Abstract
In proliferative diabetic retinopathy (PDR), vascular endothelial growth factor (VEGF) and CCN2 (connective tissue growth factor; CTGF) cause blindness by neovascularization and subsequent fibrosis. This angio-fibrotic switch is associated with a shift in the balance between vitreous levels of CCN2 and VEGF in the eye. Here, we investigated the possible involvement of other important mediators of fibrosis, tissue inhibitor of metalloproteinases (TIMP)-1 and transforming growth factor (TGF)-β2, and of the matrix metalloproteinases (MMP)-2 and MMP-9, in the natural course of PDR. TIMP-1, activated TGF-β2, CCN2 and VEGF levels were measured by ELISA in 78 vitreous samples of patients with PDR (n = 28), diabetic patients without PDR (n = 24), and patients with the diabetes-unrelated retinal conditions macular hole (n = 10) or macular pucker (n = 16), and were related to MMP-2 and MMP-9 activity on zymograms and to clinical data, including degree of intra-ocular neovascularization and fibrosis. TIMP-1, CCN2 and VEGF levels, but not activated TGF-β2 levels, were significantly increased in the vitreous of diabetic patients, with the highest levels in PDR patients. CCN2 and the CCN2/VEGF ratio were the strongest predictors of degree of fibrosis. In diabetic patients with or without PDR, activated TGF-β2 levels correlated with TIMP-1 levels, whereas in PDR patients, TIMP-1 levels, MMP-2 and proMMP-9 were associated with degree of neovascularization, like VEGF levels, but not with fibrosis. We confirm here our previous findings that retinal fibrosis in PDR patients is significantly correlated with vitreous CCN2 levels and the CCN2/VEGF ratio. In contrast, TIMP-1, MMP-2 and MMP-9 appear to have a role in the angiogenic phase rather than in the fibrotic phase of PDR.
Keywords: Diabetic retinopathy, CCN2, VEGF, TGF-β, TIMP-1, MMP-2, MMP-9, Neovascularization, Fibrosis
Introduction
In proliferative diabetic retinopathy (PDR), a major cause of blindness (Frank 2004), uncontrolled retinal neovascularization is followed by fibrosis, scarring, and tractional retinal detachment. PDR patients with established neovascularization and imminent fibrosis have a poor prognosis, despite aggressive laser treatment or surgical procedures. The major mediator of vascular leakage and angiogenesis in PDR is vascular endothelial growth factor (VEGF)-A, which is overexpressed in the ischemic retina (Aiello et al. 1994; Aiello and Wong 2000; Schlingemann and van Hinsbergh 1997; Witmer et al. 2003). CCN2, also known as connective tissue growth factor (CTGF), is involved in the fibrotic phase of PDR where it acts as a mitogen for fibroblasts and induces increased ECM production (Babic et al. 1999; Fan et al. 2000; Moussad and Brigstock 2000; Shimo et al. 1999; Suzuma et al. 2000). CCN2 has been shown to be pro-fibrotic in various organs, and is associated with pathological fibrosis, including vitreoretinal disorders, such as PDR (Kuiper et al. 2006; Leask et al. 2009). In a previous study, we reported that VEGF and CCN2 levels in the vitreous of patients with PDR correlate with neovascularization and fibrosis, respectively, but that the ratio of CCN2 and VEGF levels is the strongest predictor of fibrosis in these patients (Kuiper et al. 2008). We concluded that a shift in the balance between these growth factors causes the switch from angiogenesis to fibrosis in PDR, the so-called “angio-fibrotic switch”. Our concept predicts that anti-VEGF agents, when applied in advanced cases of PDR, may lead to increased fibrosis due to an increased CCN2/VEGF ratio, a phenomenon that indeed is observed in the clinic (Van Geest et al. 2012).
In the course of PDR development, VEGF and CCN2 induce expression of many downstream mediators such as proteases and their inhibitors in the cells of fibrovascular membranes. Levels of one of these, tissue inhibitor of metalloproteinases (TIMP)-1, were previously found to be elevated in PDR together with that of matrix metalloproteinase (MMP)-9, an enzyme associated with extracellular matrix degradation (Descamps et al. 2006; Jacqueminet et al. 2006; Matsuo et al. 1998; Mook et al. 2004; Salzmann et al. 2000; Sethi et al. 2000; Yoshida et al. 2010). This led the authors to suggest a regulating role of TIMP-1 in the onset of neovascularization in PDR.
