Abstract
The involvement of phosphoinositides (PI) signal transduction pathway and related molecules, such as the Phosphoinositide-specific Phospholipase C (PI-PLC) enzymes, in the pathophysiology of mood disorders is corroborated by a number of recent evidences. Our previous works identified the deletion of PLCB1 gene, which codifies for the PI-PLC β1 enzyme, in 4 out 15 patients affected with schizophrenia, and no deletion both in major depression affected patients and in normal controls. By using interphase fluorescent in situ hybridization methodology, we analyzed PLCB1 in paraffin embedded samples of orbito-frontal cortex of 15 patients affected with bipolar disorder. Deletion of PLCB1 was identified in one female patient.
Keywords: Genetics, Bipolar disorder, Signaling, I-FISH, Phosholipase C beta1
Introduction
Abnormalities in signal transduction are supposed to play a role in the pathogenesis of mood disorders. The cyclic-AMP, phosphoinositides (PI), mitogen-activated protein kinase (MAPK), and glycogen synthase kinase cascades were mainly indicated as pivotal actors (Tanis et al. 2007; Jope et al. 1996; Ebstein et al. 1988; Pacheco et al. 1996). PI signal transduction pathway is involved in a variety of cell functions such as hormone secretion, neurotransmitter signal transduction, cell growth, membrane trafficking, ion channel activity, cytoskeleton regulation, cell cycle control, apoptosis, cell and tissue polarity (Comer et al. 2007; Suh et al. 2008).
The role of PI in signal transduction was first described in 1953 (Hokin et al. 1953). PI metabolism is involved in processes such as differentiation, proliferation (Noh et al. 1995; Berridge et al. 1984). A combination of compartmentalized and temporal changes in molecules belonging to the PI system, such as phosphatidyl inositol (4,5) bisphosphate (PIP2) or phosphatidyl inositol (3,4,5) trisphosphate (PIP3), elicit different cellular responses, including regulation of gene expression, DNA replication, or chromatin degradation. PIP2 directly regulates a number of cell functions, including cytoskeleton reorganization, cytokinesis, membrane dynamics, nuclear events and channels activity (Bunney et al. 2011). Therefore, strict regulation of PIP2 levels by means of converting enzymes, such as Phosphoinositide-specific Phospholipase C (PI-PLC) family is necessary for homeostasis (Berridge et al. 1984).
PI-PLC β1 isoform is activated by G-protein-coupled receptors that signal through Gq/11 (Bunney et al. 2011). PI-PLC β1 is highly expressed in the nervous system, mainly the cerebral cortex and hippocampus (Ross et al. 1989), and mediates activity-dependent cortical development and synaptic plasticity (Hannan et al. 1998; Spires et al. 2005). PI-PLC β1-knockout mice developed epilepsy, minor abnormalities in the hippocampus (Kim et al. 1997), as well as specific behavioural deficits in location recognition, probably due to excess in neurogenesis and aberrant migration of adult-born neurons (Wallace et al. 1990; Choi et al. 1989). Activity-dependent regulation of synapse and dendritic spine morphology in developing barrel cortex requires PI-PLC β1 (Spires et al. 2005).
PI-PLC β1 was recently suggested to be involved in human diseases affecting the nervous system. A further report described a male child affected with epileptic encephalopathy associated with loss-of-function mutation in the gene which codifies for PI-PLC β1 enzyme (PLCB1, OMIM *607120) (Kurian et al. 2010). Moreover, PI-PLC β1 is considered to represent a molecular convergence point of several neurotransmitter pathways implicated in schizophrenia (Wallace et al. 1990; Kim et al. 1997; Choi et al. 1989). Recent evidences supported this hypothesis (Lo Vasco et al. 2010; Udawela et al. 2011; Lo Vasco et al. 2012). The nature, meaning, and developmental period of PI-PLC involvement are largely unclear and will require further studies.
Our previous work identified the deletion of PLCB1 in 4 out 15 schizophrenia affected patients (Lo Vasco et al. 2012). PLCB1 is constituted from 36 small exons and introns, and was located on the short arm of human chromosome 20, in a region frequently rearranged in mental illnesses (20p12, nearby markers D20S917 and D20S177) (Peruzzi et al. 2000; Fanous et al. 2008; Hovatta et al. 1998).
In order to analyze the deletion of PLCB1 gene in bipolar disorder affected patients, we used interphase fluorescence in situ hybridization (I-FISH) technique (Lengauer et al. 1990).
