Abstract
The present study reports the natural occurrence and pathomorphological alterations of Capillaria hepatica infection alone and in concurrence with Cysticercus fasciolaris infection in the liver of Bandicota bengalensis. Out of the eighteen mature male B. bengalensis autopsied, livers of eight rats (44.4 %) were found infected with parasites comprising two (11.1 %) rats infected with C. hepatica alone, four (22.2 %) infected with C. fasciolaris alone and two (11.1 %) infected with C. hepatica in concurrence with C. fasciolaris. Gross lesions comprising of pale cystic areas or streaks on the surface of liver in rats revealed the presence of eggs of C. hepatica scattered in the parenchyma of the liver. Histologically, granulomatous reaction around the eggs, adult worms and dead components of parasites were observed.
Keywords: Bandicota bengalensis, Capillaria hepatica, Concurrent infection, Cysticercus fasciolaris, Rodents
Introduction
Rodents play a significant role in public health, chiefly due to their role as carriers or reservoirs of microbes and parasites of zoonotic importance. Thus, they pose a potential risk to the health of humans and domestic animals. Increased rodent population in an area can be directly related to the increased zoonotic diseases in human population (Bradshaw 1999). The lesser bandicoot rat, Bandicota bengalensis is the predominant rodent pest species found throughout India including Punjab state. In addition to causing huge losses to food grains, it is also a major source of transmission of parasitic infections to humans (Singla et al. 2008).
Capillaria hepatica is a nematode found in the liver of a wide range of mammalian species. The distribution of C. hepatica is ubiquitous and has been recorded throughout Europe, North and South America, Africa, Asia, and Australia (Levine 1968; Spratt and Singleton 2001). The usual hosts for the adult parasite are rodents, particularly rats and mice (Kelly 1992), but infections have also been recorded in other animals including the dog (Lloyd et al. 2002), the horse (Munroe 1984), the rabbit (Mowat et al. 2009) and the man (Spratt and Singleton 2001; Krauss et al. 2003). C. hepatica is the only nematode parasite that inhabits the liver of the host during adult stage of the life cycle (Kelly 1992). The female worm deposits clusters of eggs in the liver parenchyma, which become encapsulated as a result of the chronic inflammatory response of the host and do not pass out in the faeces. Humans can be infected by ingesting water or foods contaminated with embryonated eggs or larvae of C. hepatica previously released from the host liver through cannibalism, predation or decomposition of carcasses (Cameron 1956; Farhang-Azad 1977; Spratt and Singleton 2001). The first human case of hepatic capillariasis, one of the fatal zoonotic infections (Sun 1999) was reported in Korea (Choe et al. 1993). The disease, however, usually remains misdiagnosed or undetected (Slais 1974).
Cysticercus fasciolaris, the metacestode of Taenia taeniaeformis, is found in the liver of rodents in the form of cysts (Soulsby 1982). C. fasciolaris infection is clinically asymptomatic and is considered harmless (Singla et al. 2003). The adult worm is frequently encountered in the small intestine of the cat and wild felids and occasionally in the dog and other carnivores.
The present study reports the natural occurrence of C. hepatica infection alone and in concurrence with C. fasciolaris in the liver of B. bengalensis and the pathomorphological alterations thereof.
Materials and methods
Eighteen mature male B. bengalensis were live trapped from premises near railway station at Ludhiana, Punjab. In the laboratory, rats were kept individually in cages of size 23″ × 13″ × 13″ with food and water provided ad libitum. Food consisted of cracked wheat smeared with powdered sugar and groundnut oil in ratio 96:2:2. No bedding material was provided to rats. The rats were kept and maintained as per the guidelines of Institutional Animal Ethics Committee. After about 5–6 months of collection, rats were autopsied. Lesions comprising pale cystic areas or streaks randomly scattered on the surface of the liver (Fig. 1) along with whitish cysts on or around the liver were found in some rats. A microscopic examination of cystic area of liver tissue was performed by tissue press technique for identifying the infection. The liver of these animals were removed, fixed in 10 % formal saline solution and processed into paraffin wax (Humason 1979). Sections (6 μ) were routinely cut, stained with hematoxylin and eosin (H&E) and studied under light microscope.
Fig. 1.

Liver of B. bengalensis showing yellowish white patches or streaks on the liver. (Color figure online)
Results and discussion
During necropsy investigations, gross lesions comprising of irregular pale cystic areas or streaks were found randomly scattered on the liver surface (Fig. 1) of four animals (22.2 %). On further investigation, it became apparent that these animals were infected with C. hepatica. The eggs of C. hepatica accumulate in liver in the form of irregular white or yellow patches and streaks on the external surface (Gotardo et al. 2000). Out of the four animals infected with C. hepatica, two (11.1 %) were also having an oval whitish cyst on the liver which contained C. fasciolaris. In addition to this, another four animals (22.2 %) were found infected only with C. fasciolaris out of which, three were having single cysts on the liver and one having a bunch of ten small cysts hanging from the liver. Size of these cysts varied from 2 to 4 mm. Their identity was revealed upon opening the cysts, when the larvae burst out. Larvae were opaque white and immature. Their length averaged 22.8 ± 0.7 mm.