However, TIMP-1 has also been associated with fibrosis, and its expression is induced by TGF-β, a major causal factor in fibrosis and scarring processes in the skin and other organs, including the eye (Chapman 2004; Connor et al. 1989; Guerin et al. 2001). TIMP-1 is one of the 4 members of the TIMP family, all natural inhibitors of the MMPs. TIMP-1 expression can be induced by many growth factors, including VEGF, TGF-β and CCN2 (Kuiper et al. 2007; McLennan et al. 2004; Zhou et al. 2007). This strongly indicates that TIMP-1 may be involved in the fibrotic stage of PDR as well, and may even mediate the effects of CCN2 in fibrosis in the eye. The inactive form of the TGF-β2 isoform is constitutively present in the human vitreous and may be activated by displaced retinal pigment epithelial cells, as occurs in proliferative vitreoretinopathy (Hinton et al. 2002). Activated TGF-β2 is therefore a possible inducer of CCN2 expression in proliferative vitreoretinopathies (Chapman 2004; Guerin et al. 2001; Hinton et al. 2002). CCN2 expression is induced by TGF-β in retinal pericytes (Van Geest et al. 2010). Increased levels of activated TGF-β2 have also been found in vitreous of PDR patients (Kita et al. 2008).
To investigate the possible roles of TIMP-1, TGF-β2, CCN2 and VEGF in the progression of PDR and the angio-fibrotic switch in more detail, we analyzed their vitreous levels in a series of patients with PDR and of diabetic and non-diabetic patients with macular hole or macular pucker, and were associated with the degree of fibrosis and neovascularization. Moreover, we investigated MMP-2 and MMP-9 levels in the vitreous of the 3 groups of patients using zymography to establish a potential effect of TIMP-1 expression on MMP-2 and/or MMP-9 activity in vitreous.
Materials and methods
Patients
Seventy-eight vitreous samples of patients with macular hole or idiopathic macular pucker (control group, total n = 26), diabetic patients with macular hole or idiopathic macular pucker but without PDR (DM group, total n = 24), and diabetic patients with PDR (PDR group, n = 28) were collected. A macular hole is a small break in the centre of the retina as a result of traction of the vitreous; a macular pucker is preretinal scar tissue (fibrosis) that is formed as a result of local damage caused by traction of the vitreous. In the DM group, 22 patients had no clinical signs of DR, and two patients had mild non-proliferative DR. The study was conducted according to the tenets of the Declaration of Helsinki and informed consent was obtained from each patient. The study was approved by the institutional review board of the Academic Medical Center at the University of Amsterdam.
Clinical data on grading of fibrosis, activity of neovascularization, presence of haemorrhage, and presence and type of diabetes, were obtained both per-operatively using a standardized form and from the patient files, and are shown in Table 1. Fibrosis was graded as 0 when there was no fibrosis (as in macular hole), as 1 when there were a few pre-retinal membranes (as limited as in macular pucker), as 2 when white preretinal fibrotic membranes with limited extension into the vitreous were present, and as 3 when abundant white membranes reaching into the vitreous body were observed. Neovascularization was graded as 0 when absent, as 1 (quiescent) when only non-perfused vessels were present, and as 2 (active) when there were perfused preretinal capillaries. Vitreous haemorrhage was graded as 0 when all media were clear and all fundus details were visible, and as 1 when media were clouded by haemorrhage.
Table 1.
