Abstract
Minimal change disease (MCD), the most common idiopathic nephrotic syndrome in children, is characterized by proteinuria and loss of glomerular visceral epithelial cell (podocyte) ultrastructure. Lipopolysaccharide (LPS) and puromycin aminonucleoside (PAN) are used to study podocyte injury in models of MCD in vivo and in vitro. We hypothesized that LPS and PAN influence components of the innate immune system in podocytes such as the Toll-Like Receptor (TLRs), TLR adapter molecules, and associated cytokines. Our results show that cultured human podocytes constitutively express TLRs 1–6 and TLR-10, but not TLRs 7–9. LPS (25 μg/ml) or PAN (60 μg/ml) caused comparable derangement of the actin cytoskeleton in podocytes. Quantitative RT-PCR analysis show that LPS differentially up-regulated the expression of genes for TLRs (1 > 4 ≥ 2 > 3 > 6 > 5), the adapter molecule, MyD88, and transcription factor NF-κB within one hour. LPS also caused increased levels of IL-6, IL-8 and MCP1 without exerting any effect on TNF-α, IFN-α or TGF-β1 at 24 h. Immunofluorescence intensity analysis of confocal microscopy images showed that LPS induced a significant increase in nuclear translocation of NF-κB by 6 h. In contrast, PAN-induced only small changes in the expression of TLRs 2–6 that included a persistent increase in TLRs 2 and 5, a transient increase in TLR-4, and a gradual increase in TLRs 3 and 6 between 1 and 6 h. Correspondingly, it did not alter pro-inflammatory cytokine levels in podocytes. However, PAN induced a low but significant increase in NF-κB nuclear translocation within one hour that remained unchanged up to 6 h. In summary, these novel findings show that LPS, a known TLR-4 ligand, induced the gene expression of multiple TLRs with maximum effect on the expression of TLR-1 suggesting a loss of receptor selectivity and induction of receptor interactions in podocytes. A comparable derangement of the podocyte cytoskeleton and significant increase in the nuclear translocation of NF-κB by PAN suggest that disparate but complementary mechanisms may contribute to the development of podocytopathy in MCD.
Keywords: Innate immunity, Toll-like receptors, Cytokines, Lipopolysaccharide, Puromycin Aminonucleoside, Minimal Change Disease, Glomerular filtration barrier, Podocytes
Introduction
Minimal change disease (MCD), characterized by proteinuria without evidence of renal damage at the level of light microscopy, is the most common diagnosis for idiopathic nephrotic syndrome in children (Srivastava et al. 1999). Immunological factors are implicated in pathogenesis of MCD because effective control of proteinuria is achieved with immunosuppressive agents, and relapse occurs following viral or bacterial infection (Neuhaus et al. 1994; MacDonald et al. 1986). Several non-immunoglobulin mediators including cytokines, T cell products and vasoactive agents are associated with MCD. However, renal biopsies from MCD patients do not show inflammatory cells, complement proteins or immune deposits (Mathieson 2007). Therefore, the etiology of MCD requires further investigation.
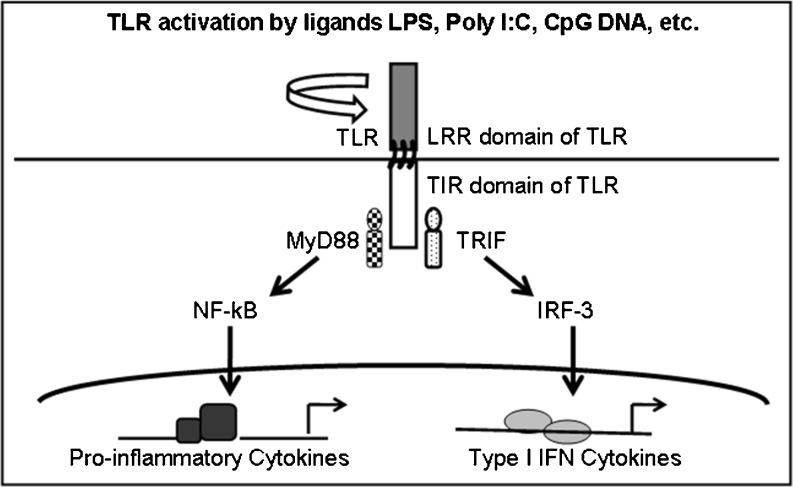
A significant role for the innate immune system in the onset and progression of MCD is implied by the available evidence but the nature of the participating molecules and signaling pathways remains unclear. The innate immune system relies on an extensive network of molecules and signaling mechanisms, many of which involve the pattern-recognizing Toll-like receptors (TLRs) 1–10 (Takeda and Akira 2004). Figure 1 summarizes TLR-mediated signaling through either of the two major adapter molecules, myeloid differentiation factor (MyD88-dependent) or Toll-Interleukin 1 Receptor (TIR) domain-containing adapter-inducing interferon-β (TRIF, MyD88-independent). MyD88-dependent signaling leads to translocation of NF-κB and transcription of the genes for proinflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-10. The TRIF-dependent pathway causes transcription of Type-I interferons (IFN), such as IFN-α and IFN-β (Yamamoto et al. 2003; Carmody and Chen 2007). Signaling pathways of the innate immune system have largely been studied in leukocytes, but recent studies point to the importance of understanding TLR signaling in epithelial cells exposed to injury or infection (Gribar et al. 2008).
Fig. 1.
Schematic representation of major TLR signaling pathways. The toll-like receptor (TLR) signaling pathways converge through two major signaling molecules, MyD88 or TRIF. The MyD88-dependent pathway activates NF-κB and transcription of inflammatory cytokines. The TRIF pathway leads to transcription of type I Interferons. [MyD88: myeloid differentiation primary-response protein 88, IFN: Interferon, IRF-3: IFN-regulatory factor-3, NF-κB: Nuclear factor-κB, LRR: leucine rich repeats, TIR: Toll/interleukin-1 receptor, TRIF: TIR-domain-containing adaptor protein inducing IFN-β]
Podocytes (visceral epithelial cells) with the basement membrane and capillary endothelium constitute the glomerular filtration barrier that regulates the passage of plasma protein into urine. The location and function of podocytes expose them to metabolic toxins, bacterial and viral products. Electron microscopy of renal biopsies from MCD patients reveals extensive foot process effacement and condensation of the actin cytoskeleton in podocytes. These ultrastructural changes are sufficient to explain the increased glomerular permeability to macromolecules and the amount of urinary protein observed in MCD (Lane and Kaskel 2009).
Puromycin aminonucleoside (PAN) and lipopolysaccharide (LPS) are unrelated molecules that cause comparable podocyte injury in vitro and in vivo. LPS, a complex of lipid A (endotoxin) and oligosaccharide from gram negative bacterial cell wall, is a known ligand of TLR-4 that activates leukocytes at very low concentration quantities (nanograms/mL); however, microgram/mL quantities of LPS are required to induce injury to podocytes in current models of MCD. Previous work has focused on TLR-4 as the only receptor of LPS (Reiser et al. 2004; Brown et al. 2007; Banas et al. 2008). PAN, an aminonucleoside, is not known to activate TLRs. A likely effect of the amount of LPS used in models of MCD on multiple TLR subtypes and, on its specificity for TLR-4 has not been addressed. Although LPS and PAN induce cellular injury through distinct mechanisms, they result in comparable changes in cell morphology and cytoskeleton suggesting a convergence of pathways. We, for the first time, carried out a head to head comparison of the effect of LPS and PAN on the gene expression of TLR subtypes, signaling elements and on the production of pro-inflammatory cytokines in cultured podocytes.
