Abstract
Present study has been undertaken to evaluate antimalarial potential and safety of artesunate based combination therapy with homeopathic medicine china (ϕ/30 potency) against Plasmodium berghei (NK-65), a lethal rodent malaria parasite. In combination therapy, the oral administration of artesunate (100 mg/kg) + china ϕ/30 proved to be highly efficacious as it completely cleared the blood stage infection. During the follow up period up to day 28, no recrudescence was observed and the survival rate was 100 %. Combination did not disturb the normal functioning of liver and kidney, as evident from the normal activity of ALP (190.5 ± 0.2 and 174.2 ± 9.12 IU/l), level of bilirubin (0.6 ± 0.33 and 0.73 ± 0.1 mg/dl), urea (28 ± 0.51 and 29.1 ± 0.03 mg/dl) and creatinine (0.9 ± 0.62 and 1.1 ± 0.1 mg/dl) in serum of treated mice on day 7 and 28 respectively. Present study points to better efficacy of china as an alternative drug partner in combination to enhance antimalarial efficacy of artesunate without affecting the liver and kidney functions of P. berghei infected BALB/c mice.
Keywords: Plasmodium berghei, Recrudescence, Artesunate, Homeopathy, Cinchona officinalis
Introduction
Artemisinin-based combinations provide mutual protection against multi drug resistant parasite, high antimalarial efficacy, excellent tolerability and reduced transmissibility (Bangchang and Congpuong 2007; Orerell et al. 2008). World Health Organization had banned the use of artemisinin in monotherapy and recommended combination therapy (ACT) to delay the development of drug resistance and to improve cure rates (WHO 2006; Robert et al. 2001). However, resistance to artemisinin has been reported around Thai Cambodian border recently (Müller et al. 2009).
Homeopathy is a method of traditional medicine that uses ultra # low doses of highly diluted natural substances originating from plants, minerals or animals. It is a nontoxic form of alternative medicine which can be combined with classical treatment methods to ensure adequate global healthcare and for avoiding the risk of adverse effects (WHO 2002). Mice have been used as a model for homeopathy research in relation to cytotoxicity, genotoxicity and carcinogenesis. Several potentized homeopathic drugs have been tested against many toxic chemical compounds and have been reported to be capable of acting by triggering the regulatory action of master genes (Khuda-Bukhsh 2009).
Homeopathic medicine china, produced from bark of Cinchona officinalis has been reported to cure low forms of fever (remittent or intermittent or malarial) and also kills parasites (Robin Murphy 2004; Shah 1999; Lessell 1993; Kent 1989). The Cinchona bark is the source of a variety of alkaloids which have been used as anti-fever agents and in treating malaria (Yarnell and Abascal 2004). China mother tincture (ϕ) has been reported to reduce Plasmodium berghei infection in Balb/c mice and also enhanced the mean survival time of treated mice. The drug completely cleared the infection when it was given in combination with standard antimalarial artesunate (100 mg/Kg). Both the monotherapy and combination were reported to be safe on the host’s blood cells (Rajan and Bagai 2011). The homeopathic medicines can recondition entire human body through life force and by stimulating the natural defense mechanism. They strengthen the dynamism of internal system which changes the susceptibility of body towards pathogen (Saxena 2006).
Present study was undertaken to evaluate anti-plasmodial efficacy along with the hepatoprotective and nephroprotective role of artesunate based combination therapy (ACT) with homeopathic medicine china ϕ and 30 potency against Plasmodium berghei (NK-65), a lethal rodent malaria parasite.
Materials and methods
Animals/parasite
Albino mice Mus musculus of BALB/c strain (weighing 22–26 g and 4–6 weeks old) of either sex were obtained from the Central Animal House, Panjab University, Chandigarh. They were maintained on a standard pellet diet and water ad libitum. A chloroquine sensitive strain of P. berghei (NK-65) was maintained by intraperitoneal inoculation of 1 × 106 infected erythrocytes to naïve mice (Santiyanont 1985). Parasitaemia was checked by preparing Giemsa stained thin blood smears on glass slides through tail vein incision of infected mice. The treatment of mice was according to the guidelines of institutional ethical committee, Panjab University, Chandigarh, India (Reg No. 45/1999/CPCSEA).