Materials and methods
Probe extraction
The probe was extracted as previously described (van Dekken et al. 1990). Briefly, defrosted strains of E. Coli bacteria transformed with PAC P1 881E24/Kan+ were cultured overnight in LB Broth/30μg/ml Kanamicin at 37 °C (Sigma-Aldrich, St Louis, MO). The next day, the pellet was accurately resuspended in 50 mM Tris pH 8.0/10 mM EDTA/100 μg/μl RnaseA solution. A solution of 0.2 N NaOH/1 % SDS was added to each tube, and after 15 m Proteinase K was also mixed. After 5 m, a solution of 3 M K acetate pH 4.8–5.5 was slowly added (Sigma-Aldrich, St Louis, MO), vigorously shaken, centrifuged at 4 °C, and to the supernatant 20 μg/ml glycogen was added. Iced isopropanol was added and suspensions were centrifuged 30 m. The DNA jellyfish containing the probe was carefully allowed to dry for no more than 20 m and resuspended in a small quantity of dH2O. When it was completely dissolved, 1/10 TE was added. The probe was biotin-labeled by nick translation (Invitrogen, Carlsbad, CA) following manufacturer’s indications.
Samples
Human post-mortem brain biopsies were obtained from the Stanley Brain Medical Research Laboratory (Bethesda, MD). Samples were divided in two anonymous groups constituted by 15 bipolar disorder affected patients and 15 donors, free from mood disorders. Bipolar disorder affected patients aged from 14 to 52, with female gender prevalence (10/15). 15 μm thick formalin-fixed (mean fixation time 8 months) paraffin-embedded slices of orbito-frontal cortex were used to extract nuclei as previously described (Lo Vasco and Polonia 2012). Briefly, slices were deparaffinized, rehydrated, and mechanically detached. The tissue was incubated at 37 °C for 1 h in a moist chamber with proteinase K solution (Boehringer 745723). The suspension was collected and passed through a 50 μm nylon mesh. The remaining nuclei were washed with PBS and pelleted by centrifugation. Supernatant was removed and replaced with Carnoy II fixative. Aliquots of the suspension were put on a slide and allowed to dry at room temperature.
I-FISH procedure
Nuclei underwent interphase fluorescent in situ hybridization (I-FISH) procedure as previously described (Lo Vasco and Polonia 2012). Briefly, slides were rinsed several times in PBS, and dehydrated in ethanol series. Biotinylated PLCB1 probe was added to each slide. Co-denaturation was performed at 72–80 °C and then slides were incubated in humidified chamber overnight at 37 °C. Slides were washed in 50 % formamide/2X SSC and several times in 2X SSC. The hybridized biotin-labeled probe was detected with FITC-conjugated streptavidin (Sigma-Aldrich, St Louis, MO). Nuclei were counterstained with DAPI (Oncor/Resnova, Italy) and slides were observed with a fluorescence microscope (Leica DM 4000, DE) equipped with image analysis and acquisition program (IAS 2000, Delta Sistemi, IT). From 95 to 100 nuclei were processed for each sample.
Results
Two signals were detected in 100 % of the evaluable nuclei in 14/15 samples of patients affected with bipolar disorder. One signal was detected in the nuclei of one female patient aged 23 affected with bipolar disorder, deceased for causes independent from the disease (Fig. 1). Two signals of the PLCB1 gene were detected in 100 % of the evaluated nuclei in 15/15 samples of the normal donors control group.
Fig. 1.
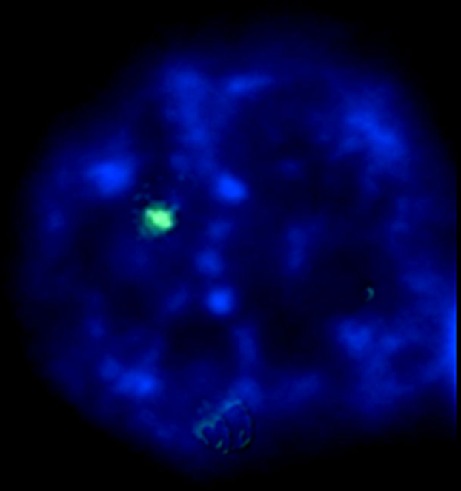
I-FISH of extracted nucleus of one patient affected with bipolar disorder bearing PLCB1 deletion: only one signal is detected
Discussion
Changes in the expression and activity of a number of genes were described in limbic and cortical brain regions of patients affected with mood disorders. Different therapies act regulating many of these changes (Tanis et al. 2007). A number of signaling pathways play a role in the pathogenesis of mood disorders (Tanis et al. 2007).
