Abstract
A total of 1920 faecal samples of sheep (960) and goats (960) of stationary flocks of the middle agro-climatic zone of Jammu province were examined, out of which 67.24 % animals were positive for helminthic infections. The different nematodes observed were strongyles (50.1 %), trichurids (12.1 %) and Strongyloides spp. (4.2 %). Trematode ova recorded were of amphistomes (8.3 %), Fasciola spp. (8.2 %) and Dicrocoelium spp. (5.4 %). No significant difference was observed between the infection level in sheep (68.54 %) and goats (65.94 %) which could be attributed to mixed grazing and sharing of pastures/sheds. Significantly (p < 0.05) higher infection was observed in monsoon as compared to winter. Strongyles were predominant during all the seasons, but significantly (p < 0.05) higher infection was observed in monsoon as compared to winter. Coproculture studies revealed that Haemonchus contortus (61.18 %) predominated during all the seasons, followed by Trichostrongylus spp. (13.67 %), Ostertagia spp. (12.17 %), Strongyloides spp. (4.14 %), Oesophagostomum spp. (3.84 %) and Bunostomum spp. (3.83 %). Eggs per gram of faeces (EPG) were the highest (sheep 1883.33 ± 117.6 and goats 1800 ± 110.21) during monsoon and the lowest during winter (sheep 640 ± 41.29 and goats 556.67 ± 33.01). Two peaks of EPG (the first in May and the second in August) were recorded during the 1 year study period. Infection was significantly (p < 0.05) higher in young (73.22 %) as compared to adults (61.25 %). Females showed a higher infection (73.33 %) as compared to males (61.14 %). The effect of prevailing agro-climatic conditions on the prevalence of gastrointestinal helminths has been discussed.
Keywords: Sheep, Goats, Prevalence, Helminths, Jammu
Introduction
Sheep and goat rearing provides livelihood to millions of people, especially to the poor and downtrodden population in the developing and under developed countries. In Jammu and Kashmir, rearing of sheep and goats is a traditional profession of most of the nomadic tribes. Parasitic diseases have got unique importance as they cause high morbidity and huge economic losses (ranging from 20 to 25 %) in the form of low wool, meat and milk production, retarded growth, morbidity and mortalities (Gupta 2006).
Among parasitic diseases, helminths are the major constraint in survival and productivity of these animals. Gastrointestinal (GI) nematodes rank highest on global index with Haemonchus contortus on top (Perry et al. 2002). There are many reports of prevalence of helminths in small ruminants from India (Thapar 1956; Singla 1995; Singh et al. 1997; Katoch et al. 1998; Godara and Sharma 2010) and different parts of Jammu and Kashmir (Makhdoomi et al. 1995; Khajuria and Kapoor 2003; Yadav et al. 2006; Shahnawaz et al. 2011). Among the three agro-climatic zones of Jammu province, 44.19 % sheep and 55.83 % goats are reared in middle agro-climatic zone (altitude 800–1500 m above sea level). However, no report is available on the prevalence of GI helminths in small ruminants from middle agro-climatic zone of Jammu province. Therefore, on the basis of coprological examination, the present investigations record the prevalence together with the associated epidemiology of GI helminths of small ruminants of middle agro-climatic zone of Jammu province.
Materials and methods
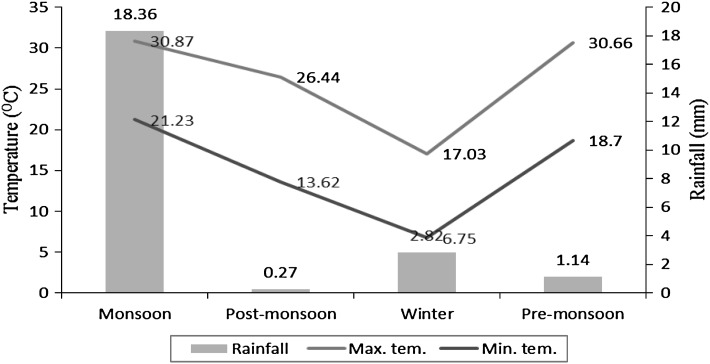
During 1 year of study period (July 2008–June 2009), a total of 1920 faecal samples (sheep 960 and goats 960) from four selected villages located distantly apart in the study area were collected and examined by direct smear method as well as floatation and sedimentation methods (Soulsby 1982). Eggs per gram (EPG) of faecal samples were calculated by Modified McMaster method (Coles et al. 1992). Petri dish culture method was used for coproculture studies. Meteorological data was collected from Division of Meteorology, SKUAST-J, Chatha (Fig. 1).