Materials and methods
Cell culture
Conditionally immortalized transgenic human podocytes enabled via a thermosensitive variant of SV-40 were provided by Dr. Moin Saleem. Briefly, podocytes were cultured in RPMI-1640 with insulin (10 μg/ml), transferrin (5.5 μg/ml), selenium (5 ng/ml Na selenite), heat-inactivated fetal bovine serum (10 % v/v), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen, Carlsbad, CA) at 33 °C under a humidified atmosphere of 95 % air and 5 % CO2 with change of the medium every 2 days. Podocytes at 50–60 % confluence were thermo-switched from 33 °C to 37 °C for differentiation under non-permissive conditions for 14 days. Differentiated podocytes were used in all experiments (Saleem et al. 2002).
Two cell types were used as reference controls for comparing PCR results. Human monocytic cells (THP-1) and human foreskin fibroblasts (HFF) were purchased from ATCC (Manassas, VA) and grown in RPMI or DMEM, respectively, with 2 mM L-gluatamine, 250 μg/ml amphotericin, 100U/ml penicillin, 0.1 mg/ml streptomycin and 10 % fetal bovine serum at 37 °C in humidified atmosphere of 95 % air and 5 % CO2. At 90 % confluence, THP-1 and HFF cells at passages 10–12 were plated at a density of ~105 cells/well in 12-well plates with serum-free RPMI overnight prior to experimental treatment(s).
Treatment of cultured podocytes using Puromycin Aminonucleoside (PAN) and Lipopolysaccharide (LPS)
Cells were maintained in serum-free RPMI-1644 medium for 24 h prior to treatment with either PAN (Sigma Chemical Company, St. Louis, MO) or purified E. Coli LPS from E. Coli O111:B4 strain (InvivoGen, San Diego, CA). During preliminary work, podocytes were treated with several concentrations of PAN (0–90 μg/ml) or LPS (0–50 μg/ml) for up to 24 h to assess changes in cell morphology (0.1 % crystal violet stain) and actin cytoskeleton (phalloidin conjugated Alexa Fluor 568, Invitrogen, Carlsbad, CA) to determine optimum concentrations for subsequent studies. Further experiments were conducted in triplicates using 60 μg/ml PAN or 25 μg/ml LPS to study TLR mRNA expression by quantitative real time polymerase chain reaction (qRT-PCR) at 1 h (early) and 6 h (late), and secreted cytokines into the medium at 6 h (early) and 24 h (late). The concentrations of LPS and PAN used in these studies are within the range used in other acute in vitro studies. Currently established models of podocyte injury use comparable or higher doses of PAN and LPS (Reiser et al. 2004; Banas et al. 2008; Suzuki et al. 2001; Marshall et al. 2006; Gloy et al. 2000; Coers et al. 1994).
Phalloidin staining for F-actin and crystal violet staining for cell morphology in podocyte
Fluorescence or bright field microscopy was used to evaluate the changes in cell structure and the actin cytoskeleton caused by LPS or PAN at the concentrations indicated. Prior to phalloidin staining for F-actin, cells were washed with phosphate buffered saline (PBS) and fixed with 4 % paraformaldehyde. Phalloidin conjugated with Alexa Fluor 568 dye (Invitrogen A12380) was used to assess the actin cytoskeleton in podocytes. Cell morphology was assessed by first fixing PBS-washed cells in 2 % glutaraldehyde for 10 min followed by staining with 0.1 % crystal violet for 20 min. Images were obtained from 10–20 random fields in a masked manner using Olympus BX60 fluorescence microscope (Hamburg, Germany).
RNA extraction and qualitative reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was used to evaluate the expression of TLRs 1–10 and their principal signal elements MD-1 (lymphocyte antigen 86, LY86) and MD-2 (lymphocyte antigen 96, LY96), the cysteine-rich molecules that bind the leucine-rich region of TLRs and mediate interaction with LPS. The expression of TLRs in HFF and THP-1 cells has been previously described and these cells were used as reference quality controls (Yew et al. 2010).
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The concentration and quality of RNA was determined spectrophotometrically at 260/280 nm using Nanodrop spectrophotometer (Pharmacia Biotech, Piscataway, NJ). Total RNA was reverse-transcribed to generate cDNA using Qiagen Sensiscript Reverse Transcriptase for RT-PCR using standard method and cDNA was stored at−20 °C until use. The cDNA generated from reverse transcription of RNA was amplified by PCR for 40 cycles (initial denaturation at 95 °C for 10 min, followed by 40 cycles at 94 °C (30 sec), 60 °C (30 sec), 72 °C (30 sec) and the final extension at 72 °C for 10 min) with specific primer sets (Table 1). The forward and reverse primers used are shown in Table 1. PCR products were separated by electrophoresis in 2 % agarose gel for RT-PCR, exposed to ethidium bromide and fluorescent images were obtained. RT-PCR data were analyzed qualitatively and scored using an arbitrary numeric scale (0–4+) in a masked manner.
Table 1.
Forward and reverse primers used in reverse transcription polymerase chain reaction (RT-PCR) and quantitative real time polymerase chain reaction (qRT-PCR) for different components of TLR signaling pathway in podocyte
| Forward primer | Reverse primer | PCR size (bp) | |
|---|---|---|---|
| TLR1 | TCAAGCACTTGGACCTGTCA | GCAATTGGCAGCACACTAGA | 133 |
| TLR2 | GGGTTGAAGCACTGGACAAT | CTGCCCTTGCAGATACCATT | 134 |
| TLR3 | CCGCCAACTTCACAAGGTAT | AGCTCATTGTGCTGGAGGTT | 130 |
| TLR4 | CGGAGGCCATTATGCTATGT | TCCCTTCCTCCTTTTCCCTA | 141 |
| TLR5 | ATCGTTTCTGCAACCTCACC | AAGGGGAAGGATGAAGCAGT | 106 |
| TLR6 | AGGGCTGGCCTGATTCTTAT | TGGTGACGATCAGCAGAGTT | 114 |
| TLR7 | TGCTGTGTGGTTTGTCTGGT | GATCACACTTTGGCCCTTGT | 109 |
| TLR8 | CATCATCGACAACCTCATGC | TAGCCTCTGCAAAGCCAAGT | 121 |
| TLR9 | AATTCCCATCTCTCCCTGCT | TCCTTCACCCCTTCCTCTTT | 135 |
| TLR10 | ACTTTGCCCACCACAATCTC | CTTGGGCCATTCCAAGTATG | 146 |
| MD-1 | GGGCCTGTCAATAATCCTGA | TTTGCTACAGGCCACAGTCA | 137 |
| MD-2 | CCGAGGATCTGATGACGATT | TGGGCTCCCAGAAATAGCTT | 142 |
| TRIF | ACGCCACTCCAACTTTCTGT | TCAGGTGAGCTGAACAAGGA | 136 |
| IRF-3 | GATGCACAGCAGGAGGATTT | TCTGCTAAACGCAACCCTTC | 149 |
| MyD88 | TGCAGAGCAAGGAATGTGAC | AGGATGCTGGGGAACTCTTT | 121 |
| NF-κB p65 | TGGAGTCTGGGAAGGATTTG | CGAAGCTGGACAAACACAGA | 129 |
Quantitative real time polymerase chain reaction (qRT-PCR)
We used qRT-PCR to evaluate the effect of LPS or PAN on TLR expression. Total RNA was extracted and purified as described. PCR amplifications were performed using a BioRad iCycler® (Hercules, CA) sequence detection system. A 50 μl reaction mixture including 10X Gold Buffer, 25 mm MgCl2, 2.5 mM dNTPs, 10X SYBR Green, Taq Gold polymerase, dH2O, DNA template and 10 μM of each primer was used for PCR. Amplification was performed by initial polymerase activation (10 min) at 95 °C, and 40 cycles of denaturation at 95 °C (15 sec), annealing at 60 °C (20 sec) and elongation (30 sec) at 72 °C. PCR efficiency was examined by serially diluting the template cDNA and PCR specificity by obtaining melting curve data. Proper controls were included in each assay. Gene-specific mRNA expression was calculated with reference to the standard cDNA dilution curve and expressed as mol/μL. The relative gene-specific mRNA expression (RE) was normalized against mRNA expression for β-actin as follows: RE for gene-specific mRNA = gene-specific mRNA [mol/μL] ÷ β-actin mRNA [mol/μL].