The PI pathway contributes an important intracellular system involved in a variety of cell functions, including the neurotransmitter signal transduction (Suh et al. 2008; Lo Vasco 2012). PI-PLC plays a central role by regulating the spatio-temporal balance of PI metabolism. When a signaling molecule binds a G-protein-linked receptor in the plasma membrane (Gq), activated PI-PLC in less than a second cleaves PIP2 into inositol-trisphosphate (IP3) and diacylglycerol (DAG), both crucial molecules in signal transduction (Suh et al. 2008). IP3, a small water-soluble molecule, diffuses rapidly to the cytoplasm, where calcium is released from the endoplasmic reticulum after binding to IP3-gated calcium-release channels. DAG can be further cleaved to release arachidonic acid. DAG can also activate serine/threonine calcium-dependent protein kinases C (Suh et al. 2008; Bunney et al. 2011).
PI-PLC enzymes are divided into six sub-families comprising thirteen isoforms, classified on the basis of amino acid sequence, domain structure and mechanism of recruitment in response to activated receptors (Suh et al. 2008; Bunney et al. 2011). PI-PLC enzymes are strictly tissue specific and different expression of some isoforms was described in pathological with respect to normal tissues. Different Pi-plc β1 isoform expression was described both in rat astrocytoma and activated astrocytes with respect to the quiescent counterpart (Lo Vasco et al. 2007; Lo Vasco et al. 2010). A role for PI-PLC β1 in mood disorders was also suggested (Udawela et al. 2011; Lo Vasco 2012; Lo Vasco et al. 2012). This hypothesis corroborated data obtained from Pi-plc β1 knock-out mice, widely used as schizophrenia experimental models (Mc Omish et al. 2008).
PI-PLC β1 was suggested to represent a molecular convergence point of several neurotransmitter pathways implicated in schizophrenia (Wallace et al. 1990; Kim et al. 1997; Choi et al. 1989). A recent report described a male child affected with epileptic encephalopathy associated with loss-of-function mutation in the PLCB1 gene (Kurian et al. 2010).
In the present study, we performed I-FISH in the orbitofrontal cortex paraffin-embedded samples of 15 bipolar disorder affected patients and 15 normal controls, intended as donors not presenting with psychiatric or neurologic disorders. Both copies of PLCB1 gene were detected in about 100 % (range 89–100 %) of the processed nuclei (Fig. 1) in 14/15 samples of patients affected with bipolar disorder. Both copies of the PLCB1 gene were detected in 100 % of the processed nuclei in the normal donors control group. In one female patient, aged 23, I-FISH homogeneously detected exclusively one signal, indicating that the analyzed nuclei presented the deletion of one PLCB1 allele. In previous studies (Lo Vasco and Polonia 2012; Lo Vasco et al. 2012), as well as in the present, we did not detect deletions of PLCB1 in normal controls. However, the P1 881E24 probe is 115.611 bp long. The present study is able to exclude wide deletions of PLCB1. The present technique cannot exclude further abnormalities, such as single point mutation or shorter deletions, undetectable with molecular cytogenetic techniques.
The present results corroborate previous observations in schizophrenia. A similar PLCB1 deletion was detected in patients affected with schizophrenia (Lo Vasco et al. 2012). The involvement of PI-PLC β1 isoform in schizophrenia was also suggested by an independent study using different methodology (Udawela et al., 2011). By contrast, in our present and previous studies we did not find PLCB1 deletion in normal controls and in patients affected with major depression. Although the I-FISH methodology does not allow the detection of small deletions, the present results suggest that PI-PLC β1 enzyme might be involved in both schizophrenia and bipolar disorder. Although the role of PI-PLC β1 is not elucidated, these data suggest that PI-PLC enzymes might be involved in the pathogenesis or progression of affective disorders.
However, the nature, meaning, and developmental period of PI-PLC involvement are still largely unclear and will require further studies. Although significant progresses were achieved in studying the PI system and its alterations in human brain, many issues remain to be addressed with special regard to the possible involvement in mood disorders. In fact, further studies are required both to refine the analysis of PLCB1 gene, of the PI-PLC β1 protein and to extend the examination to other, more recently discovered molecules involved in the PI signaling.
