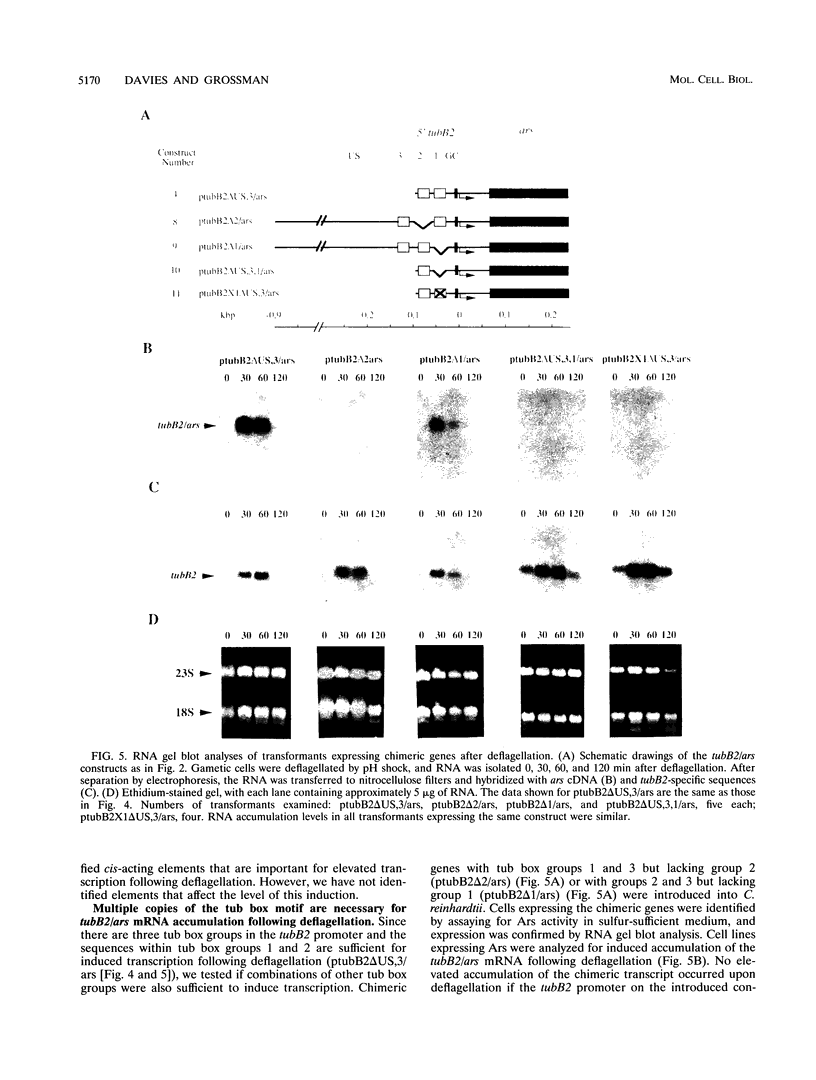
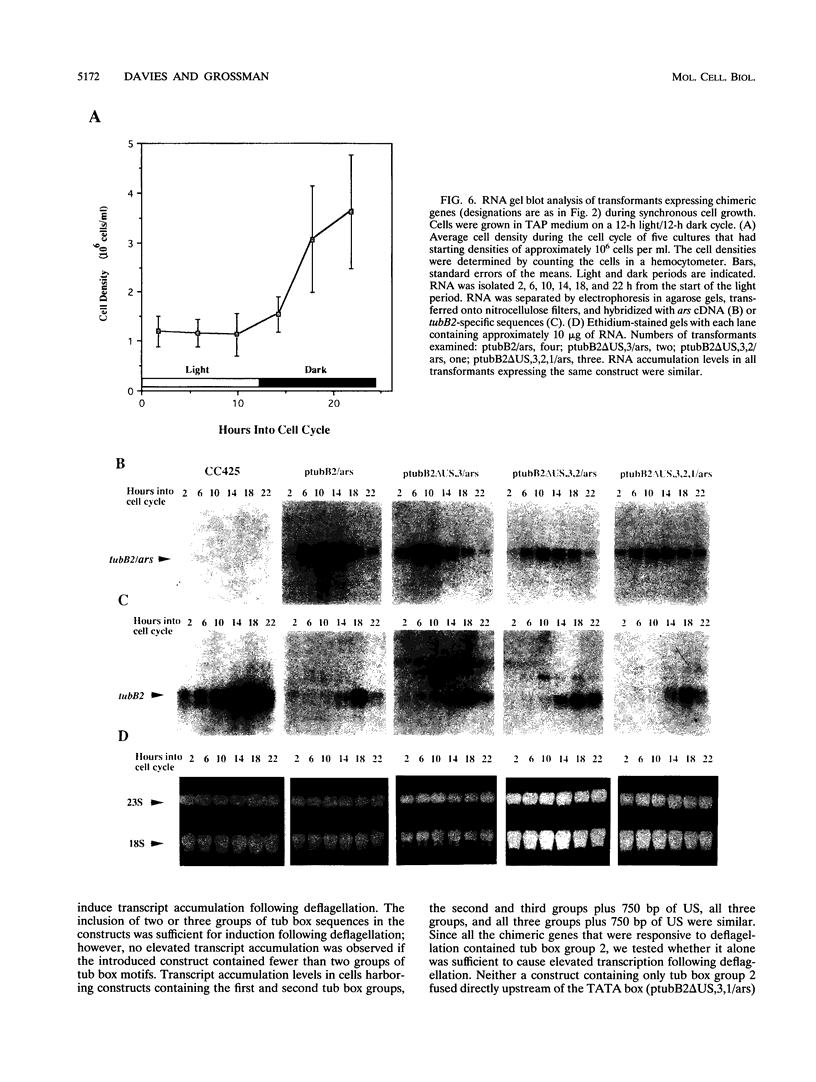
In Chlamydomonas reinhardtii, transcripts from the beta 2-tubulin gene (tubB2), as well as those from other tubulin-encoding genes, accumulate immediately after flagellar excision as well as at a specific time in the cell cycle. Control of tubB2 transcript accumulation following deflagellation is regulated, at least partially, at the transcriptional level. We have fused the tubB2 promoter to the arylsulfatase (ars) reporter gene, introduced this construct into C. reinhardtii, and compared expression of the chimeric gene with that of the endogenous tubB2 gene. After flagellar excision, transcripts from the tubB2/ars chimeric gene accumulate with kinetics similar to those of transcripts from the endogenous tubB2 gene. The tubB2/ars transcripts also accumulate in a cell cycle-specific manner; however, chimeric transcripts are more abundant earlier in the cell cycle than the endogenous tubB2 transcripts. To elucidate transcriptional control of tubB2, we have mutated or removed sequences in the tubB2 promoter and examined the effect on transcription. The tubB2 promoter shares features with the promoters of other tubulin-encoding genes; these include a GC-rich region between the TATA box and the transcription initiation site and multiple copies of a 10-bp sequence motif that we call the tub box. The tubB2 gene contains seven tub box motifs. Changing the GC-rich region to an AT-rich region or removing three of the seven tub box motifs did not significantly affect transcription of the chimeric gene. However, removing four or five tub box motifs prevented increased transcription following deflagellation and diminished cell cycle-regulated transcription from the tubB2 promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Howell S. H. Cell cycle stage-specific accumulation of mRNAs encoding tubulin and other polypeptides in Chlamydomonas. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5577–5581. doi: 10.1073/pnas.79.18.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. J., Schloss J. A., Rosenbaum J. L. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J Cell Biol. 1984 Dec;99(6):2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandziulis R. J., Rosenbaum J. L. Novel control elements in the alpha-1 tubulin gene promoter from Chlamydomonas reinhardii. Mol Gen Genet. 1988 Oct;214(2):204–212. doi: 10.1007/BF00337712. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Anthony J. G., Sternberg E. J., Weeks D. P. Repeated consensus sequence and pseudopromoters in the four coordinately regulated tubulin genes of Chlamydomonas reinhardi. Mol Cell Biol. 1984 Jun;4(6):1115–1124. doi: 10.1128/mcb.4.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Young E. E., Buchbinder B. U., Weeks D. P. Coordinate regulation of the four tubulin genes of Chlamydomonas reinhardi. Nucleic Acids Res. 1982 Feb 25;10(4):1295–1310. doi: 10.1093/nar/10.