Nuclear translocation of NF-κB
NF-κB nuclear translocation in podocytes were determined by indirect immunofluorescence followed by specific measurement of NF-κB immunorectivity overlapping with DAPI-labeled nuclei relative to NF-κB immunoreactivity in non-nuclear (cytosolic) compartments. Immunofluorescence was performed using primary rabbit anti-NF-κB antibody at 1:1,000 (#04-235 from Millipore Corporation, Billerica, MA) and secondary goat anti-rabbit IgG at 1:2,500 (Invitrogen). Images for analysis were acquired using a Zeiss LSM-510 laser scanning confocal microscope at 63X magnification. Z-stack images were obtained and resulting maximum intensity projection images were used for analysis.
Multiplex bead-based immunoassay for secreted cytokines
Secreted cytokines in the medium were assayed to determine the effect of PAN or LPS on podocytes at 6 h and 24 h using a Luminex 200TM platform (Invitrogen). First, the supernatants were concentrated 20-fold by centrifugation-based filtration using Amicon Centricon-10 (Millipore Corporation, Billerica, MA) with a molecular weight cut-off of 10 kD. The medium was not concentrated for IL-6 assay. Concentrated media (50 μl) or standards of known protein concentrations were added to antibody-conjugated beads and incubated for 2 h. Beads were washed and incubated with protein-specific biotinylated detector antibodies for 1 h, washed and mixed with streptavidin-conjugated R-phycoerythrin to form a four-member solid phase sandwich. The Luminex 200 TM dual laser identifies the bead by its spectral property and the protein concentration is determined by extrapolating the amount of R-phycoerythrin fluorescence to the standard curve. We measured IL-6, IL-8, MCP-1, MIP-1α, IP-10, TNF-α, IFN-α, and TGF-β1 in duplicates in the medium. IFN-β was measured by an ELISA kit (R&D Systems, Piscataway, NJ) following the manufacturer’s instructions. Measured cytokine was corrected for DNA content of lysate, and was expressed as pg cytokine/μg DNA.
Statistical analysis
Data were analyzed using the SPSS 18.0 statistical software (SPSS Inc., Chicago, IL) for three group comparison. A p < 0.05 value was considered significant.
Results
Toll-Like Receptors (TLRs) are expressed in human podocytes
Table 2 shows a comparison of the gene expression for TLRs, MD-1 and MD-2 in podocytes cultured with or without FBS. Podocytes showed constitutive expression of the genes for TLR-1, 2, 3, 4, 5, 6 and 10, but not for TLR-7, 8 or 9. Most TLRs are transmembrane cell surface receptors, but TLR-7, 8 and 9 are exclusively endosomal receptors, and TLR-3 has been localized at both sub-cellular sites. Thus, cell surface rather than endosomal TLRs are dominantly expressed in cultured human podocytes. Table 2 also shows that podocytes express cofactor MD-2 (Lymphocyte antigen 96), but not MD-1 (Lymphocyte antigen 86). MD2 associates with TLR-4 to form homodimers and, with TLR-2 to form heterodimers with TLR-1 or TLR-6.
Table 2.
Semi-quantitative gene expression 0 to 4+ by visual estimation in human podocyte, human foreskin fibroblast cell line (HFF) and human monocytic cell line (THP-1) for Toll like receptor (TLR) TLR1 to TLR10, and cofactors MD-1 and MD-2 on reverse transcription polymerase chain reaction (RT-PCR). Podocytes were also tested with and without 10 % fetal bovine serum in media
| Podocyte (serum free) | Podocyte (with serum) | HFF | THP-1 | |
|---|---|---|---|---|
| TLR1 | 2+ | 3+ | 2+ | 3+ |
| TLR2 | 2+ | 2+ | 2+ | 3+ |
| TLR3 | 2+ | 2+ | 3+ | 2+ |
| TLR4 | 2+ | 3+ | 4+ | 4+ |
| TLR5 | 2+ | 3+ | 3+ | 3+ |
| TLR6 | 3+ | 3+ | 4+ | 4+ |
| TLR7 | 0 | 0 | 0 | 3+ |
| TLR8 | 0 | 0 | 0 | 3+ |
| TLR9 | 0 | 0 | 0 | 3+ |
| TLR10 | 2+ | 2+ | 3+ | 3+ |
| MD-1 | 0 | 0 | 0 | 3+ |
| MD-2 | 3+ | 3+ | 4+ | 4+ |
Table 2 presents a comparison of the effect of FBS in the cell culture medium on TLR expression in podocytes. Exclusion of FBS in the medium caused only a slight decrease in the expression of TLR-1, 4 and 5. Thus, changes observed in the following experiments were free of interference from serum in the cell culture medium.
Table 2 also shows the expression of TLR subtypes in THP-1 and HFF cells. These cells are routinely used in our laboratory as reference for comparing TLR gene expression using a numeric scale (0–4+) (Yew et al. 2010). The THP-1 cell line expresses all of the known 10 human TLRs, MD-1 and MD-2. Overall expression of TLRs in podocytes was comparable to that observed in the HFF cell line.