Fig. 1.
Maximum and minimum temperatures (°C) and rainfall (mm) in middle agro-climatic zone of Jammu province during 2008–2009
The period of study was divided into four seasons viz. monsoon (July–September), post-monsoon (October–November), winter (December–February) and pre-monsoon/summer (March–June). One-way ANOVA and Chi-square test (Snedecor and Cochran 1989) were used for analyzing the data. A p value of <0.05 was considered significant.
Results and discussion
Out of the total animals (1,920) examined, 67.24 % were positive for helminthic infections either by direct smear or by concentration methods. Ova of strongyles, Trichuris spp. and Strongyloides spp. were observed in 50.1, 12.1, and 4.2 % animals, respectively. The other helminthic ova in descending order were of amphistomes (8.3 %), Fasciola spp. (8.2 %), Dicrocoelium spp. (5.4 %), and anoplocephalids (1.7 %) (Table 1).
Table 1.
Seasonal prevalence of gastrointestinal helminths of sheep and goats of middle agro-climatic zone of Jammu province
| Season | Species | No. examined | Infected (%) | Prevalence (%) of helminths | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strongyle | Strongyloides | Trichuris | Fasciola | Dicrocoelium | Amphistomes | Anoplocephalids | ||||
| Monsoon | Sheep | 240 | 190 (79.17) | 57.9 | 4.6 | 15.0 | 12.9 | 3.8 | 10.8 | 1.3 |
| Goats | 240 | 184 (76.67) | 63.3 | 3.3 | 16.3 | 10.8 | 5.0 | 11.7 | 1.3 | |
| Post-monsoon | Sheep | 160 | 104 (65.0) | 46.3 | 4.4 | 8.8 | 15.6 | 6.3 | 13.13 | 2.5 |
| Goats | 160 | 103 (64.38) | 44.4 | 3.8 | 8.1 | 13.13 | 8.1 | 12.5 | 1.9 | |
| Winter | Sheep | 240 | 135 (56.25) | 35.0 | 4.6 | 6.3 | 7.9 | 8.3 | 3.8 | 0.8 |
| Goats | 240 | 128 (53.33) | 35.4 | 4.6 | 8.3 | 5.4 | 10.0 | 4.6 | 0.8 | |
| Pre-monsoon | Sheep | 320 | 229 (71.56) | 57.5 | 4.7 | 14.1 | 4.4 | 2.2 | 6.6 | 2.5 |
| Goats | 320 | 218 (68.13) | 54.1 | 14.1 | 15.9 | 2.5 | 2.5 | 7.2 | 2.2 | |
| Total | 1,920 | 1,291 (67.24) | 50.1 | 4.2 | 12.1 | 8.2 | 5.4 | 8.3 | 1.7 | |
Similar findings were recorded from middle agro-climatic zone of Himachal Pradesh (Katoch et al. 1999; Devina et al. 2007). Earlier, Yadav et al. (2006) reported 82.44 and 83.63 % helminthic infections, respectively in sheep and goats from low altitude zone of Jammu province.
There was no significant difference in the infection pattern of sheep and goats. The reason could be that samples were collected from the livestock of stationary breeders having mixed flocks. Grazing pattern and managerial practices followed by these breeders are almost same for both the species. Sheds, pastures and water places are shared by these animals and this could be an important factor responsible for similar pattern of infection in both the species.