Patient characteristics and data
| Patient characteristics (n = 78) | Subcategory | Non-diabetic (n = 26) Macular hole (n = 10) Pucker (n = 16) | DM (no PDR) (n = 24) Macular hole (n = 4) Pucker (n = 20) | PDR (n = 28) |
|---|---|---|---|---|
| Age in years (mean ± SD) | 67.4 ± 5.3 | 71.9 ± 7.5 | 58.6 ± 12.3 | |
| Gender | Male | n = 16 | n = 18 | n = 15 |
| Female | 10 | 6 | 13 | |
| Patients with diabetes | Total | 0 | 24 | 28 |
| Type I | 0 | 1 | 7 | |
| Type II | 0 | 23 | 21 | |
| Haemorrhage | No haemorrhage (0) | 26 | 24 | 5 |
| Haemorrhage (1) | 0 | 0 | 23 | |
| Degree of neovascularization | No neovascularization (0) | 26 | 24 | 0 |
| Quiescent neovascularization (1) | 0 | 0 | 7 | |
| Active neovascularization (2) | 0 | 0 | 21 | |
| Degree of fibrosis | No fibrosis (0) | 10 | 4 | 10 |
| Only few pre-retinal membranes (1) | 16 | 20 | 3 | |
| Some proliferative membranes (2) | 0 | 0 | 13 | |
| Abundant proliferative membranes (3) | 0 | 0 | 2 | |
| TIMP-1 (geom. mean, 95 % Cl) | 14.1 ng/ml [10.3–19.3] | 32.5 ng/ml [22.4–47.2] | 76.7 ng/ml [58.1–101.3] | |
| CCN2 (geom. mean, 95 % Cl) | 4.4 ng/ml [3.6–5.5] | 7.3 ng/ml [5.6–9.6] | 10.6 ng/ml [8.7–13.0] | |
| VEGF (geom. mean, 95 % Cl) | 9.8 pg/ml [8.7–11.1] | 30.2 pg/ml [14.2–64.37] | 625.5 pg/ml [413.7–945.7] | |
| TGF-β2, activated (geom. mean, 95 % Cl) | 85.4 pg/ml [53.1–137.6] | 129.6 pg/ml [77.8–215.9] | 151.2 pg/ml [96.5–236.9] |
VEGF data were not normally distributed. Therefore, geometric means with 95 % confidence intervals were calculated for all proteins investigated
Sample collection
Undiluted vitreous samples (0.5–1 ml) were obtained by using a vitrectome at the start of a three-port pars plana vitrectomy with the infusion line in position but not opened. The vitreous was transferred to sterile Eppendorf tubes and immediately frozen in dry ice in duplicate. The samples were kept at −80 °C until assayed.
Enzyme-linked immunosorbent assays (ELISAs)
After thawing, vitreous samples were centrifuged at 20,000 × g for 15 min at 4 °C, and supernatant was collected. Concentrations of VEGF165, TIMP-1 and activated TGF-β2 were determined by Quantikine ELISA assays according to the manufacturer’s protocol (R&D Systems, Minneapolis MS, USA). Concentrations of CCN2 were determined by sandwich ELISA, using two distinct monoclonal antibodies specifically recognizing the N-terminal part of the CCN2 protein (FibroGen, San Francisco CA, USA) as described previously (Kuiper et al. 2006). This assay detects both CCN2 N-terminal fragments as well as full-length CCN2. Purified recombinant human CCN2 (FibroGen) was used as standard.
Gelatin zymography
Activity of MMP-2 and MMP-9 was determined using a gelatin-zymography kit (Primary Cell, Hokkaido, Japan; Frederiks and Mook 2004; Mook et al. 2003). Vitreous samples of three non-diabetic, three diabetic patients without PDR and five samples of PDR patients were used. Of all samples, 10 μl mixed with 10 μl loading buffer and 10 μl MMP marker were loaded onto a precast gel and processed according to the manufacturer’s directions. Bands on the zymograms were quantified using densitometry (Alphease; AlphaInnoTech, San Leandro CA, USA).
Please note that inhibitors such as TIMP-1 dissociate from MMP during electrophoresis and do not interfere with the detection of MMP activity on zymograms (Frederiks and Mook 2004).
Statistical analysis
The growth factor levels in vitreous were tested for normal distribution using histograms and the Shapiro-Wilk test. VEGF showed a left skewed distribution. Therefore, VEGF data were log10 transformed where appropriate, and for vitreous levels of all proteins investigated geometric means were calculated. Differences between the groups in age were assessed by ANOVA. Differences in gender, diabetes type, degree of neovascularization, degree of fibrosis and haemorrhage were assessed by Chi-square or Fischer’s exact test. Differences in growth factor levels were assessed by nonparametric tests (Kruskal-Wallis variance analysis, followed by pair wise comparison using the Mann Whitney U test). Correlations between protein levels and proMMP-2, MMP-2 and proMMP-9 activity were expressed as Spearman’s correlation coefficient, with a ρ of 0.5 or greater considered to be relevant. Univariate and multiple ordinal logistic regression analyses were performed with degree of fibrosis and neovascularization as dependent variable, and associations were expressed as odds ratios with a 95 % confidence interval. A two-tailed p-value <0.05 was considered to indicate statistical differences. All analyses were carried out using PASW Statistics (Version 18) software (SPSS, Chicago IL, USA).