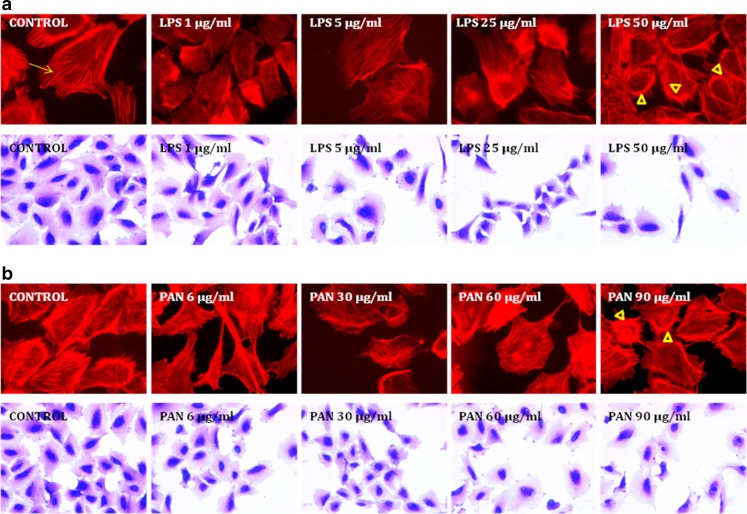
Puromycin aminonucleoside (PAN) and lipopolysaccharide (LPS) deform podocyte morphology and disrupt the actin cytoskeleton
Both LPS and PAN elicited a similar dose-dependent response in the podocyte cytoskeleton. LPS (1–50 μg/ml) caused dose-dependent changes in the actin cytoskeleton and podocyte morphology (Fig. 2a). PAN (6 to 90 μg/ml) also caused an incremental loss of fine F-actin stress fibers in the actin cytoskeleton as observed by Phalloidin staining (Fig. 2b). At higher concentrations, both LPS and PAN caused formation of thick cortical actin network after a 24-h exposure (arrow heads, Fig. 2a and b). These observations were further corroborated by crystal violet staining which showed blunting and/or loss of cytoplasmic processes in podocytes exposed to PAN for 24 h (Fig. 2b). LPS- or PAN-induced changes in podocyte morphology and actin cytoskeleton were virtually indistinguishable by microscopy. Thus, PAN at 60 μg/ml or LPS at 25 μg/ml appeared to induce changes in the actin cytoskeleton and podocyte morphology without causing cell lysis. Subsequent experiments were carried out using PAN or LPS at these concentrations to determine the effects of these treatments on TLR expression and cytokine production.
Fig. 2.
Effect of LPS or PAN on actin cytoskeleton and cell morphology. Control untreated podocytes show fine cytoplasmic F-actin stress fibers with F-actin phalloidin staining. Podocytes exposed to LPS or PAN show loss of fine F-actin stress fibers (arrow) and appearance of thick cortical distribution (arrowheads) at the highest concentration of each agent at the end of 24 h incubation with LPS or PAN (upper panels, 2A and 2B). The change in actin cytoskeleton reflected a change in cell morphology by crystal violet (0.1 %) staining (lower panels, 2A and 2B)
LPS, but not PAN, activates the MyD88-dependent TLR signaling pathway
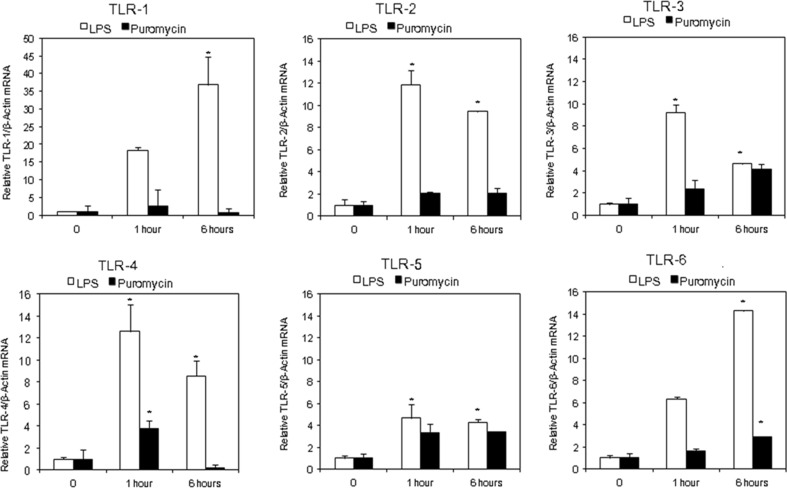
Figures 3 and 4 show the relative gene-specific mRNA expression (RE) for experimental genes of interest normalized against β-actin, a housekeeping gene that should not be acutely affected. LPS caused time-dependent changes in TLR expression profile at 0, 1 and 6 h. Results presented in Figs. 3 and 4 are annotated in Table 3 for a convenient summary.
Fig. 3.
Effect of lipopolysaccharide (LPS) or puromycin (PAN) for 1 h or 6 h on gene expression of Toll-like Receptors (TLR 1–6). Quantitative RT-PCR analysis results are expressed as mean fold-change in experimental groups compared to control untreated cells. β-actin was used as control. Results presented as mean fold-change in gene expression. (n = 3; *p < 0.05 compare to untreated cells)
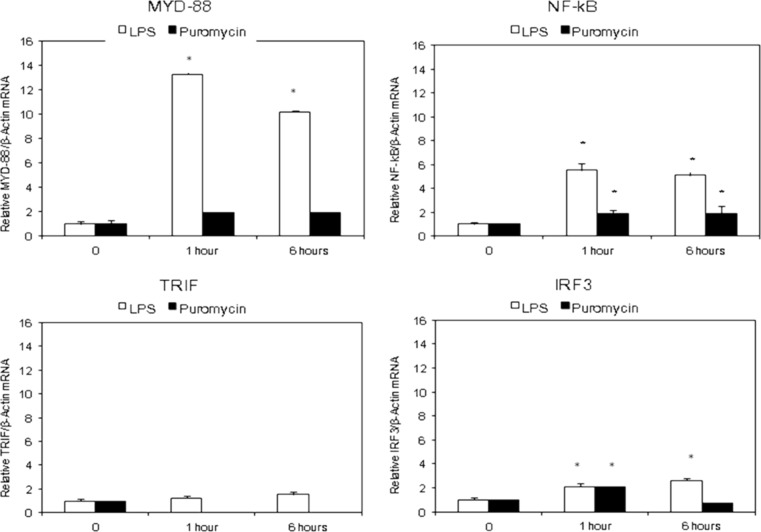
Fig. 4.
Effect of lipopolysaccharide (LPS) or puromycin (PAN) for 1 h and 6 h on mRNA expression of MyD88 dependent [upper panel] and TRIF dependent TLR signaling pathways [lower panel]. Quantitative RT-PCR analysis results are expressed as mean fold-change in experimental groups compared to untreated control group. β-actin was used as control. (n = 3; *p < 0.05 compare to untreated cells)
Table 3.