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland T. G., Gull K., Schedl T., Boston R. S., Dove W. F. Cell type-dependent expression of tubulins in Physarum. J Cell Biol. 1983 Dec;97(6):1852–1859. doi: 10.1083/jcb.97.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshire J. L., Keller L. R. Uncoupling of Chlamydomonas flagellar gene expression and outgrowth from flagellar excision by manipulation of Ca2+. J Cell Biol. 1991 Dec;115(6):1651–1659. doi: 10.1083/jcb.115.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988 Sep;13(9):339–343. doi: 10.1016/0968-0004(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Curry A. M., Williams B. D., Rosenbaum J. L. Sequence analysis reveals homology between two proteins of the flagellar radial spoke. Mol Cell Biol. 1992 Sep;12(9):3967–3977. doi: 10.1128/mcb.12.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. P., Weeks D. P., Grossman A. R. Expression of the arylsulfatase gene from the beta 2-tubulin promoter in Chlamydomonas reinhardtii. Nucleic Acids Res. 1992 Jun 25;20(12):2959–2965. doi: 10.1093/nar/20.12.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. P., Yildiz F., Grossman A. R. Mutants of Chlamydomonas with Aberrant Responses to Sulfur Deprivation. Plant Cell. 1994 Jan;6(1):53–63. doi: 10.1105/tpc.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuchy R., Purton S., Rochaix J. D. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989 Oct;8(10):2803–2809. doi: 10.1002/j.1460-2075.1989.tb08426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi P., Bossie C. A., Doonan J. H., May G. S., Morris N. R. Two alpha-tubulin genes of Aspergillus nidulans encode divergent proteins. Mol Gen Genet. 1991 Jan;225(1):129–141. doi: 10.1007/BF00282651. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Yen T. J., Lau J. T., Cleveland D. W. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987 Aug 28;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbault P., Wittemer C., Johanningmeier U., Jacobs J. D., Howell S. H. Structure of the Chlamydomonas reinhardtii cabII-1 gene encoding a chlorophyll-a/b-binding protein. Gene. 1988 Dec 20;73(2):397–407. doi: 10.1016/0378-1119(88)90504-5. [DOI] [PubMed] [Google Scholar]
- Keller L. R., Schloss J. A., Silflow C. D., Rosenbaum J. L. Transcription of alpha- and beta-tubulin genes in vitro in isolated Chlamydomonas reinhardi nuclei. J Cell Biol. 1984 Mar;98(3):1138–1143. doi: 10.1083/jcb.98.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K. E., Yu S. M., Heruth D. P., Kelly A. A., Gorovsky M. A. Regulation and evolution of the single alpha-tubulin gene of the ciliate Tetrahymena thermophila. Cell Motil Cytoskeleton. 1994;27(3):272–283. doi: 10.1002/cm.970270308. [DOI] [PubMed] [Google Scholar]
- Minami S. A., Collis P. S., Young E. E., Weeks D. P. Tubulin induction in C. reinhardii: requirement for tubulin mRNA synthesis. Cell. 1981 Apr;24(1):89–95. doi: 10.1016/0092-8674(81)90504-3. [DOI] [PubMed] [Google Scholar]