In overall, 44.4 % rats were having liver infected with parasites. 11.1 % were having infection of C. hepatica alone, 22.2 % having infection of C. fasciolaris alone and 11.1 % having concurrent infection of C. hepatica and C. fasciolaris. Prior to necropsy, the animals had exhibited no adverse clinical signs. Claveria et al. (2005) reported 100 % infection of Rattus spp. with C. hepatica and C. fasciolaris in Philippines. C.hepatica infections and their concurrence with C. fasciolaris have been reported in India from Bandicota indica and Rattusrattus (Anantaraman 1966; Bhattacharya et al. 1998; Raut et al. 2003). Concurrent infections with these two parasites do not appear to have been reported previously in B. bengalensis.
In the present study, massive deposition of eggs was found in the liver tissue of rats infected with C. hepatica. Typical eggs with bipolar caps were found scattered in the parenchyma of the liver and granulomatous reaction was seen around them giving rise to several micro granulomas or coalesced macrogranulomas (Fig. 2). The micro granulomas consisted of aggregates of eggs surrounded by mature fibrous connective tissue containing a few lympho mononuclear cells (Fig. 3). Additionally, the surrounding parenchyma showed granular degeneration. Average size of eggs was found to be 40–55 μm in length and 25–35 μm in breadth. Li et al. (2010) measured the C. hepatica egg to be about 48–66 × 28–36 μm and Wright (1961) measured them to be 54 × 31 μm on average. Adult and gravid female worms with eggs in the uterus (Figs. 4, 5) along with dead and disintegrating parts of adult worms were also observed in the liver tissue sections in present studies. The inflammatory reaction around the adult parasites consisted of macrophages and only few eosinophils. The adjacent parenchyma revealed congestion, necrosis and micro granulomas (Fig. 4). In general, the histopathologic response was more profound surrounding the dead C. hepatica stages (Fig. 6).
Fig. 2.
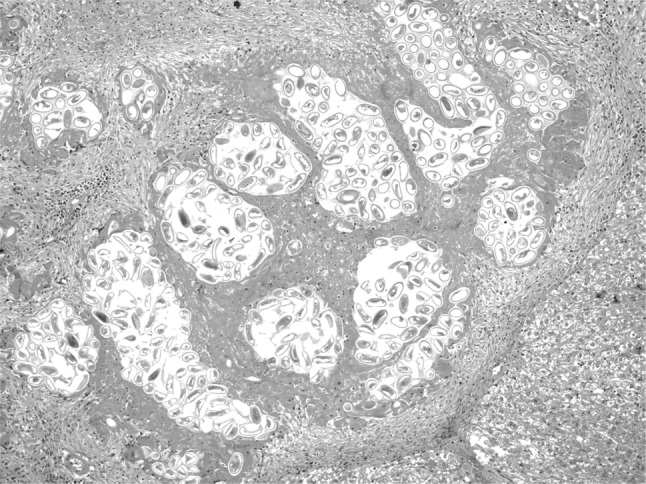
Section of liver showing multiple coalescing micro granulomas containing numerous eggs of C. hepatica forming an encapsulated macro granuloma (H&E ×100)
Fig. 3.
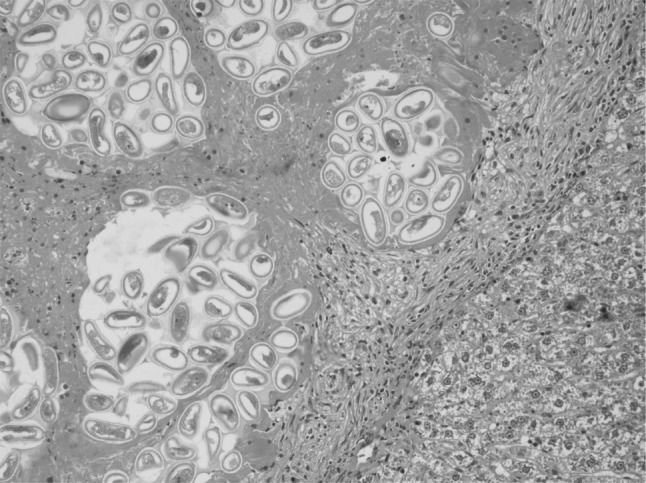
Higher magnification of the Fig. 2 showing changes and live and degenerating eggs with polar plugs more clearly (H&E ×200)
Fig. 4.