Results from Figs. 3 and 4 are summarized to highlight the differences between the effects of LPS and PAN on TLRs, MyD88, NF-kB, IRF and TRIF at 1 and 6 h
| A | B | C | D | |
|---|---|---|---|---|
| Time | LPS/Control ≥3-fold | PAN/Control ≥3-fold | LPS/PAN ≥3-fold | LPS/PAN <3-fold |
| 1 h | TLR 1 > 4 ≥ 2 > 3 > 6 > 5 | TLR-4, 5 | TLR-1, 2, 3, 4 and 6 | TLR-5 |
| MyD88, NF-κB | MYD88, NF-κB | IRF3, TRIF | ||
| 6 h | TLR 1 > 6 > 2 ≥ 4 > 3 ≈ 5 | TLR-3, 5 | TLR-1, 2, 4 and 6 | TLR-5 and 3 |
| MyD88, NF-κB | MYD88, NF-κB | IRF3, TRIF | ||
Columns A and B: LPS caused greater upregulation of TLR-1 than of other subtypes at both 1 and 6 h. PAN consistently upregulated TLR-5 at both time points. LPS upregulated the expression of MyD88 and NF-κB but PAN did not. TLR-5 was least affected by LPS but most affected by PAN at both time points
Columns C and D: Expression of TLR-5 was comparable in LPS and PAN treated cell at both 1 and 6 h
Treatment of podocytes with LPS caused more than an 8-fold increase in the gene expression of TLRs-1, 2, 3 and 4 (TLR1 > TLR4 ≥ TLR2 > TLR3) and a 4 to 6-fold increase in TLR-6 and 5 within 1 h. After 6 h, the expression of TLRs-2, 3, 4 decreased but that of TLR-1 and TLR-6 continued to increase. The overall profile of TLRs following stimulation with LPS was (TLR-1 > 6 > 2 ≥ 4 > 3 ≈ 5). LPS also caused a smaller increase in TLR-5 expression at both time points. Thus, LPS at concentrations commonly used in models of MCD, stimulated the expression of all transmembrane TLRs in podocytes by at least 4-fold at both 1 and 6 h. However, it did not induce the expression of endosomal TLRs 7–9. In contrast to LPS, PAN did not cause greater than a 4-fold increase in any of the TLRs at 1 or 6 h. Interestingly, both LPS and PAN caused a comparable increase in the expression of TLR-3 and −5. However, while TLR-3 and −5 were among the most up-regulated TLRs by PAN, these TLRs were among the least up-regulated by LPS. The expression of TLR-1 was least affected by PAN, and, in contrast, it was the TLR most up-regulated by LPS. Thus, PAN induced a small but persistent increase in TLRs 2 and 5, a transient increase in TLR-4 and a gradual increase in TLRs 3 and 6 between 1 and 6 h.
Figure 4 shows the effect of PAN or LPS on the expression of the adaptor molecules involved in TLR signaling at 0, 1 and 6 h. LPS caused up-regulation of MyD88 at 1 h by more than 12-fold, and these levels were maintained at 6 h. The corresponding increase in the expression of NF-κB was approximately 4-fold at both 1 and 6 h (p < 0.05 vs. Control). In keeping with the mild increase in TLR-3 expression, LPS induced only a minimal increase in the expression of TRIF and IRF3. In contrast, PAN did not induce a notable change in the expression of MyD88 or TRIF, suggesting that the observed small increase in TLRs (Fig. 3) did not generate a significant downstream signaling response. Thus, LPS primarily activated the MyD88-dependent TLR signaling pathway involving NF-κB in human podocytes while PAN did not produce a comparable effect.
Nuclear translocation of NF-κB
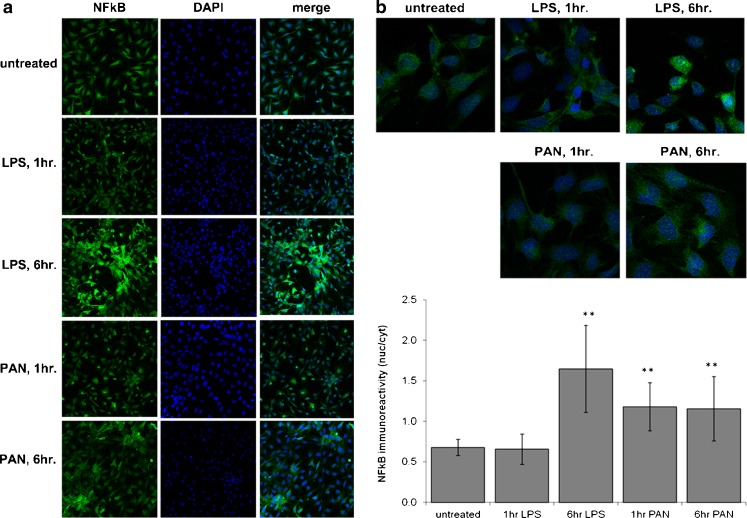
In order to verify that LPS treatment of podocytes leads to activation of the canonical signaling TLR pathway, we measured the LPS-elicited NF-κB nuclear translocation. Treatment of podocytes with LPS for 6 h resulted in a significant nuclear translocation of NF-κB (Fig. 5). The maximum intensity as measured (mean ± SD) by confocal microscopy for the whole cell in control podocytes was 3.4 ± 0.9, LPS at 1 h 2.5 ± 0.7, LPS at 6 h 4.2 ± 0.3, PAN at 1 h 2.3 ± 0.2 and PAN at 6 h 3.9 ± 0.34, with a nuclear/cytoplasmic ratio of 0.7 ± 0.1, LPS at 1 h 0.7 ± 0.2, LPS at 6 h 1.7 ± 0.5, PAN at 1 h 1.2 ± 0.3 and PAN at 6 h 1.2 ± 0.4, which would give a mean NF-κB nuclear activity based on immunofluorescence intensity for control podocytes as 2.3 ± 0.3, LPS at 1 h 1.6 ± 0.5 (p = 0.64), LPS at 6 h 6.8 ± 2.2 (p < 0.0001), PAN at 1 h 2.6 ± 0.7 (p < 0.0001) and PAN at 6 h 4.5 ± 1.6 (p = 0.003). Interestingly, PAN caused a rapid but less robust nuclear translocation of NF-κB than LPS (translocation within 1 h versus 6 h) (Fig. 5a and b).
Fig. 5.
Effect of lipopolysaccharide (LPS) or puromycin (PAN) on NF-κB nuclear translocation. Figure 5a shows separate and merged images of NF-κB fluorescence in the cytoplasmic and nuclear compartments at 1 and 6 h. Figure 5b shows representative images and numeric presentation of fluorescence intensity. Confocal microscopy results were analyzed for the nuclear/cytoplasmic ratio of fluorescence intensity presented in the bar graph. LPS caused a gradual increase in nuclear intensity by 6 h. PAN caused a lower but significant increase in fluorescence within 1 h that did not show further increase by 6 h. (**, p < 0.001)
PAN and LPS differ in their effects on cytokine production
Table 4 summarizes the effect of PAN and LPS on cytokines in podocytes. LPS-treated podocytes showed a significant increase in IL-6, IL-8, and MCP1, but little change was observed for MIP1α, IP-10 and IFN-β (Table 4). LPS caused significant increase in IL-6, IL-8 and MCP1 at both 6 h and 24 h (p < 0.05). However, TNFα, IFNα and TGFβ1 were not detectable (ND). Lack of change in IFN-α or IFN-β suggested that LPS was eliciting the cytokine response in accordance with its known effects. In contrast, in accordance with the small changes in the adapter molecules, PAN also did not cause significant elevation in the secreted cytokines IL-6, IL-8, MCP1, MIP-1α, IP-10 or IFN-β. Finally, TNF-α, IFN-α and TGF-β1 were not detectable (ND) in the supernatants of PAN-treated podocytes (Table 4).
Table 4.