Significantly (p < 0.05) higher helminthic infection (77.92 %) was observed in monsoon season as compared to post-monsoon (64.69 %, χ2 = 16.24) and winter (54.79 %, χ2 = 56.45) and pre-monsoon (69.84 %, χ2 = 8.72). However, the difference was non- significant between post-monsoon and pre-monsoon. Higher rainfall during monsoon season provides suitable molarity of salts in soil which is an important factor for ecdysis (Soulsby 1966). It also helps in larval dispersion on pasture and increases the chances of contact between host and infective larvae (Katoch 1998). Higher temperature and rainfall cause stress to the host which lowers its immunity and predisposes it to a heavy infection (Hawkins 1945).
Strongyles were predominant during all seasons. But significantly (p < 0.05) higher infection was observed in monsoon season as compared to winter, post-monsoon and pre- monsoon (Table 1). Almost similar type of findings was recorded by Devina et al. (2007) in Gaddi breed of sheep from middle agro-climatic zone of Himachal Pradesh. Like present observations, ova of Strongyloides spp. and trichurids have been reported from Jammu district by Khajuria and Kapoor (2003) and Yadav et al. (2006).
Amphistomes, Fasciola spp., Dicrocoelium spp. and anoplocephalids were observed in 8.3, 8.2, 5.4, and 1.7 % of faecal samples, respectively. Higher infection of amphistomes was recorded during winter, whereas the highest infection of Fasciola spp. was found during post-monsoon and the lowest during pre-monsoon.
Ollerenshaw and Rowland (1959) described two annual cycles of infection in snails viz. summer and winter, out of which summer infection is more important. Eggs deposited in pasture during spring and over wintered eggs hatch and finally miracidia infect the snails in spring. On the contrary, due to longer intra-molluscan developmental period of larvae of Fasciola spp. (especially F. gigantica) (Soulsby 1982), the infection passes onto herbage in late summer and autumn, results in losses during early October and continue throughout whole winter season. In case of amphistomes, infection passes to herbage in early monsoon and losses may occur in monsoon and post-monsoon seasons. In winter infection, snails are infected in late summer or early autumn. Winter development of larvae in snails is inhibited and recommences in the spring. Moreover, snails undergo hibernation and many of them die during extreme winter. In this case, infection passes to herbage in late spring or early summer and gives rise to the disease from July to October in case of Fasciola spp. and about 1–2 months earlier in case of amphistomes. This could be the possible reason for different patterns of infection of Fasciola spp. and amphistomes in different seasons of the year.
Prevalence of anoplocephalids was more in pre- and post-monsoon seasons, as climatic factors during this period were more conductive for development of oribatid mites, the intermediate host for anoplocephalids (Soulsby 1982).
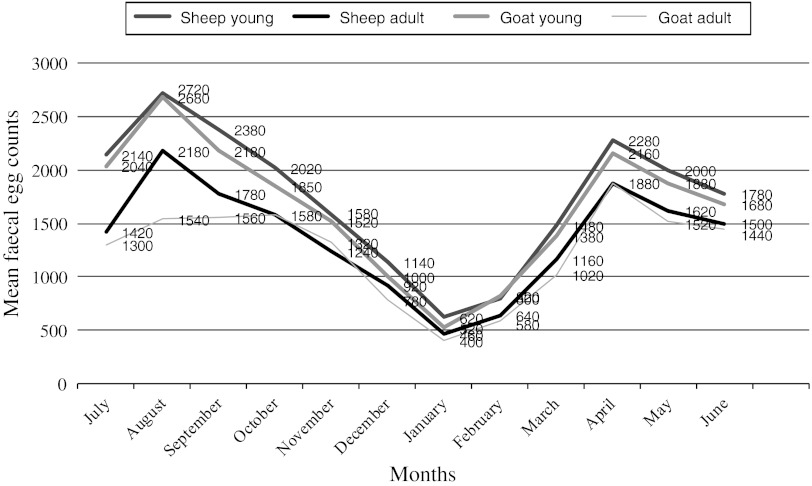
Quantitative analysis of faecal samples of sheep and goats revealed the highest EPG (1883.33 ± 117.6 in sheep and 1800 ± 110.21 in goats) during monsoon and the lowest during winter (640 ± 41.29 and 556.67 ± 33.01, respectively in sheep and goats). When compared month wise, the lowest EPG was recorded during January and the highest during August. Two peaks of EPG were recorded during the period of study, one during August and the second during May (Fig. 2). In young animals (<1 year), mean EPG was significantly (p < 0.05) higher as compared to adults (>1 year), irrespective of host and season (Fig. 2). High EPG during monsoon could be attributed to many other factors. Majority of these flocks graze on road side grasses near water bodies and are partially stall fed. The lambs and kids born in February–March are on weaning side and the innate immunity is on reducing side, whereas acquired immunity is in initial phase. This is taken as advantage by the infective stages of nematodes and other helminths during this season (Katoch et al. 1999; Devina et al. 2007). As the temperature increases from March, the over wintered larvae start moulting and become infective. Post-parturition in February–March, leads to high shedding of eggs of GI nematodes, thereby increasing pasture infectivity leading to high EPG during the month of April–May.