Experimental design
Ten groups (G-1 to G-10) having 10 mice each (same sex and age) were used for the present study (Table 1). All groups except G-1 were injected with 1 × 106P. berghei parasitized erythrocytes on 1st day (D0). Dose of various antimalarials/vehicles (0.2 ml/mouse/day) was administered orally to mice of different groups for 7 days (D0–D6). Falcigo tablet containing 50 mg artesunate base (Cadilla Pharmaceutical Company, India) was suspended in 1 ml of (5 %) Na2HCO3 and 5 ml of (0.9 %) NaCl. Artesunate (AS) 100 mg/kg was administered orally bi daily (BD) on 1st day and once a day (OD) for another 6 days. Standard recommended dose of AS (4 mg/kg for 3 days) + SP # (1.2 mg/kg as single dose) was administered orally (Laridox containing 500 mg Sulphadoxine and 25 mg Pyremethamine). Homeopathic medicine china ϕ and 30 potency (Dr. Willmar Schwabe India Pvt. Ltd., Noida) were used. In present study, mother tincture consisted of 1 part drug in 10 parts of absolute alcohol, and 30 potency was obtained after potentisation and it denoted 30 dilutions in centesimal scale. China ϕ and china 30 were diluted in distilled water in ratio of 1:2 and administered OD for 7 days (0.2 ml/mice/day), 1–2 h after the administration of AS in combination therapy.
Table 1.
Experimental groups for present study showing dose regimens and Mean Survival Time (MST)
| Group n = 10 | G-1 | G-2 | G-3 | G-4 | G-5 | G-6 | G-7 | G-8 | G-9 | G-10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug/vehicle 0.2 ml/day/mouse | DW | DW | china ϕ (1:2) | china 30 (1:2) | Absolute alcohol | ASa (100 mg/kg) | 5 % NaHCO3 + 0.9 %NaCl | AS + chinab ϕ | AS + chinab 30 | AS (4 mg/kg) + SP(1.2 mg/kg) |
| MST (days) | 28 ± 0 | 7 ± 0 | 24.5 ± 3.5 | 26.8 ± 1.2 | 5 ± 0 | 28 ± 0 | 6.5 ± 0.5 | 28 ± 0 | 28 ± 0 | 28 ± 0 |
All the groups except G-1 were injected with 1 × 106 infected RBCs on day 0
aAdministered BD on day 0 and OD on D1-D6
bchina was given 1 h after the oral administration of artesunate
Parasitaemia was monitored on day 3, 5, 7 and thereafter weekly during 1 month follow up period. The mean survival time (MST) of mice in each group was calculated.
Liver and kidney function tests
The activity of alkaline phosphatase (ALP) (p-NPP method), concentration of bilirubin (Jendrassik and Grof method), urea (modified Berthelot method) and creatinine (alkaline picrate method) were checked in serum of mice using ENZOPAK/CHEMPAK reagent kits (Reckon Diagnostic Pvt. Ltd., Gorwa, Baroda, India) and manufacturer’s instructions were followed. Biochemical assays were performed in serum of normal (G-1) and P. berghei infected mice (G-2) on day 7, whereas, in experimental groups, it was done twice on day 7 and 28.
Statistical analysis
Data has been presented as mean and standard deviation (SD). Statistical evaluation of differences between the experimental groups was determined by the Student’s t test with the level of significance of p < 0.05 using GraphPad Software (San Diego, California, USA). The experiments were repeated for validation of results.