Cytokines IL-6, IL-8, MCP1, MIP-1α, IP-10, IFN-β, IFN-α, TNF-α and TGF-β1 concentrations in podocyte supernatant media following treatment with lipopolysaccharide (LPS) or puromycin aminonucleoside (PAN) at 6 h and 24 h on a Luminex platform
| Cytokine | Control | PAN treatment | 24 h | LPS treatment | 24 h |
|---|---|---|---|---|---|
| 6 h | 6 h | ||||
| IL-6 (pg/μgDNA) | 0.66 ± 0.10 | 0.94 ± 0.18 | 1.03 ± 0.32 | 1.46 ± 0.15* | 2.55 ± 0.62* |
| MCP1 (pg/μgDNA) | 4.47 ± 1.03 | 1.45 ± 0.12 | 3.64 ± 1.29 | 19.54 ± 2.29* | 32.87 ± 1.15*‡ |
| IL-8 (pg/μgDNA) | 13.36 ± 3.67 | 1.97 ± 0.26 | 6.82 ± 3.93 | 32.01 ± 1.97* | 59.47 ± 3.06*‡ |
| MIP-1α (pg/μgDNA) | 0.033 ± 0.003 | 0.027 ± 0.001 | 0.043 ± 0.009 | 0.037 ± 0.005 | 0.042 ± 0.004 |
| IP-10 (pg/μgDNA) | 0.073 ± 0.028 | 0.031 ± 0.012 | 0.315 ± 0.291 | 0.08 ± 0.002 | 0.061 ± 0.018 |
| IFN-β (pg/μgDNA) | 0.52 ± 0.13 | 0.43 ± 0.01 | 0.45 ± 0.05 | 0.51 ± 0.13 | 0.36 ± 0.02 |
| IFN-α (pg/μgDNA) | ND | ND | ND | ND | ND |
| TNF-α (pg/μgDNA) | ND | ND | ND | ND | ND |
| TGF-β1 (pg/μgDNA) | ND | ND | ND | ND | ND |
Values as mean ± SE; ND = Not detected; *, p < 0.05 vs. Control; ‡, p < 0.05 vs. 6 h
Discussion
MCD is the most common form of nephrotic syndrome in children and is associated with ultrastructural changes in podocytes but its etiology remains unknown. Involvement of the immune system in the origin and/or progression of MCD is suggested by several observations including its occurrence with malignancy, immunization, infections and allergies (Lane and Kaskel 2009) and effective control of proteinuria by immunosuppressive drugs (Saha and Singh 2006). However, a role of the adaptive immune system has been difficult to prove since LPS causes proteinuria with cytoskeletal changes in podocytes and induces CD80 in T and B cell deficient SCID mice (Reiser et al. 2004). The innate immune system in epithelia with barrier function has received increasing attention (Gribar et al. 2008; Anders and Schlöndorff 2007) and may prove valuable in understanding the etiology of podocyte injury in MCD (Brown et al. 2007; Banas et al. 2008; Coers et al. 1994; Van Den Berg et al. 2000).
Sufficient quantities of several agents may increase glomerular protein permeability (McCarthy et al. 1998; Savin et al. 2008) and derange podocyte structural characteristics both in vitro and in vivo. These agents include free radicals, cytokine IL-13 (Yap et al. 1999), complement membrane attack complex C5b-9 (Couser 2012), immature differentiating cells (Sellier-Leclerc et al. 2007), PAN and LPS (Pippin et al. 2009). PAN-induced nephrosis (Frenk et al. 1955; Lannigan 1963) and podocyte injury are also known to be mediated by oxidants (Suzuki et al. 2001; Marshall et al. 2006; Diamond et al. 1986; Shiiki et al. 1998) and inhibition of the NOTCH signaling pathway (Ding et al. 2011). LPS, a TLR-4 ligand, causes podocyte injury that results in decreased cell numbers, dysregulated gene expression and a disrupted protein-protein network leading to albuminuria (Sun et al. 2009; Pawar et al. 2009). LPS-induced podocyte injury also involves generation of oxygen radicals and leads to activation of NF-κB (Greiber et al. 2002). Thus, podocyte injury in models of MCD may incorporate diverse mechanisms, each of which finally result in the same phenotype.
Podocyte cytoskeletal derangement serves as a useful surrogate of cellular injury and a marker of the phenotype. Both LPS and PAN caused an incremental dose-dependent loss of fine F-actin stress fibers and appearance of thickbundles of cortical actin filaments in podocytes (Fig. 2). Similar changes were reported by other investigators using 50 μg/ml LPS for up to 72 h (Reiser et al. 2004; Marshall et al. 2006; Gloy et al. 2000; Smoyer and Ransom 2002). We have observed similar injurious effects of fluid flow shear stress on mouse podocytes (Srivastava et al. 2010). We used cytoskeletal changes as preliminary evidence to present comparable effects of LPS and PAN.
However, the in vivo models of MCD and many in vitro studies of podocytes employ large quantities of PAN or LPS. We surmised that barrier epithelia may be less sensitive to microbial products than leukocytes. Constant exposure to filtered toxins and microbial products in the Bowman’s capsule may require podocytes to possess natural resistance to such molecules. We did not notice cell lysis at PAN concentrations under 100 μg/ml for up to 48 h (unpublished observation) and other investigators did not find DNA fragmentation (Suzuki et al. 2001). PAN is typically used at 50–100 μg/ml for 24–48 h or at lower concentration (5 μg/ml) for longer period (7 days) for in vitro study of podocytes [Gloy et al. 2000; Coers et al. 1994; Smoyer and Ransom 2002). Likewise, LPS was also used as 50 μg/ml levels to study its effect on TLR-4 (Reiser et al. 2004; Banas et al. 2008). In contrast, only nanogram/mL quantities of LPS are sufficient to stimulate vascular endothelial cells or leukocytes (Walia et al. 2012). These observations led us to consider the possibility that the high quantities of LPS employed for inducing podocyte injury may alter the expression of multiple TLRs within the innate immune system and that even PAN may alter TLR expression through heretofore unknown mechanism(s).
As a first step, present experiments showed that podocytes constitutively express TLRs 1–6 and 10 but not the endosomal TLRs 7–9 (Table 2). This profile of TLRs in cultured human podocytes resembles that reported in human tissues (Zarember and Godowski 2002; Medzhitov et al. 1997; Nishiya and DeFranco 2004; Matsumoto et al. 2003). These findings are similar to a recent report with the exception that we did not detect TLR-9. However, when stimulated with LPS, culture podocytes responded through an unanticipated and complex change in TLR profile that has not been described previously. Briefly, LPS induced an increase in TLRs 1–4 genes expression within 1 h. However, by 6 h, the expression of TLRs 2–4 had declined but that of TLR-1 and TLR-6 continued to increase (Fig. 3, Table 3). In summary, LPS (25 μg/mL) caused a minimum of 4-fold increase in TLRs by 6 h with minimum effect on TLRs 3 and 5. Increased expression TLR-4 by LPS has been reported in models of podocyte/glomerular injury (Reiser et al. 2004; Brown et al. 2007; Banas et al. 2008; Shimada et al. 2012; Tipping 2008; Kistler and Reiser 2010). We, for the first time, show that LPS-induced change in podocytes are most noticeable in TLR-1 and not in TLR-4. In contrast, PAN induced only a small increase in TLR at 1 h and in TLRs 3, 5 and 6 at 6 h. Of interest, TLRs 3, 5 and 6 were least affected by LPS (Fig. 3). The observed translocation of NF-κB (Fig. 5) did not involve upregulation of the downstream signaling elements of TLR pathway. Therefore, it is either an effect of PAN-induced oxidative stress and/or involves a mechanism unclear presently. These preliminary findings will indeed require further validation. Detailed studies using siRNA to inhibit mRNA for individual TLRs and antibodies to block respective proteins will be valuable in identifying and confirming the role of TLRs in mediating the observed effect of LPS or PAN on NF-κB. Such approach will further elucidate the differences between epithelial cells and other cell typess that respond to very low concentrations of LPS e.g., immune or endothelial cells.