Fig. 2.
Monthly mean faecal egg counts of gastrointestinal nematodes of sheep and goats in middle agro-climatic zone of Jammu province during 2008–2009
The low level of EPG (556.67 ± 33.0 to 640.0 ± 41.29) and infection (54.79 %) during winter could be due to adverse climatic conditions in winter months, which help in arrested development in host and environment (Hutchinson et al. 1972). In addition, short photoperiod in winter reduces the grazing period and helps in reducing the chance of contact between the host and parasite. Majority of ewes during this period are pregnant and hormonal impact results in low faecal egg output and contributes to the less availability of infection in pastures as reported earlier from different parts of country (Jithendran 1998; Devina 2004; Shugufta et al. 2005; Yadav et al. 2006).
Age-wise analysis of both the species revealed that the overall helminthic infection was significantly higher (73.22 %) in young animals than in adults (61.25 %). Singh et al. (1997) recorded similar type of findings in sheep from Rajasthan. They reported higher EPG (3,896.01 ± 662.1) in young animals than adults (1,812.5 ± 472.3). However, from the same region Swarnkar et al. (1996) reported higher prevalence of GI nematodes in adults, followed by hoggets and weaners. Higher rate of nematode infection in young animals could be attributed to the low level of immunity in young animals as compared to that in adults.
The rate of infection was significantly (p < 0.05) higher in females (73.33 %) as compared to that in males (61.14 %). It was observed that in ewes, there was a slight increase in EPG and the level was maintained up to 9 weeks post-lambing. From Kashmir, Wani et al. (2011) and Shahnawaz et al. (2011) also reported higher prevalence of GI helminths in female sheep than in males, but the difference was not significant. The hypobiotic larvae in the final host during winter get released and develop faster in the animals with impaired immunity and further add to higher level of infection in female sheep. In conclusion, two annual anthelminthic treatments during the months of May and August for nematodes and one treatment during the month of October for trematodes would help to minimise the infection and optimum growth and productivity of small ruminants in the region.
Acknowledgments
The authors are highly thankful to the Director, Sheep Husbandry Department and the concerned staff for providing cooperation and facilities during the collection of faecal samples from different areas.