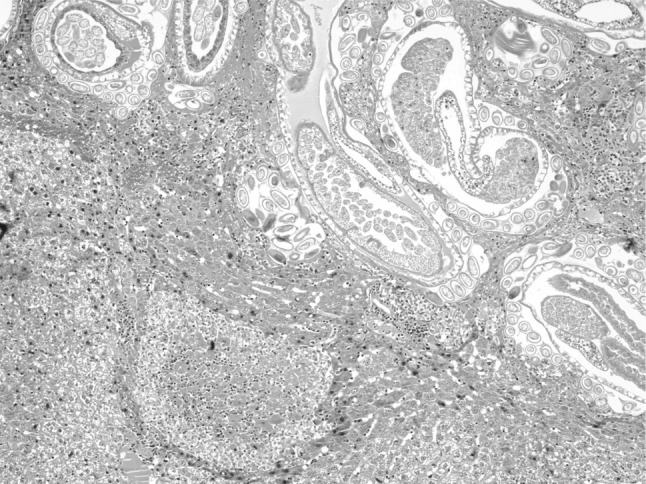
Section of liver showing adult C. hepatica and the surrounding parenchyma showing congestion, necrosis and micro granuloma (H&E ×100)
Fig. 5.
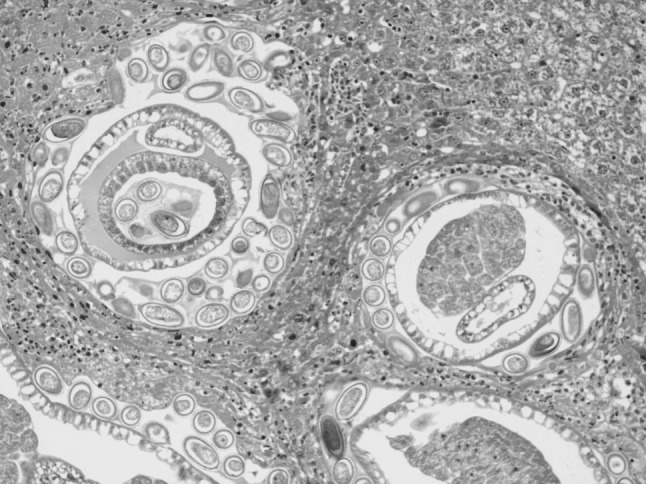
Adult and gravid female C. hepatica containing eggs in uterus and surrounded by mixed population of inflammatory cells including eosinophils (H&E ×200)
Fig. 6.
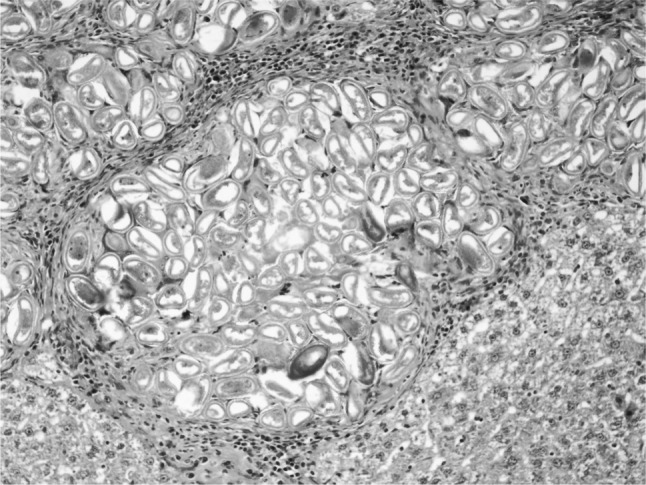
Numerous live and dead eggs of C. hepatica showing more pronounced inflammatory and immune response around them (H&E ×200)
After ingestion by the definitive host, the embryonated eggs hatch in the small intestine or caecum (Levine 1968), penetrate the intestinal mucosa and pass to the liver via the portal or mesenteric veins within 2–3 days post infection. The final moulting of the larva to produce the male or female adults occurs at 18–20 days post-infection (Spratt and Singleton 2001). The female worms after fertilization, deposit eggs in the liver and subsequently, the adult worms start dying and disintegrating. The adult male parasites are reported to die approximately 40 days post-infection and adult females about 60 days after infection (Wright 1961). Clear cut presence of gravid females containing eggs in the uterus in present studies suggest that the infection of the animals might have occurred some weeks earlier. But since the rats were autopsied after about 5–6 months of their collection from the areas near the railway station and there were no chances of infection in laboratory (because the rats were kept individually and no bedding material was provided), these rats might have ingested the embryonated eggs before being trapped. Thus the reason for the presence of gravid female worms in present studies after about 5–6 months of infection is not known.