LPS-induced up-regulation of multiple TLRs is an interesting finding in the light of some recent reports that suggest interaction among different TLR subtypes. For example, while TLR-4 functions as a homodimer and, TLR-2 forms a heterodimer with TLR-1 or TLR-6 (Adeline et al. 2001; Farhat et al. 2008). Additionally, TLR-1 appears to have an antagonistic effect on TLR-4 in endothelial cells (Spitzer et al. 2002). Parallel increase in TLR-1 expression may attenuate the effects of TLR-4 activation that is considered to mediate podocyte injury caused by LPS (Reiser et al. 2004; Banas et al. 2008). However, a persistent increase in TLR-1 expression by LPS leads us to speculate that podocyte injury by LPS involves upregulation of TLR-1 and its endogenous ligand(s). A specific endogenous ligand for TLR-1 or TLR-1/TLR-2 is not known although several molecules including heat-shock protein, heparin sulfate and fibronectin function as endogenous ligands for TLRs (Ospelt and Gay 2010). These findings suggest that LPS-induced changes in podocytes accompany a loss of exclusivity in receptor-ligand binding and activation of multiple TLRs that may result into activation of diverse signaling events. Recent reports suggest that activation of TLRs by antigens, cytokines or viral ligands results in proteinuria and urinary CD80 (B7-1) in MCD (Shimada et al. 2012; Ishimoto et al. 2011; Shimada et al. 2011). In contrast, others did not detect LPS-induced changes in CD80 despite severe glomerular stress and decreased podocyte numbers (Sun et al. 2009). These diverse and contradicting findings support our view that the established models of podocyte injury involve complex and seemingly antagonistic processes. Non-specific effects of high LPS or PAN levels may induce heretofore unrecognized cross-talk between signaling pathways. Our experiments provide a clear indication of changes initiated by the activation of multiple TLRs that may foretell the complex role of innate immune system in models of MCD in vitro or in vivo.
LPS induced the upregulation of MyD88-NF-κB expression was accompanied by only small increases in TRIF and IRF3 expression (Fig. 1 and 4) while PAN did not cause comparable changes in these adapters. We analyzed the cell culture medium for proinflammatory induced by MyD88-NF-κB pathway and cytokines known to change in MCD (Woroniecki et al. 2008; Araya et al. 2006; Suranyi et al. 1993; Wasilewska et al. 2011). We found that cytokines IL-6, IL-8 and MCP1 were increased at both 6 and 24 h following stimulation with LPS, and these changes reached statistical significance at 24 h. Conversely, we did not detect changes in IFN-α or IFN-β, key elements of the TRIF-IRF3 pathway. In addition, we did not detect a change in IP-10 (CXCL-10), TNFα or TGFβ1 in the medium. Although the effect of TNFα on podocytes and its role in renal disease has been widely reported but podocytes express TNFα only under specific conditions (Sanchez-Niño et al. 2010). PAN did not induce pro-inflammatory cytokines release despite causing an increase in TLR-5 and TLR-3 expression (Table 4) suggesting that these receptor subtypes engage in a heretofore unknown signaling cross-talk. Thus, LPS-mediates its effects in podocytes by the canonical TLR-MyD88-NF-κB signaling whereas the translocation of NF-κB induced by PAN appears to be the result of oxidative stress, a known effect of PAN (Suzuki et al. 2001; Marshall et al. 2006; Diamond et al. 1986).
In summary, cultured podocytes express all membrane-bound TLR subtypes one of which, TLR-3, also localizes to endosomes. LPS induces upregulation of several TLRs suggesting an apparent loss of its specificity for TLR-4 at concentrations used in in vitro models of MCD. While PAN does not induce major changes in TLR expression, LPS and PAN induce comparable changes in podocytes through disparate but complementary mechanisms that increase nuclear translocation of NF-κB. These studies suggest that barrier epithelia may invoke the innate immune system in a manner distinct from immune cells or endothelial cells and that, interactions among TLR subtypes may play a role in mediating podocyte injury in MCD and other renal diseases.
Acknowledgements
The work was supported by The Paul Henson Endowed Fund for Pediatric Research and The Sam and Helen Kaplan Research Fund in Pediatric Nephrology. We thank Ashley Sherman, M.A. for assistance in statistical analysis. Parts of the work were presented at the XVth Congress of the International Pediatric Nephrology Association, New York, 30th August 2010.
Conflict of interest
None
References
- Adeline M, Hajjar D, O’Mahony S, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Functional interactions between Toll-Like Receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Schlöndorff D. Toll-like receptors: emerging concepts in kidney disease. Curr Opin Nephrol Hypertens. 2007;16:177–83. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, et al. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2006;21:603–610. doi: 10.1007/s00467-006-0026-5. [DOI] [PubMed] [Google Scholar]
- Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HJ, Lock HR, Wolfs TG, Buurman WA, Sacks SH, Robson MG. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J Am Soc Nephrol. 2007;18:1732–1739. doi: 10.1681/ASN.2006060634. [DOI] [PubMed] [Google Scholar]
- Carmody RJ, Chen YH. Nuclear factor-kappaB activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007;4:31–41. [PubMed] [Google Scholar]
- Coers W, Huitema S, van der Horst ML, Weening JJ. Puromycin aminonucleoside and adriamycin disturb cytoskeletal and extracellular matrix protein organization, but not protein synthesis of cultured glomerular epithelial cells. Exp Nephrol. 1994;2:40–50. [PubMed] [Google Scholar]
- Couser WG. Basic and translational concepts of immune-mediated glomerular disease. J Am Soc Nephrol. 2012;23:381–399. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- Diamond JR, Bonventre JV, Karnovsky MJ. A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int. 1986;29:478–483. doi: 10.1038/ki.1986.24. [DOI] [PubMed] [Google Scholar]
- Ding X, Zhu F, Li T, Zhou Q, Hou FF, Nie J. Numb protects renal proximal tubular cells from puromycin aminonucleoside-induced apoptosis through inhibiting Notch signaling pathway. Int J Biol Sci. 2011;7:269–278. doi: 10.7150/ijbs.7.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, et al. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008;83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- Frenk S, Antonowicz I, Craig JM, Metcoff J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955;89:424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- Gloy J, Reitinger S, Fischer K–G, Schreiber R, Boucherot A, Kunzelmann K, et al. Amino acid transport in mouse podocytes. Am J Physiol. 2000;278:F999–F1005. doi: 10.1152/ajprenal.2000.278.6.F999. [DOI] [PubMed] [Google Scholar]
- Greiber S, Müller B, Daemisch P, Pavenstädt H. Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony-stimulating factor. J Am Soc Nephrol. 2002;13:86–95. doi: 10.1681/ASN.V13186. [DOI] [PubMed] [Google Scholar]
- Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Shimada M, Araya CE, Huskey J, Garin EH, Johnson RJ. Minimal Change Disease: A CD80 podocytopathy? Semin Nephrol. 2011;31:320–325. doi: 10.1016/j.semnephrol.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Kistler AD, Reiser J. Maximal ‘CD80-uria’ with minimal change. Kidney Int. 2010;78:236–238. doi: 10.1038/ki.2010.148. [DOI] [PubMed] [Google Scholar]
- Lane JC, Kaskel FJ. Pediatric nephrotic syndrome: from the simple to the complex. Semin Nephrol. 2009;29:389–398. doi: 10.1016/j.semnephrol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Lannigan R. The production of chronic renal disease in rats by a single intravenous injection of aminonucleoside of puromycin and the effect of low dosage continuous hydrocortisone. Br J Exp Pathol. 1963;44:326–333. [PMC free article] [PubMed] [Google Scholar]
- MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986;108:378–382. doi: 10.1016/S0022-3476(86)80876-9. [DOI] [PubMed] [Google Scholar]
- Marshall CB, Pippin JW, Krofft RD, Shankland SJ. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 2006;70:1962–1973. doi: 10.1038/sj.ki.5001642. [DOI] [PubMed] [Google Scholar]
- Mathieson PW. Minimal change nephropathy and focal segmental glomerulosclerosis. Semin Immunopathol. 2007;29:415–426. doi: 10.1007/s00281-007-0094-z. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–62. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- McCarthy ET, Sharma R, Sharma M, Li JZ, Ge X, Dileepan KN, et al. Tumor Necrosis Factor-α increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Amer Soc Nephrol. 1998;9:433–438. doi: 10.1681/ASN.V93433. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Neuhaus TJ, Fay J, Dillon MJ, Trompeter RS, Barratt TM. Alternative treatment to corticosteroids in steroid sensitive idiopathic nephrotic syndrome. Arch Dis Child. 1994;71:522–526. doi: 10.1136/adc.71.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling proper ties of the Toll-like receptors. J Biol Chem. 2004;279:19008–19017. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- Ospelt C, Gay S. TLRs and chronic inflammation. Int J biochem Cell Biol. 2010;42:495–505. doi: 10.1016/j.biocel.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology. 2009;128(1 Suppl):e206–221. doi: 10.1111/j.1365-2567.2008.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, et al. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TC, Singh H. Minimal Change Disease: A Review. Southern Med J. 2006;99:1264–1270. doi: 10.1097/01.smj.0000243183.87381.c2. [DOI] [PubMed] [Google Scholar]
- Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Sanchez-Niño MD, Benito-Martin A, Gonçalves S, Sanz AB, Ucero AC, Izquierdo MC et al (2010) TNF superfamily: a growing saga of kidney injury modulators. Mediators Inflamm. doi:10.1055/2010/182958 [DOI] [PMC free article] [PubMed]
- Savin VJ, Sharma M, McCarthy ET, Sharma R, Reddy S, Dong JW, et al. Cardiotrophin like cytokine-1: Candidate for the focal glomerular sclerosis permeability factor. J Am Soc Neph. 2008;19:59A. doi: 10.1681/ASN.2007030276. [DOI] [Google Scholar]
- Sellier-Leclerc AL, Duval A, Riveron S, Macher MA, Deschenes G, Loirat C, et al. A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J Am Soc Nephrol. 2007;18:2732–2739. doi: 10.1681/ASN.2006121346. [DOI] [PubMed] [Google Scholar]
- Shiiki H, Sasaki Y, Nishino T, Kimura T, Kurioka H, Fujimoto S, et al. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology. 1998;66:221–229. doi: 10.1159/000028027. [DOI] [PubMed] [Google Scholar]
- Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, Garin EH. Minimal change disease: a “two-hit” podocyte immune disorder? Pediatr Nephrol. 2011;26:645–649. doi: 10.1007/s00467-010-1676-x. [DOI] [PubMed] [Google Scholar]
- Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-{kappa}B-dependent pathway. Nephrol Dial Transplant. 2012;27:81–89. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]
- Smoyer WE, Ransom RF. Hsp27 regulates podocyte cytoskeletal changes in an in vitro model of podocyte process retraction. FASEB J. 2002;16:315–326. doi: 10.1096/fj.01-0681com. [DOI] [PubMed] [Google Scholar]
- Spitzer JH, Visintin A, Mazzoni A, Kennedy MN, Segal DM. Toll-like receptor 1 inhibits Toll-like receptor 4 signaling in endothelial cells. Eur J Immunol. 2002;32:1182–1187. doi: 10.1002/1521-4141(200204)32:4<1182::AID-IMMU1182>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13:13–18. doi: 10.1007/s004670050555. [DOI] [PubMed] [Google Scholar]
- Srivastava T, McCarthy ET, Sharma R, Cudmore PA, Sharma M, Johnson ML, et al. Prostaglandin E2 is crucial in the response of podocytes to fluid flow shear stress. J Cell Commun Signal. 2010;4:79–90. doi: 10.1007/s12079-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, He L, Takemoto M, Patrakka J, Pikkarainen T, Genové G, Norlin J, et al. Glomerular transcriptome changes associated with lipopolysaccharide-induced proteinuria. Am J Nephrol. 2009;29:558–570. doi: 10.1159/000191469. [DOI] [PubMed] [Google Scholar]
- Suranyi MG, Guasch A, Hall BM, Myers BD. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am J Kidney Dis. 1993;21:251–259. doi: 10.1016/s0272-6386(12)80742-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takemura H, Noiri E, Nosaka K, Toda A, Taniguchi S, et al. Puromycin aminonucleoside induces apoptosis and increases HNE in cultured glomerular epithelial cells. Free Radic Biol Med. 2001;31:615–623. doi: 10.1016/S0891-5849(01)00641-4. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Sem Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Tipping PG. Are podocytes passive or provocative in proteinuric glomerular pathology? J Am Soc Nephrol. 2008;19:651–653. doi: 10.1681/ASN.2008020156. [DOI] [PubMed] [Google Scholar]
- Van Den Berg JG, Aten J, Chand MA, Claessen N, Dijkink L, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol. 2000;11:413–422. doi: 10.1681/ASN.V113413. [DOI] [PubMed] [Google Scholar]
- Walia D, Sharma M, Raveendran V, Zhou J, Sharma R, Stechschulte DJ et al (2012) Human Mast Cells (HMC-1 C56) Enhance Interleukin-6 production by quiescent and lipopolysaccharide-stimulated human coronary artery endothelial cells. Mediators Inflamm. doi:10.1155/2012/274347 [DOI] [PMC free article] [PubMed]
- Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Kołodziejczyk Z. Urinary monocyte chemoattractant protein-1 excretion in children with glomerular proteinuria. Scand J Urol Nephrol. 2011;45:52–59. doi: 10.3109/00365599.2010.526140. [DOI] [PubMed] [Google Scholar]
- Woroniecki RP, Shatat IF, Supe K, Du Z, Kaskel FJ. Urinary cytokines and steroid responsiveness in idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2008;28:83–90. doi: 10.1159/000109396. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999;10:529–537. doi: 10.1681/ASN.V103529. [DOI] [PubMed] [Google Scholar]
- Yew KH, Carsten B, Harrison C. Scavenger receptor A1 is required for sensing HCMV by endosomal TLR-3/-9 in monocytic THP-1 cells. Mol Immunol. 2010;47:883–893. doi: 10.1016/j.molimm.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]