Moreover, further studies might also help to clarify the complex interplay in the network of signal transduction pathways acting in the nervous system (Lo Vasco and Polonia 2012). To understand the role, the timing of action and the interplay of the signalling pathways recruited in the nervous system might allow the delineation of the pathogenesis and the clinical history of a number of nervous diseases. That will be helpful in order to define the diagnosis and prognosis, which often are difficult to define. This promising field of research might also provide useful insights in order to open the way to novel therapeutic strategies.
Although the I-FISH methodology does not allow the identification of all the possible abnormalities of the PLCB1 gene, it might be useful as a screening method. The interphase hybridisation technique, used to identify DNA sequences in nuclei offers a great advantage (Lo Vasco and Polonia 2012). In fact, I-FISH makes unnecessary the availability of live cells to culture in order to obtain metaphase chromosomes, widening the availability of human brain biopsies to analyze for population studies using stored samples.
Abbreviations
- SSC
Saline-sodium citrate buffer
- DAPI
4',6-diamino-2-phenylindole dihydrochloride
Footnotes
Submission declarations
The Corresponding Author, Dr Vincenza Rita Lo Vasco, has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to permit this article (if accepted) to be published in this journal.
The Authors disclose any commercial associations that might pose a conflict of interest in connection with the submitted manuscript and indicates that there are no conflicts.
The AUTHORS, Dr Vincenza Rita Lo Vasco and Dr Patrizia Polonia, declare that:
the submitted work has not been published previously, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Author contribution
The authors materially participated in the research and/or article preparation. Vincenza Rita Lo Vasco: participated in concept and design of the research; carrying out the experimental work; data analysis and interpretation; writing of the article. Patrizia Polonia and Lucia Longo: participated in data analysis and interpretation; writing of the article.
References
- Berridge MJ, Irvine RF. Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. PLC regulation: emerging pictures for molecular mechanisms. Trends Biochem Sci. 2011;36(2):88–96. doi: 10.1016/j.tibs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Choi WC, Gerfen CR, Suh PG, Rhee SG. Immunohistochemical localization of a brain isozyme of phospholipase C (PLC III) in astroglia in rat brain. Brain Res. 1989;499(1):193–197. doi: 10.1016/0006-8993(89)91153-0. [DOI] [PubMed] [Google Scholar]
- Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128(2):239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Lerer B, Bennett ER, Shapira B, Kindler S, Shemesh Z, Gerstenhaber N. Lithium modulation of second messenger signal amplification in man: inhibition of phosphatidylinositol-specific phospholipase C and adenylate cyclase activity. Psychiatry Res. 1988;24(1):45–52. doi: 10.1016/0165-1781(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Webb BT, Straub RE, O’Neill FA, Walsh D, Riley BP, Kendler KS. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry. 2008;64(2):121–127. doi: 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Kind PC, Blakemore C. Phospholipase C-beta1 expression correlates with neuronal differentiation and synaptic plasticity in rat somatosensory cortex. Neuropharmacology. 1998;37:593–605. doi: 10.1016/S0028-3908(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Hokin MR, Hokin LE. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953;203(2):967–977. [PubMed] [Google Scholar]
- Hovatta I, Lichtermann D, Juvonen H, Suvisaari J, Terwilliger JD, Arajärvi R, Kokko-Sahin ML, Ekelund J, Lönnqvist J, Peltonen L. Linkage analysis of putative schizophrenia gene candidate regions on chromosomes 3p, 5q, 6p, 8p, 20p and 22q in a population-based sampled Finnish family set. Mol Psychiat. 1998;3(5):452–457. doi: 10.1038/sj.mp.4000443. [DOI] [PubMed] [Google Scholar]