Results
Analysis of all patients
Patient characteristics and mean vitreous protein levels are listed in Table 1. Patients with PDR were significantly younger than the non-diabetic and diabetic patients with a macular hole or macular pucker.
Mean vitreous levels of CCN2, VEGF and TIMP-1, but not activated TGF-β2, were significantly higher in diabetic patients with a macular hole or macular pucker and in PDR patients than in non-diabetic patients (Fig. 1). PDR patients had significantly higher levels of VEGF and TIMP-1, but not of CCN2 and activated TGF-β2, than diabetic patients with macular hole or macular pucker. Univariate analysis of all 78 patients showed that CCN2, VEGF and TIMP-1 levels, and also activated TGF-β2, were significantly associated with the presence of diabetes (p-values <0.001, except for activated TGF-β2: p < 0.05; data not shown).
Fig. 1.
Vitreous protein levels of CCN2 (a), TIMP-1 (b), VEGF (c) and activated TGF-β2 (d) per group of patients that are non-diabetic (Con), diabetic without PDR (DM), and diabetic with PDR (PDR). Geometric means with 95 % confidence intervals are shown. * p < 0.01, ** p < 0.001
Analysis of all diabetic patients
Since diabetes was associated with elevated levels of all proteins, we performed further statistical analysis on the data of the diabetic patients to exclude this confounder. Moreover, diabetic patients with and without PDR were analyzed separately. In diabetic patients, a significant correlation was found between the levels of TIMP-1 and activated TGF-β2 (ρ 0.5, p < 0.001; data not shown). No relevant correlations were found between other protein levels.
The multiple ordinal regression analysis which is adjusted for the effects of other included covariates, showed that CCN2, but not VEGF, TIMP-1 and activated TGF-β2 was significantly associated with degree of fibrosis (Table 2). An association with degree of neovascularization was found for VEGF and TIMP-1, but not for CCN2 and activated TGF-β2. The effect of VEGF, however, was larger than the effect of TIMP-1.
Table 2.
Results of the multivariable ordinal logistic regression analysis (proportional odds model) with CCN2, TIMP-1, VEGF and activated TGF-β2 included in the model and degree of fibrosis and degree of neovascularization as responses in 52 diabetic patients with and without PDR
| Multiple ordinal regressiona | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Contrast | Degree of fibrosis | Degree of neovascularization | ||||
| OR | [95%Cl] | p Value | OR | [95%Cl] | p Value | ||
| CCN2 | per unit increase | 1.36 | 1.18–1.58 | <0.001 | 0.99 | 0.87–1.12 | 0.886 |
| VEGF | per 10-fold increase | 1.09 | 0.56–2.09 | 0.803 | 7.52 | 2.47–22.92 | <0.001 |
| TIMP-1 | per unit increase | 1.01 | 1.00–1.02 | 0.201 | 1.02 | 1.00–1.05 | 0.030 |
| act. TGF-β2 | per unit increase | 1.00 | 1.00–1.00 | 0.915 | 1.00 | 1.00–1.01 | 0.395 |
aCTGF, VEGF, TIMP-1, act. TGF-β2 in model
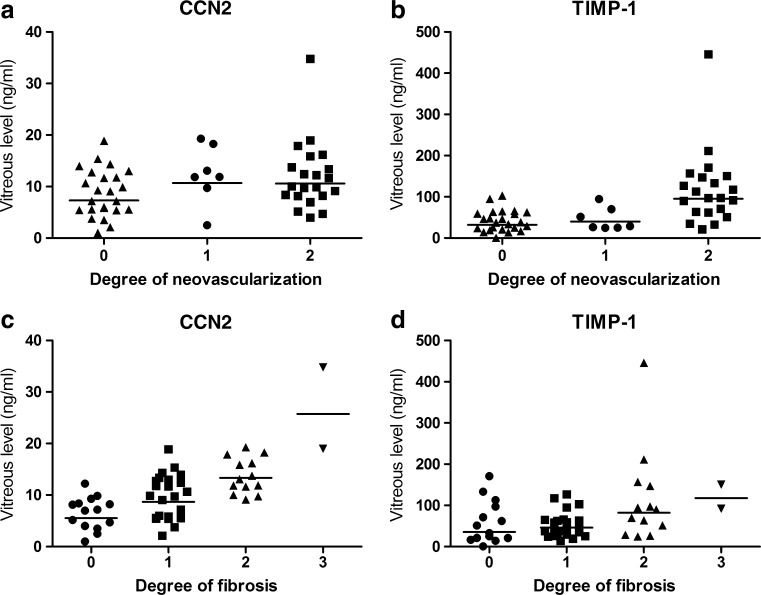
Correlations of CCN2 and TIMP-1 with degree of fibrosis and neovascularization are shown in Fig. 2.