Results
Course of parasitaemia in experimental groups
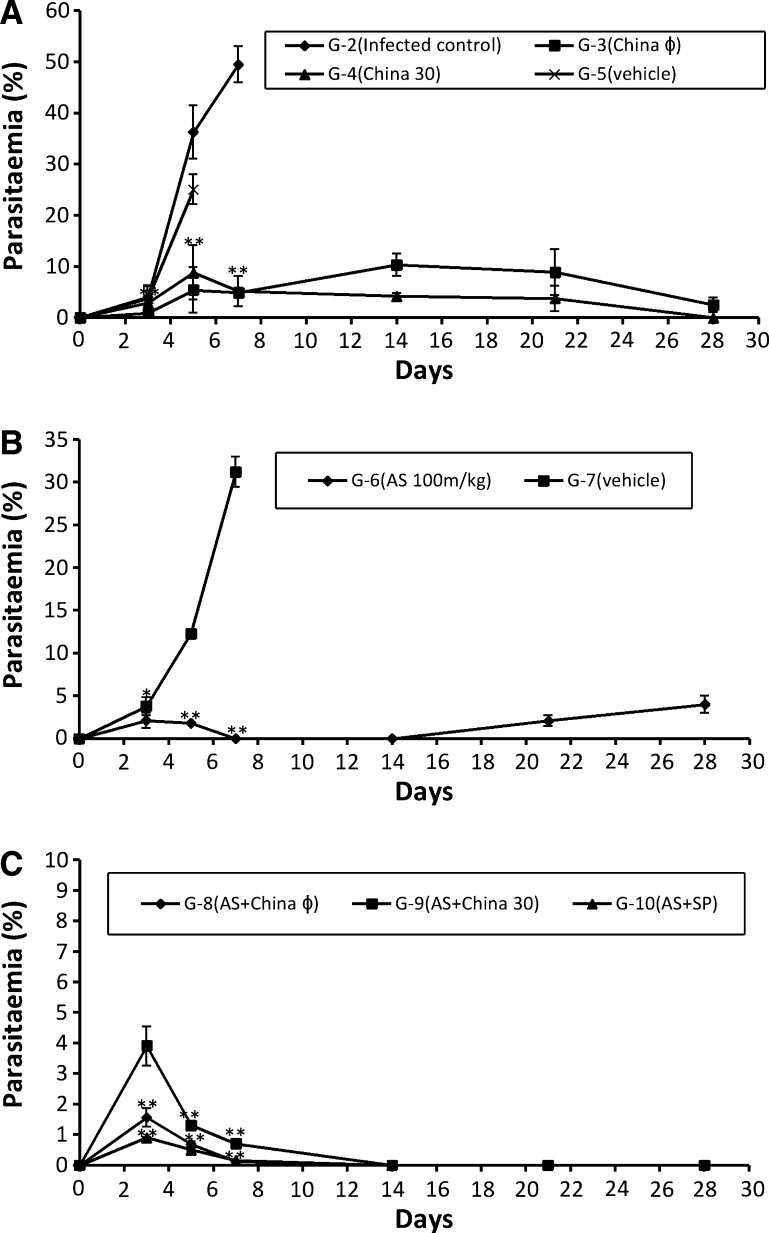
Maximum infection of 49.5 ± 3.53 % was observed in infected control (G-2) on 7th day post inoculation after which all the mice died due to heavy infection, whereas, mice treated with vehicle (G-5, G-7) died by day 5 (25.1 ± 0 %) and day 7 (31.19 ± 1.77 %) respectively. In G-3 (china ϕ), the infection was observed to increase till day 14 up to 10.3 ± 2.2 % after which it declined continuously to 2.5 ± 1.44 % (p < 0.0005) by the day 28 whereas, in G-4 (china 30) infection was completely cleared after day 21 (Fig. 1a).
Fig. 1.
Graphs showing course of parasitaemia (Mean ± SD) in various experimental groups on various days of study. Data is given as mean ± SD. p < 0.05 (# statistically significant), p value in comparison to infected control is represented as p < 0.005 (* statistically highly significant), p < 0.0005 (** statistically extremely significant). a Course of parasitaemia in groups treated with monotherapy of china (G-3 and G-4) and placebo control (G-5). b Course of parasitaemia in groups treated with monotherapy of artesunate (G-6) and vehicle treated group (G-7). c Course of parasitaemia in groups treated with combination therapy (G-8, G-9) and positive control group (G-10)
In AS monotherapy (G-6) the infection was reduced to 1.78 ± 0.05 % (p < 0.0005) by day 5 but many free merozoites outside the RBCs were observed in blood smears. Infection was completely cleared by day 7 but recrudescence was observed on day 21 with 2.07 ± 0.64 % infection which increased up to 3.99 ± 0.07 % by day 28 (Fig. 1b).