- Müller F. W., Igloi G. L., Beck C. F. Structure of a gene encoding heat-shock protein HSP70 from the unicellular alga Chlamydomonas reinhardtii. Gene. 1992 Feb 15;111(2):165–173. doi: 10.1016/0378-1119(92)90684-h. [DOI] [PubMed] [Google Scholar]
- Nagawa F., Fink G. R. The relationship between the "TATA" sequence and transcription initiation sites at the HIS4 gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8557–8561. doi: 10.1073/pnas.82.24.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl D. S., Schloss J. A., John P. C. Tubulin gene expression in the Chlamydomonas reinhardtii cell cycle: elimination of environmentally induced artifacts and the measurement of tubulin mRNA levels. J Cell Sci. 1988 Mar;89(Pt 3):397–403. doi: 10.1242/jcs.89.3.397. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Gould J., Kim S., Kane M. Y., Hereford L. Identification of sequences in a yeast histone promoter involved in periodic transcription. Cell. 1986 May 23;45(4):537–544. doi: 10.1016/0092-8674(86)90285-0. [DOI] [PubMed] [Google Scholar]
- Remillard S. P., Witman G. B. Synthesis, transport, and utilization of specific flagellar proteins during flagellar regeneration in Chlamydomonas. J Cell Biol. 1982 Jun;93(3):615–631. doi: 10.1083/jcb.93.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T., Burland T. G., Gull K., Dove W. F. Cell cycle regulation of tubulin RNA level, tubulin protein synthesis, and assembly of microtubules in Physarum. J Cell Biol. 1984 Jul;99(1 Pt 1):155–165. doi: 10.1083/jcb.99.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A. A Chlamydomonas gene encodes a G protein beta subunit-like polypeptide. Mol Gen Genet. 1990 May;221(3):443–452. doi: 10.1007/BF00259410. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Soares H., Galego L., Cóias R., Rodrigues-Pousada C. The mechanisms of tubulin messenger regulation during Tetrahymena pyriformis reciliation. J Biol Chem. 1993 Aug 5;268(22):16623–16630. [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction and synthesis of tubulin during the cell cycle and life cycle of Chlamydomonas reinhardi. Dev Biol. 1979 Apr;69(2):400–407. doi: 10.1016/0012-1606(79)90300-2. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P. S. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell. 1976 Sep;9(1):15–27. doi: 10.1016/0092-8674(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Collis P., Gealt M. A. Control of induction of tubulin synthesis in Chlamydomonas reinhardi. Nature. 1977 Aug 18;268(5621):667–668. doi: 10.1038/268667a0. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Velleca M. A., Curry A. M., Rosenbaum J. L. Molecular cloning and sequence analysis of the Chlamydomonas gene coding for radial spoke protein 3: flagellar mutation pf-14 is an ochre allele. J Cell Biol. 1989 Jul;109(1):235–245. doi: 10.1083/jcb.109.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]