- Jope RS, Song L, Li PP, Young LT, Kish SJ, Pacheco MA, Warsh JJ. The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem. 1996;66(6):2402–2409. doi: 10.1046/j.1471-4159.1996.66062402.x. [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Meyer E, Vassallo G, Morgan NV, Prakash N, Pasha S, Hai NA, Shuib S, Rahman F, Wassmer E, Cross JH, O’Callaghan FJ, Osborne PJ, Scheffer IE, Gissen P, Maher ER. Phospholipase C beta 1 deficiency is ass Sociated with early-onset epileptic encephalopathy. Brain. 2010;133:2964–2970. doi: 10.1093/brain/awq238. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Riethman H, Cremer T. Painting of human chromosomes with probes generated from hybrid cell lines by PCR with Alu and L1 primers. Hum Genet. 1990;86(1):1–6. doi: 10.1007/BF00205163. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR. The Phosphoinositide pathway and the signal transduction network in neural development. Neuroscience Bulletin (accepted on 7th, May 2012) [DOI] [PMC free article] [PubMed]
- Lo Vasco VR, Polonia P. Molecular cytogenetic interphase analysis of Phosphoinositide-specific Phospholipase C β1 gene in paraffin-embedded brain samples of major depression patients. J Affect Disord. 2012;136(1–2):177–180. doi: 10.1016/j.jad.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Fabrizi C, Artico M, Cocco L, Billi AM, Fumagalli L, Manzoli FA. Expression of phosphoinositide-specific phospholipase C isoenzymes in cultured astrocytes. J Cell Biochem. 2007;100:952–959. doi: 10.1002/jcb.21048. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Fabrizi C, Fumagalli L, Cocco L. Expression of phosphoinositide specific phospholipase C isoenzymes in cultured astrocytes activated after stimulation with Lipopolysaccharide. J Cell Biochem. 2010;109(5):1006–1012. doi: 10.1002/jcb.22480. [DOI] [PubMed] [Google Scholar]
- Lo Vasco VR, Cardinale G, Polonia P. Deletion of PLCB1 gene in schizophrenia affected patients. J Cell Mol Med. 2012;16(4):844–851. doi: 10.1111/j.1582-4934.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish CE, Burrows EL, Howard M, Hannan AJ. PLC-b1 Knockout Mice as a Model of Disrupted Cortical Development and Plasticity: Behavioral Endophenotypes and Dysregulation of RGS4 Gene Expression. Hippocampus. 2008;18(8):824–834. doi: 10.1002/hipo.20443. [DOI] [PubMed] [Google Scholar]
- Noh DY, Shin SH, Rhee SG. Phosphoinositide-specific phospholipase C and mitogenic signalling. Biochim Biophys Acta. 1995;1242:99–114. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Jope RS. Phosphoinositide signaling in human brain. Prog Neurobiol. 1996;50(2–3):255–273. doi: 10.1016/S0301-0082(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Calabrese G, Faenza I, Manzoli L, Matteucci A, Gianfrancesco F, Billi AM, Stuppia L, Palka G, Cocco L. Identification and chromosomal localisation by fluorescence in situ hybridisation of human gene of phosphoinositide-specific phospholipase Cb1. Biochim Biophys Acta. 2000;484:175–182. doi: 10.1016/s1388-1981(00)00012-3. [DOI] [PubMed] [Google Scholar]
- Ross CA, MacCumber MW, Glatt CE, Snyder SH. Brain phospholipase C isozymes: differential mRNA localizations by in situ hybridization. Proc Natl Acad Sci U S A. 1989;86:2923–2927. doi: 10.1073/pnas.86.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Molnar Z, Kind PC, Cordery PM, Upton AL, Blakemore C, Hannan AJ. Activity-dependent regulation of synapse and dendritic spine morphology in developing barrel cortex requires phospholipase C-beta1 signalling. Cereb Cortex. 2005;15:385–393. doi: 10.1093/cercor/bhh141. [DOI] [PubMed] [Google Scholar]
- Suh PG, Park J, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yuk S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Reports. 2008;41:415–434. doi: 10.5483/BMBRep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007;39(7):531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- Udawela M, Scarr E, Hannan AJ, Thomas EA, Dean B. Phospholipase C beta 1 expression in the dorsolateral prefrontal cortex from patients with schizophrenia at different stages of illness. Aust N Z J Psychiatry. 2011;45(2):140–147. doi: 10.3109/00048674.2010.533364. [DOI] [PubMed] [Google Scholar]
- van Dekken H, Pizzolo JG, Reuter VE, Melamed MR. Cytogenetic analysis of human solid tumors by in situ hybridization with a set of 12 chromosome-specific DNA probes. Cytogenet Cell Genet. 1990;54(3–4):103–107. doi: 10.1159/000132971. [DOI] [PubMed] [Google Scholar]
- Wallace MA, Claro E. A novel role for dopamine: inhibition of muscarinic cholinergic-stimulated phosphoinositide hydrolysis in rat brain cortical membranes. Neurosci Lett. 1990;110:155–161. doi: 10.1016/0304-3940(90)90804-I. [DOI] [PubMed] [Google Scholar]