In the mice treated with combination of artesunate and china (ϕ and 30 potency), the infection was observed to be 0.12 ± 0 and 0.7 ± 0 % respectively on day 7 after which complete clearance of blood stage infection was observed till day 28. In G-10 (AS + SP) also, the infection was reduced to 0.16 ± 0.2 % on day 7 after which the infection was completely cleared off (Fig. 1c).
Mean survival time
The mean survival time of experimental groups was calculated after completion of follow up period. All the mice of infected control (G-2) and placebo control groups (G-5 and G-7) died within 7 days and the mean survival time was 7 ± 0, 5 ± 0 and 6.5 ± 05 days respectively. The mean survival time of G-6, G-8, G-9 and G-10 was maximum and was comparable to normal control group G-1 (i.e. 28 days) followed by G-4 (26.8 ± 1.2 days) and G-3 (24.5 ± 3.5 days) (Table 1).
Liver function test
Three-fold increase in ALP activity of infected mice G–2 (632.9 ± 12.2 IU/l) was observed on day 7 as compared to normal control G-1 (212.5 ± 5.08 IU/l). After treating with monotherapy of china (G-3 and G-4) and artesunate (G-6), the activity of ALP was found to be less than normal on day 7 i.e. 84.75 + 2.3 IU/L (p < 0.0001), 168 ± 5.9 IU/L (p < 0.0005) and 201 ± 5.1 IU/L (p < 0.0005) respectively which decreased significantly to 135.2 ± 3.5 IU/L (p < 0.0005), 172.1 ± 2.5 IU/L (p < 0.0005) and 237 ± 2.03 IU/L (p < 0.0005) on 28th day post inoculation as compared to infected control. In G-8 and G-9 the ALP activity was found to be declined in comparison to infected control and was also comparable to the normal level on both day 7 and 28 whereas, in the group treated with AS + SP, the ALP activity was lower than that of normal mice both on day 7 and 28 (Table 2).
Table 2.
Table showing ALP activity, concentration of bilirubin, concentration of creatinine and urea in serum of mice of different treated groups on day 7 and 28
| Group | Liver function test | Kidney function test | ||||||
|---|---|---|---|---|---|---|---|---|
| ALP (IU/l) | Bilirubin (mg/dl) | Urea (mg/dl) | Creatinine (mg/dl) | |||||
| D7 | D28 | D7 | D28 | D7 | D28 | D7 | D28 | |
| G-1 | 212.5 ± 5.08 | 0.65 ± 0.37 | 32.0 ± 1.05 | 0.9 ± 0.2 | ||||
| G-2 | 632.9 ± 12.2 | 1.04 ± 0.52 | 62.6 ± 2.30 | 2.25 ± 0.7 | ||||
| G-3 | 84.75 ± 2.3** | 135.2 ± 3.5** | 0.96 ± 0.40 | 0.87 ± 0.42 | 64.4 ± 0.56 | 60.5 ± 5.1 | 0.78 ± 0.1** | 0.66 ± 0.02** |
| G-4 | 168 ± 5.9** | 172.1 ± 2.5** | 1.70 ± 0.92 | 1.5 ± 0.3 | 41.6 ± 2.26** | 40.3 ± 3.6** | 1.7 ± 0.02 | 0.8 ± 0.2** |
| G-6 | 201 ± 5.1** | 237 ± 2.03** | 1.86 ± 0.45 | 1.53 ± 0.26 | 79.4 ± 4.51** | 65.3 ± 3.6 | 2.05 ± 0.34 | 1.02 ± 0.5* |
| G-8 | 182 ± 5.1** | 190.5 ± 0.2** | 0.81 ± 0.33 | 0.6 ± 0.33 | 26.5 ± 3.2** | 28 ± 0.51** | 1.01 ± 0.54* | 0.9 ± 0.62* |
| G-9 | 170 ± 3.5** | 174.2 ± 9.1** | 1 ± 0.2 | 0.73 ± 0.1 | 33 ± 2.4** | 29.1 ± 0.03** | 1.2 ± 0.2* | 1.1 ± 0.1* |