Fig. 2.
Correlations of CCN2 (a,c) and TIMP-1 (b,d) with degree of neovascularization (a,b) and degree of fibrosis (c,d) in vitreous of 52 diabetic patients with and without PDR. The lines indicate the geometric means of protein levels per degree of neovascularization or fibrosis. CCN2 correlated significantly with fibrosis (Spearman’s ρ 0.6, p < 0.001), and TIMP-1 correlated significantly with neovascularization (Spearman’s ρ 0.6, p < 0.001)
Analysis of patients with PDR
Additional regression analysis of the 28 patients with PDR revealed that CCN2 levels were significantly associated with degree of fibrosis (OR 2.00, 95 % CI 1.28–3.15, p < 0.01), but the CCN2/VEGF ratio was the strongest predictor of degree of fibrosis in this group (OR 4.08, 95 % CI 1.68–9.90, p < 0.01). TIMP-1 was significantly associated with degree of neovascularization in this analysis (OR 1.04, 95 % CI 1.00–1.07, p < 0.05).
TIMP-1 levels were significantly correlated with activated TGF-β2 in the PDR group (ρ 0.5, p < 0.05; data not shown).
Analysis of diabetic patients without PDR
In patients with diabetes but without PDR (DM group), a significant correlation was found between TIMP-1 and activated TGF-β2 levels (Fig. 3a), and also between TIMP-1 and CCN2 levels. No relevant correlations were found between the other protein levels. Despite small numbers, TIMP-1 levels and also activated TGF-β2 levels were significantly higher in the diabetic patients with macular pucker (fibrosis grade 1) than in diabetic patients with macular hole (fibrosis grade 0; Fig. 3b), a difference not observed in the non-diabetic control patients (data not shown). CCN2 and VEGF levels did not differ significantly between macular hole and macular pucker patients whether the patients were diabetic or not.
Fig. 3.
Correlation between levels of TIMP-1 and activated TGF-β2 in vitreous of 24 diabetic patients without PDR (a), and vitreous TIMP-1 levels in macular hole and macular pucker patients with diabetes (b). a TIMP-1 and activated TGF-β2 correlated significantly (Spearman’s ρ 0.5, p < 0.01). b TIMP-1 levels were significantly higher in diabetic patients with puckers compared to macular holes. * p < 0.01. The lines indicate the geometric means of TIMP-1 levels in each group
MMP-2 and MMP-9 zymography in vitreous of all patients
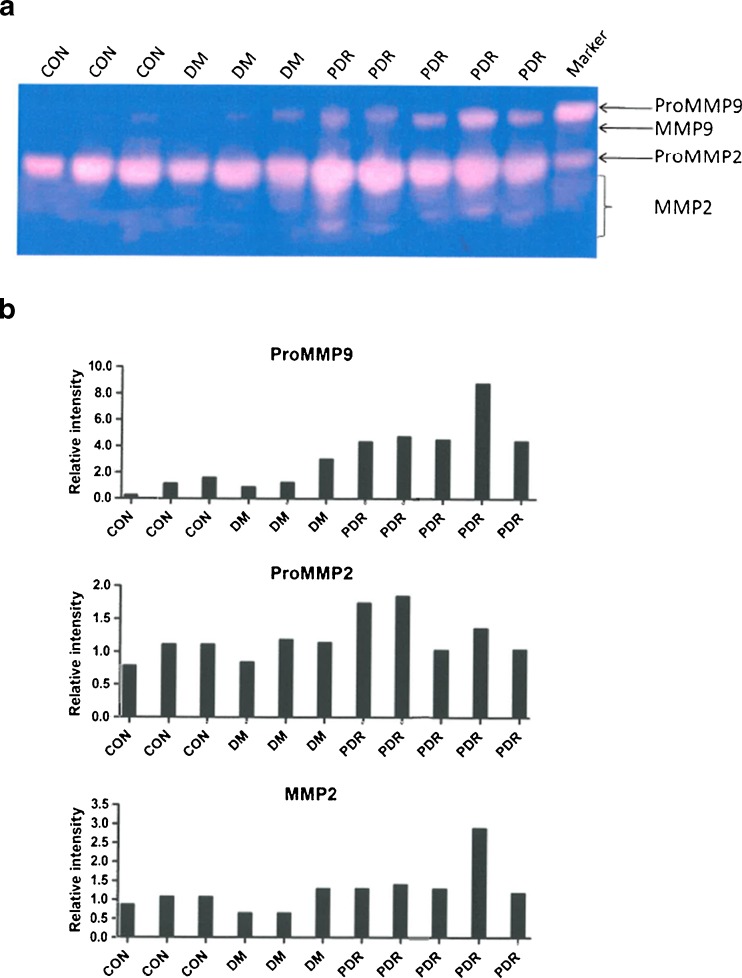
Figure 4 shows activity of proMMP-9. Active MMP-9 was not found in any of the samples to any significant extent. MMP-2 activity in the proform or active form was not found to be significantly different in vitreous of non-diabetic or diabetic patients with or without PDR. On the other hand, proMMP-9 activity was significantly higher in PDR than in non-PDR.