References
- Coles GC, Bauer G, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ. World Association for the advancement of veterinary parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-U. [DOI] [PubMed] [Google Scholar]
- Devina S (2004) Epidemiological studies on gastrointestinal helminths of sheep and chemotherapy of Haemonchus contours. MVSc thesis, CSK, Himachal Pradesh, Krishi Vishvavidyalaya, Palampur, pp 1–74
- Devina S, Katoch R, Agnihotri RK. Gastrointestinal helminths in Gaddi sheep. J Vet Parasitol. 2007;21:141–143. [Google Scholar]
- Godara R, Sharma RL. Parasitic infections in livestock at Jaipur. J Vet Parasitol. 2010;24:193–195. [Google Scholar]
- Gupta JL. Sheep production and management. 1. New Delhi: CBS Publishers and Distributors; 2006. pp. 1–239. [Google Scholar]
- Hawkins PA. Studies of sheep parasites VI. Observations on weather in relation to untreated nematode infections. J Parasitol. 1945;31:17. doi: 10.2307/3272934. [DOI] [Google Scholar]
- Hutchinson GW, Lee EH, Fernando MA. Effects of variation in temperature on infective larvae and their relationship to inhibited development of Obeliscoides cuniculi in rabbit. Parasitology. 1972;65:333–342. doi: 10.1017/S003118200004511X. [DOI] [PubMed] [Google Scholar]
- Jithendran KP. Epidemiology of gastrointestinal nematodes in migratory sheep and goats in North-Western humid Himalayan region. Indian J Anim Sci. 1998;68:894–896. [Google Scholar]
- Katoch R (1998) Epidemiological, chemotherapeutic and immunological studies on Haemonchus Contortus in goats. PhD thesis, University of Agriculture and Technology, Kanpur
- Katoch R, Mittra S, Agnihotri RK, Sharma AK. Winter strongyloidosis in sheep and goats at high altitude, a sporadic occurrence. Indian Vet J. 1998;75:361–362. [Google Scholar]
- Katoch R, Mandial RK, Nagal KB. Outbreak of Haemonchus contortus infection in sheep of Himachal Pradesh. Indian Vet J. 1999;76:932–933. [Google Scholar]
- Khajuria JK, Kapoor PR. Prevalence of parasites in sheep and goats at Kathua-Jammu. J Vet Parasitol. 2003;17:121–126. [Google Scholar]
- Makhdoomi DM, Shagufta N, Bandey SD, Moulvi B. Incidence of different ovine gastrointestinal parasites in Kashmir. Indian Vet J. 1995;72:898–900. [Google Scholar]
- Ollerenshaw CB, Rowland LP. A method of forecasting the incidence of fascioliasis in Anglesey. Vet Rec. 1959;71:591–598. [Google Scholar]
- Perry BD, Randolph RFMC, Dermott JJ, Sones KR, Thornton PK. Investing in animal health research to alleviate poverty. Research proceedings. Nairobi: International Livestock Research Institute (ILRI); 2002. p. 148. [Google Scholar]
- Shahnawaz M, Shahardar RA, Wani ZA. Seasonal prevalence of platyhelminthosis of sheep in Ganderbal area of Kashmir valley. J Vet Parasitol. 2011;25:59–62. [Google Scholar]
- Shugufta N, Jeelani SG, Hakeem M. Incidence of gastrointestinal nematodes in sheep in Kashmir Valley. J Vet Parasitol. 2005;19:27–29. [Google Scholar]
- Singh D, Swarnkar CP, Khan FA, Srivastava CP, Bhagwan PSK. Epidemiology of ovine gastrointestinal nematodes at a organized farm in Ranchi. Small Rum Res. 1997;26:31–37. doi: 10.1016/S0921-4488(96)00988-1. [DOI] [Google Scholar]
- Singla LD. A note on subclinical intestinal parasitism in sheep and goats in Ludhiana and Faridkot districts of Punjab. Indian Vet Med J. 1995;19:61–62. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: The Iowa State University Press; 1989. [Google Scholar]
- Soulsby EJL. Biology of parasites. New York and London: Academic Press; 1966. pp. 185–196. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: The English Language Book Society; 1982. pp. 126–173. [Google Scholar]
- Swarnkar CP, Khan FA, Singh D, Bhagwan PSK. Multiple anthelmintic resistance in Haemonchus contortus of sheep. Indian J Anim Sci. 1996;69:547–549. [Google Scholar]
- Thapar GS. Systematic survey of helminth parasites of domesticated animals in India. Indian J Vet Sci. 1956;26:211–271. [Google Scholar]
- Wani ZA, Shahardar RA, Shahnawaz M. Prevalence of nemathelminth parasites in sheep of Ganderbal district of Kashmir valley. J Vet Parasitol. 2011;25:26–29. [Google Scholar]
- Yadav Anish, Khajuria JK, Raina AK. Seasonal prevalence of gastrointestinal parasites in sheep and goats of Jammu. Indian Vet J. 2006;20:65–68. [Google Scholar]