| G-10 | 315 ± 13.5** | 270.1 ± 0.4** | 1.4 ± 0.4 | 0.8 ± 0.4 | 21 ± 4.24** | 18 ± 0.5** | 1.26 ± 0.1* | 1.02 ± 0.04* |
Data is presented as mean ± SD of two separate experiments. p value in comparison to infected control is shown as p < 0.05 (# statistically significant), p < 0.005 (* statistically highly significant), p < 0.0005 (** statistically extremely significant)
The amount of total bilirubin was found to be 0.65 ± 0.37 mg/dl in G-1 which increased to 1.04 ± 0.52 mg/dl in infected control (G-2). In G-3, the concentration of bilirubin was found to be comparable to the infected level with no statistically significant difference i.e. 0.96 ± 0.40 mg/dl whereas, it increased up to 1.70 ± 0.92 mg/dl in G-4 on day 7. In G-6 also it increased up to 1.86 ± 0.45 mg/dl on day 7 when compared to the infected control. Decreased level of bilirubin was detected in G-8 and G-9 on day 7 and 28 as compared to infected control but in AS + SP treated mice, the level of total bilirubin was found to be more on day 7 (1.4 ± 0.4 mg/dl) which decreased to 0.8 ± 0.4 mg/dl by day 28, however, both the levels were more than the normal value.
Kidney function test
In normal mice (G-1), the concentration of urea in serum was found to be 32.0 ± 1.05 mg/dl which increased to 62.6 ± 2.30 mg/dl in infected control (G-2). In G-3 the concentration of urea in serum increased to 64.4 ± 0.56 mg/dl on 7th day post inoculation but, in G-4 the concentration of urea was found to be decreased as compared to infected i.e. 41.6 ± 2.26 mg/dl (p < 0.0005). In the groups treated with AS monotherapy, the concentration of urea on day 7 was found to be more than the infected control i.e. 79.4 ± 4.51 mg/dl (p < 0.0005). Whereas, in groups treated with combination of china ϕ/30 with 100 mg/kg of AS, the levels of urea were found to decline significantly on both the days when compared to the infected control. Both the values were comparable to the normal level. In G-10 (AS + SP) the amount of urea on day 7 was 21 ± 4.24 mg/dl which decreased to 18 ± 0.5 mg/dl on day 28. Both the values were extremely statistically significant (p < 0.0005) when compared to the infected control (Table 2).
In normal mice, the creatinine level was found to be 0.9 ± 0.2 mg/dl whereas, in G-2 the creatinine level increased up to 2.25 ± 0.7 mg/dl. In G-3 (china ϕ) the creatinine level was 0.78 ± 0.1 mg/dl (p < 0.0005), but in G-4, it was slightly elevated to 1.7 ± 0.02 mg/dl. However, it significantly reduced to 0.8 ± 0.2 mg/dl (p < 0.0005) by day 28. In G-6, the creatinine levels on day 7 was comparable to the levels in infected group i.e. 2.05 ± 0.34 mg/dl, however, it declined to 1.02 ± 0.5 mg/dl (p < 0.005) by day 28. In G-10, the concentration of creatinine were significantly lower (p < 0.005) than the infected and comparable to normal levels on both day 7 (1.26 ± 0.1 mg/dl) and day 28 (1.02 ± 0.04 mg/dl).