Fig. 4.
ProMMP-9, proMMP-2 and MMP-2 activity in vitreous. a Gelatin zymography showing activity of proMMP-9, proMMP-2 and active MMP-2 in samples of vitreous of non-diabetic patients (CON), diabetic patients without PDR (DM) and diabetic patients with PDR (PDR). MMP-9 activity was hardly present. b Relative intensity of bands of proMMP-9, proMMP-2 and MMP-2 activity in zymograms as compared to the mean values of the controls
A strong correlation was found between TIMP-1 expression and proMMP-9 activity in all patients (ρ = 0.764, p = 0.006; data not shown) as well as in the PDR patients alone (ρ = 0.900, p = 0.037; data not shown). In PDR, a correlation was found between MMP2 and the degree of neovascularization (ρ = 0.889, p = 0.04; data not shown), whereas proMMP-9 and the degree of neovascularization were associated (ρ = 0.866), but not statistically significant (p = 0.058; data not shown). In all patients together, both proMMP-9 (ρ = 0.905, p < 0.001; data not shown) and MMP2 (ρ = 0.825, p = 0.002; data not shown) were correlated with neovascularisation.
Discussion
In the present study, we confirmed our previous findings that the degree of fibrosis in retinas of PDR patients is significantly related to vitreous levels of CCN2 and even stronger with the ratio of CCN2 and VEGF levels (Kuiper et al. 2008; Van Geest et al. 2012). We also show here that the levels of a major protein involved in tissue remodeling, wound healing and fibrosis, TIMP-1, are increased in the vitreous of diabetic and PDR patients, and that TIMP-1 is associated mainly with active neovascularization rather than with fibrosis, in a similar way as levels of activated TGF-β2.
We investigated whether there was evidence that TGF-β2, in addition to VEGF, acts as an upstream inducer of CCN2 in PDR, and TIMP-1 as a downstream effector of CCN2 in PDR. However, the relationship between the four proteins is complex. For example, TIMP-1 expression is known to be induced by TGF-β, but also by CCN2 and VEGF (Kuiper et al. 2007; McLennan et al. 2004; Zhou et al. 2007). Feedback mechanisms between VEGF and CCN2 are also known: VEGF can induce CCN2, and CCN2 can inhibit the actions of VEGF by direct protein-protein binding (Inoki et al. 2002; Jang et al. 2004). Despite these complex interactions, we were able to show novel associations between vitreous levels of these proteins and the degree of fibrosis and neovascularization of the retina that may help to understand their role in the development of PDR and the contribution to the angio-fibrotic switch.