Discussion
Plasmodium berghei (NK-65) has again been confirmed to be lethal to albino mice of BALB/c strain. All the mice, injected with 1 × 106P. berghei infected RBCs, died by day 7 post inoculation, due to heavy infection in infected as well as vehicle treated groups. The infection was observed to increase continuously in vehicle treated groups (G-5) showing that there is no placebo effect against P. berghei infection as a result of which all the mice died within 7 days due to heavy infection. A placebo is an inert substance (e.g. sugar pill, alcohol, saline injection) or sham physical/electrical manipulation that is believed to have no chemical, electrical, or physical effect on the patient (Arnstein 2003). It was confirmed that china 30 potency in monotherapy was capable of complete clearance of parasite with survival of mice up to 1 month follow up period. In earlier studies, monotherapy of china ϕ was also reported to have statistically extremely significant effect on the reduction of P. berghei infection in BALB/c mice (Rajan and Bagai 2011). China is also recommended in homeopathic medical repertories against recurring, relapsing and intermittent type of malaria (Robert et al. 2001; Kent 1989). The present study is also in accordance with the homeopathic doctrine of centesimal dilutions, which generally believes that ‘higher the potency (dilutions) stronger is the effect (Hahnemann 1960).
Artesunate monotherapy (100 mg/kg) completely cleared the parasitaemia by day 7 but recrudescence occured by day 21. In a previous study the maximum cumulative dose of oral artesunate used was 600 mg which was given over 5 days, but it was followed by a recrudescence (Bonginkosi et al. 2003). 14 days treatment with artesunate (100 mg/kg) was reported to completely prevent parasitaemia and enhance survival up to 60 days. No toxicity was observed at a total dose of artesunate of 1400 mg/kg given over 14 days in same study (WHO 2005). In the present study, combination therapy of artesunate (100 mg/kg) with china (ϕ/30) led to complete clearance of parasite and absence of recrudescence during the follow up period. The survival rate has also been 100 %. The results were in comparison to the positive control group treated with standard antimalarial combination of AS + SP. In earlier studies also, the administration of AS + SP to P. berghei infected mice was found effective with mean parasitaemia reduction of 100 % by day 7 (Georgewill and Ebong 2012).
In another study the homeopathic medicine eucalyptus ϕ was found to be effective in combination with artesunate in clearing P. berghei infection in BALB/c mice. It also protected recrudescence caused by AS monotherapy up to 1 month follow up period (Bagai et al. 2008). It has also been reported by WHO, that the artesunate is much effective in combination therapies (CT) as compared to artesunate alone which leads to the problem of recrudescence because of its short half life period (WHO 2004).
Hydrolytic enzymes play an important part in digestion and elimination of pathogen from host. Baheti et al. (2003) have reported increased ALP activity in infected red blood cells. Present study confirms that parasite alters the enzyme activity of host tissue as P. berghei infected mice were observed to record 3-fold rise in serum ALP activity. It was reported earlier also that liver integrity is compromised during malaria infection, as reflected by increased level of alkaline phosphatase and bilirubin (Ibrahim and Ubon 2005). The elevation in serum ALP activity is commonly observed during malaria, which is an indication of hepatocytes damage leading to leakage of this enzyme into blood (Noppadon et al. 2006). In case of P. berghei infected mice (G-2) the amount of total bilirubin increased slightly to 1.04 + 0.52 g/dl. Bilirubin levels ≥6 mg%) and hyperbilirubinaemia (6–38 mg%) has been reported (Baheti et al. 2003).
In present study, the amount of total bilirubin and ALP activity was found to be normal on both day 7 and 28 confirming that ACT with china (ϕ/30) does not harm the integrity of liver. Bilirubin in serum is estimated in order to assess the extent of liver damage (Garba et al. 2006). Elevated Bilirubin levels also indicate liver disease or bile duct blockage because, RBCs are being broken down too fast for the liver to process (Aikawa et al. 1980). The increased bilirubin level in AS + SP treated group as compared to infected control indicated jaundice like symptoms pointing towards impairment of liver function after the treatment with standard drug combination.
The bilirubin levels were comparable to normal in the combination therapy treated groups indicating the normal functioning of liver and confirming that artesunate and china act in synergy to protect liver against parasite when given in combination. It has also been reported that elevated levels of serum bilirubin and ALP returns to normal within few weeks after treatment with therapies based on artemisinin derivatives (Das et al. 2007).