First, we found that vitreous levels of CCN2, VEGF and TIMP-1 but not of activated TGF-β2 were significantly higher in patients with diabetes and in patients with PDR. Second, VEGF was associated with neovascularisation in diabetic patients. Although a role in active PDR has also been suggested for CCN2 (Hinton et al. 2004), the increased CCN2 levels in our PDR patients did not differ between quiescent and active neovascularization. CCN2 was associated only with fibrosis, as was shown by us in previous reports (Kuiper et al. 2006, 2008; Van Geest et al. 2012). Third, TIMP-1 was associated with neovascularization, and not with fibrosis. This suggests that in the natural course of PDR from an angiogenic phase to a fibrotic phase via the angio-fibrotic switch, TIMP-1 has an early role in angiogenesis and may not act as a downstream mediator of CCN2 in the fibrotic phase. A similar upregulation of TIMP-1 levels was reported recently that was associated with angiogenesis in wound healing (Mayrand et al. 2012). Seemingly in contrast to the conclusion that TIMP-1 has a role in angiogenesis is our finding that in PDR patients, TIMP-1 correlated with active TGF-β2, a major growth factor known to cause fibrosis and scarring (Chapman 2004; Connor et al. 1989; Guerin et al. 2001). However, we did not find a correlation of active TGF-β2 with fibrosis in PDR patients, indicating that this growth factor, like TIMP-1, has no role in the angio-fibrotic switch and the subsequent fibrotic phase of PDR.
Although not the main focus of this study, it is interesting to note that in the group of diabetic patients without PDR of which almost all had no retinopathy at all, TIMP-1 was also increased and correlated with activated TGF-β2. This suggests that the expression of TIMP-1 is under the control of TGF-β2 in diabetic patients with or without PDR. In the patients without PDR, TIMP-1 and activated TGF-β2 were both significantly higher in eyes with macular pucker than in macular holes, a pattern not seen in non-diabetic controls. Macular pucker is associated with fibrosis whereas macular hole is not. This suggests that TIMP-1 and active TGF-β2 have a role in glial cell activation and pre-retinal fibrosis only in a diabetic environment, and that the pathogenesis of pre-retinal fibrosis may differ between diabetic and non-diabetic subjects. Moreover, the role of activated TGF-β2 in early phases of DR rather than in the late phase of PDR fit in the role of TGF-β2 signaling in preclinical DR (Van Geest et al. 2010).
We can only speculate how TIMP-1 functions exactly in the context and course of development of PDR. TIMPs are endogenous inhibitors of MMPs, which have an important role in connective tissue remodelling and in the degradation of the basal lamina and surrounding ECM during angiogenesis (Sethi et al. 2000). Matrix remodeling, necessary for sprouting angiogenesis, is dependent on well-coordinated interactions between MMPs and TIMPs. TIMP-1 is increased in PDR (Matsuo et al. 1998; Salzmann et al. 2000; Sethi et al. 2000; Yoshida et al. 2010), and can inhibit MMP-9 activation in vitreous (Descamps et al. 2006). We found that levels of proMMP-2 and active MMP-2 were not different in any of the patient groups. Moreover, no relation was observed of TIMP-1 expression and MMP-2 activity in the vitreous of these patient groups. However, as was found previously, MMP-9 in its proform was correlated with TIMP-1 levels in the vitreous of PDR patients (Descamps et al. 2006; Yoshida et al. 2010), indicating that MMP-9 and TIMP-1 are involved in the early angiogenic phase of PDR. In addition, both proMMP-9 and MMP-2, like TIMP-1, were associated with the degree of neovascularization and not with the degree of fibrosis.
It is important to note that MMP-9 is exclusively expressed in leukocytes and glial cells (Mook et al. 2004). Increased expression of TIMP-1 may therefore have a counter balancing effect on pro-angiogenic factors such as MMPs and VEGF, and thereby a regulatory anti-angiogenic effect. These observations in the literature are in line with our finding that TIMP-1 appears to have a role in the angiogenic phase rather than in the later scarring phases of PDR.
In summary, our study indicates that in the complex context of the development of PDR from an angiogenic phase to fibrosis and scarring, TIMP-1, a major regulatory protein in tissue remodeling and wound healing, is mainly involved in the process of angiogenesis after induction by VEGF and TGF-β2.
Acknowledgements
This study was supported by grant 2005.00.042 from the Diabetes Fonds Nederland. The preparation of the manuscript by Monique Arendse is gratefully acknowledged.