Quantitative estimation of urea and creatinine was also done to check the affect of different therapies on kidney function of host. In infected control group, the concentration of urea was found to be increased (62.6 ± 2.30 mg/dl). In case of Ch 30 monotherapy the level of urea reduced in comparison to infected. In case of combination therapies, the concentration of urea was also observed to be significantly (p < 0.0005) reduced as compared to infected control showing that the combination of artesunate and china does not harm the functioning of kidneys. It is also reported in earlier studies that creatinine concentration does not get altered unless the kidney function is reduced more than 50 % (Sitprija 1988). This indicates that the monotherapy and combination therapy used in the present study are not nephrotoxic.
Present study points to the better efficacy of AS + china (ϕ/30) in the clearance of P. berghei infection in BALB/c mice when given orally for 7 days. It was further confirmed by liver and kidney function tests that the combination does not harm the functional organization of these organs which are otherwise badly affected during malarial infection. However, it was confirmed that artesunate in monotherapy causes recrudescence within 3 weeks of treatment. More clinical studies are required to establish homeopathic preparation of Cinchona officinalis (china) as combination partner for artesunate against human malaria parasite.
Acknowledgments
The authors are grateful to UGC-CAS programme of the Department of Zoology, Panjab University, Chandigarh for financial support. Ms Aswathy Rajan is thankful to University Grants Commission, New Delhi, India for award of Rajiv Gandhi National Fellowship.
References
- Aikawa M, Suzuki M, Guttierrez Y. Pathology of malaria. In: Krier JP, editor. Malaria. New York: Academic Press; 1980. pp. 47–102. [Google Scholar]
- Arnstein PRN. The placebo effect. Semin Integr Med. 2003;1(3):125–135. doi: 10.1016/S1543-1150(03)00026-7. [DOI] [Google Scholar]
- Bagai U, Rajan A, Chandel S (2008) Efficacy of Eucalyptus (a homeopathic medicine) in combination with artesunate to clear Plasmodium berghei infection in Balb/C mice. In: Veena Tandon, Arun K Yadav, Bishnupada Roy (eds) Current trends in parasitology, Proceedings, 20th National Congress of Parasitology, November 3–5, 2008 NEHU Shillong India, 2008 pp 203–211
- Baheti R, Laddha P, Gehlot RS. Liver involvement in falciparum Malaria: a histopathological analysis. JIACM. 2003;4(1):34–38. [Google Scholar]
- Bangchang KN, Congpuong B. Current malaria status and distribution of drug resistance in East and Southeast Asia with special focus to Thailand. Tohoku J Exp Med. 2007;211:99–113. doi: 10.1620/tjem.211.99. [DOI] [PubMed] [Google Scholar]
- Bonginkosi G, Peter F, Bernhard R. Oral artesunate prevents Plasmodium berghei ANKA infection in mice. Parasitol Int. 2003;52:53–59. doi: 10.1016/S1383-5769(02)00081-8. [DOI] [PubMed] [Google Scholar]
- Das SN, Mohapatra B, Mohanty R, Dash PC, Kar K, Dash P. Malarial hepatitis as a component of multiorgan failure: a bad prognostic sign. J Indian Med Assoc. 2007;105(5):247–250. [PubMed] [Google Scholar]
- Garba IH, Gatsing D, Ubon G. Elevated total and isoenzyme forms of acid phosphatase in falciparum malaria. Comtes Rendus Biologies. 2006;329:75–78. doi: 10.1016/j.crvi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Georgewill UO, Ebong OO. A comparative study on the efficacy of some artemisinin combination therapies on Plasmodium berghei in swiss albino mice. Pharmacol Pharma. 2012;3:109–112. doi: 10.4236/pp.2012.31016. [DOI] [Google Scholar]