References
- Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000;77:S113–S119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA. Disorders of lung matrix remodeling. J Clin Invest. 2004;113:148–157. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TB, Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, De Bustros S, Enger C, Kato H, Lansing M, Hayashi H, Glaser BM. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps FJ, Martens E, Kangave D, Struyf S, Geboes K, Van Damme J, Opdenakker G, Abu El-Asrar AM. The activated form of gelatinase B/matrix metalloproteinase-9 is associated with diabetic vitreous hemorrhage. Exp Eye Res. 2006;83:401–407. doi: 10.1016/j.exer.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Mook OR. Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histchem Cytochem. 2004;52:711–722. doi: 10.1369/jhc.4R6251.2004. [DOI] [PubMed] [Google Scholar]
- Guerin CJ, Hu L, Scicli G, Scicli AG. Transforming growth factor beta in experimentally detached retina and periretinal membranes. Exp Eye Res. 2001;73:753–764. doi: 10.1006/exer.2001.1095. [DOI] [PubMed] [Google Scholar]
- Hinton DR, He S, Jin ML, Barron E, Ryan SJ. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye (Lond) 2002;16:422–428. doi: 10.1038/sj.eye.6700190. [DOI] [PubMed] [Google Scholar]
- Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, Oliver N, Lim JI. Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2004;27:758–764. doi: 10.2337/diacare.27.3.758. [DOI] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Jacqueminet S, Ben AO, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi BJL. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta. 2006;367:103–107. doi: 10.1016/j.cca.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Jang HS, Kim HJ, Kim JM, Lee YS, Kim KL, Kim JA, Lee JY, Suh W, Choi JH, Jeon ES, Byun J, Kim DK. A novel ex vivo angiogenesis assay based on electroporation-mediated delivery of naked plasmid DNA to skeletal muscle. Mol Ther. 2004;9:464–474. doi: 10.1016/j.ymthe.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, Mochizuki Y, Enaida H, Goto Y, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci U S A. 2008;105:17504–17509. doi: 10.1073/pnas.0804054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper EJ, de Smet MD, van Meurs JC, Tan HS, Tanck MW, Oliver N, Van Nieuwenhoven FA, Goldschmeding R, Schlingemann RO. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124:1457–1462. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Hughes JM, Van Geest RJ, Vogels IMC, Goldschmeding R, Van Noorden CJF, Schlingemann RO, Klaassen I. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest Ophthalmol Vis Sci. 2007;48:4267–4276. doi: 10.1167/iovs.06-0804. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJF, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Okada Y, Shiraga F, Yanagawa T. TIMP-1 and TIMP-2 levels in vitreous and subretinal fluid. Jpn J Ophthalmol. 1998;42:377–380. doi: 10.1016/S0021-5155(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Mayrand D, Laforce-Lavoie A, Larochelle S, Langlois A, Genest H, Roy M, Moulin VJ. Angiogenic properties of myofibroblasts isolated from normal human skin wounds. Angiogenesis. 2012;15:199–212. doi: 10.1007/s10456-012-9253-5. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM. Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology. 2004;145:5646–5655. doi: 10.1210/en.2004-0436. [DOI] [PubMed] [Google Scholar]
- Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;38:295–304. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- Mook OR, Frederiks WM, Van Noorden CJF. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Salzmann J, Limb GA, Khaw PT, Gregor ZJ, Webster L, Chignell AH, Charteris DG. Matrix metalloproteinases and their natural inhibitors in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 2000;84:1091–1096. doi: 10.1136/bjo.84.10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, van Hinsbergh VW. Role of vascular permeability factor/vascular endothelial growth factor in eye disease. Br J Ophthalmol. 1997;81:501–512. doi: 10.1136/bjo.81.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi CS, Bailey TA, Luthert PJ, Chong NH. Matrix metalloproteinase biology applied to vitreoretinal disorders. Br J Ophthalmol. 2000;84:654–666. doi: 10.1136/bjo.84.6.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem. 2000;275:40725–40731. doi: 10.1074/jbc.M006509200. [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO. Differential TGF-β signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJF, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Progr Retin Eye Res. 2003;22:1–29. doi: 10.1016/S1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ishikawa K, Matsumoto T, Yoshida A, Ishibashi T, Kono T. Reduced concentrations of angiogenesis-related factors in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:799–804. doi: 10.1007/s00417-010-1301-5. [DOI] [PubMed] [Google Scholar]
- Zhou X, Hu H, Huynh ML, Kotaru C, Balzar S, Trudeau JB, Wenzel SE. Mechanisms of tissue inhibitor of metalloproteinase 1 augmentation by IL-13 on TGF-beta 1-stimulated primary human fibroblasts. J Allergy Clin Immunol. 2007;119:1388–1397. doi: 10.1016/j.jaci.2007.02.011. [DOI] [PubMed] [Google Scholar]