- Hahnemann S (1960) In: Organon of medicine. First Indian edn, M. Bhattacharyya and Co., Calcutta, pp 1–314
- Ibrahim HG, Ubon G. Serum alkaline phosphatase activity as a potential biomarker for the integrity of the hepatic drainage system in acute falciparum malaria infection. Internet J Infect Dis. 2005;ISSN:1528–8366. [Google Scholar]
- Kent JT (1989) Repertory of the homeopathic Materia Medica. B. Jain publishers (P) Ltd, New-Delhi, ISBN 81-7021-083-6 pp 1–253
- Khuda-Bukhsh AR. Mice as a model for homeopathy research. Homeopathy. 2009;98:267–279. doi: 10.1016/j.homp.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Lessell C. Handbook of the homeopathic traveler’s manual. Bromley: Winter Press; 1993. pp. 21–33. [Google Scholar]
- Müller O, Sié A, Meissner P, Schirmer RH, Kouyaté B. Artemisinin resistance on the Thai-Cambodian border. Lancet. 2009;374(9686):266–277. doi: 10.1016/S0140-6736(09)61857-2. [DOI] [PubMed] [Google Scholar]
- Noppadon T, Thanachartwet V, Krudsood S, Luplertlop N, Pornpininworakij N, Chalermrut K, Phokham S, Kano S, Looareesuwan S, Wilairatana P. Minor liver profile dysfunction in Plasmodium vivax, P. malariae and P. ovale patients and normalization after treatment. Korean J Parasitol. 2006;44(4):295–302. doi: 10.3347/kjp.2006.44.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orerell C, Little F, Smith P. Pharmakokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur J Clin Pharmacol. 2008;64:683–690. doi: 10.1007/s00228-007-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Bagai U. SEM studies on blood cells of Plasmodium berghei infected Balb/c mice treated with artesunate and homeopathic medicine china. J Parasit Dis. 2011;35(2):134–139. doi: 10.1007/s12639-011-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Benoil-Vical F, Carbarat DO. From classical antimalarial drugs to new compounds based on the mechanism of action of artemisinin. Pure Appl Chem. 2001;73(7):1173–1188. doi: 10.1351/pac200173071173. [DOI] [Google Scholar]
- Robin Murphy ND (2004) Homeopathic medical repertory. B Jain publishers (P) Ltd. New-Delhi, ISBN 81-8056-079-1, pp 1–1950
- Santiyanont R (1985) Parasite identification, counting and staining: Application of genetic engineering techniques in tropical diseases, pathogens with special reference to plasmodia. A laboratory manual of selected techniques. UNDP/World Bank/WHO special program for research and techniques in tropical diseases, Bangkok Thailand, 8.7, pp 413–448
- Saxena R (2006) Role of homeopathy in infectious diseases (philosophy and therapeutics). B. Jain publishers (P) Ltd, New-Delhi, ISBN 81-8056-701-X, pp 1–181
- Shah H (1999) Malaria and homeopathy. Mayur Jain. Indian Books and Periodicals Publishers, ISBN 81-7467-057-2, pp 1–233
- Sitprija V. Nephropathy in falciparum malaria. Kidney Int. 1988;33:867–877. doi: 10.1038/ki.1988.262. [DOI] [PubMed] [Google Scholar]
- WHO (2002) WHO traditional medicine strategy 2002–2005. Geneva, Switzerland, Report No. WHO/EDM/TRM/2002.1
- WHO (2004) First meeting of regional technical advisory group on malaria, Manesar, Haryana, 15–17 December 2004, World Health Organisation, Regional Office for South East Asia, New Delhi, SEA MAL 239, pp 1–38
- WHO (2005) Malaria risk and malaria control in Asian countries affected by the tsunami disaster. Roll Back Malaria Department, Report No. WHO/HQ
- WHO (2006) Guidelines for the treatment of malaria. Roll Back Malaria, World Health Organisation and United Nations Children Funds (UNICEF) 2005 Report No. WHO/HTM/MAL/2005.11.02
- Yarnell E, Abascal K (2004) Botanical treatment and prevention of malaria. Part 2-Selected Botanical Alt Compl Therapies, pp 277–284