In two rats, containing both cysts and yellowish streaks on the liver, concurrent infection of C. hepatica with C. fasciolaris was detected. Size of the cysts of C. fasciolaris was 10 and 12 mm in diameter and each cyst contained a single live characteristic strobilocercus larva. Tyagi and Mishra (1978) observed the size of the cyst of C. fasciolaris to be 2–20 mm. Histopathologically, the larva of C. fasciolaris was encapsulated by connective tissue capsule (Fig. 7). No reaction was seen around the cyst of C. fasciolaris whereas, little inflammatory reaction was observed around live and dead eggs of C. hepatica. The adjacent parenchyma had a micro granuloma possibly due to dead parasitic components (Figs. 7, 8). From the present study the overall observation was that the adult parasites of C. hepatica evoked minimal inflammatory and immune response followed by more response by live eggs and the maximum response induced by dead stages of the parasites.
Fig. 7.
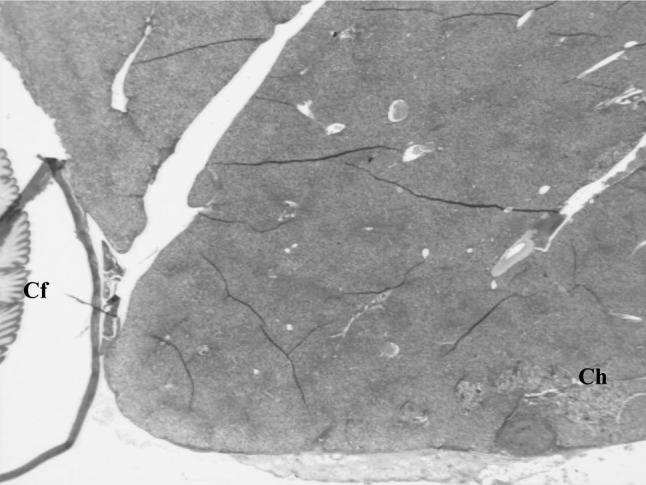
Over all view of liver of B. bengalensis showing concurrent infection of C. hepatica (Ch) and C. fasciolaris (Cf) (H&E ×40)
Fig. 8.
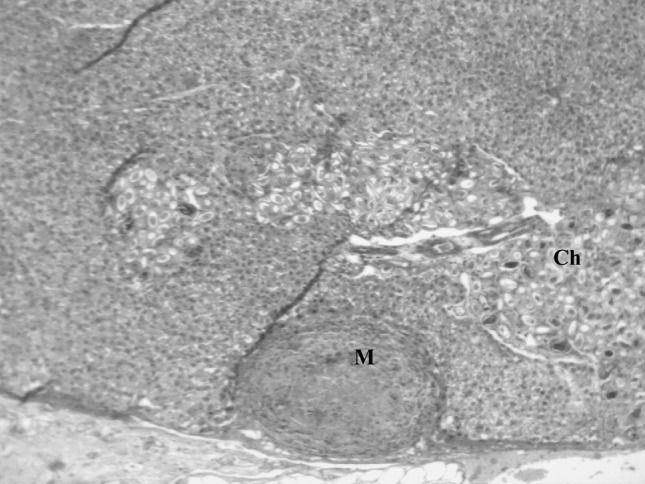
Higher magnification of Fig. 7 showing live and dead eggs of C. hepatica (Ch) and a micro granuloma (M) in the adjacent parenchyma possibly having dead parasitic component (H&E ×100)
Despite the marked hepatic tissue damage owing to severe capillariasis and strobilocercus larval infection in present studies, all the rats appeared healthy and active, suggesting a well established rat host-parasite relationship. The co-existence of rats with diverse parasitic species is reflective of host’s capability to support parasites behavioural, physiological and developmental needs (Claveria et al. 2005). Keeping in view the great potential of adult female C. hepatica worms for production of large number of eggs and their zoonotic importance, it is necessary to prevent the incidence of this disease by taking proper sanitation and rodent control measures around animal and human dwellings.
Acknowledgments
Authors are thankful to Indian Council of Agricultural Research, New Delhi, India for providing financial support.
References
- Anantaraman M (1966) Parasites in indian rodents, with special reference to disease in man and animals. In: Proceedings of Indian rodent symposium, Calcutta, p 8–11
- Bhattacharya D, Sikadar A, Sarma U, Ghosh AK, Biswas G. Concurrent infection of Capillaria hepatica and Cysticercus fasciolaris in rat (Rattus rattus): a preliminary note. Indian Vet J. 1998;75:486. [Google Scholar]
- Bradshaw J. Know your enemy. Environ Health. 1999;107:126–128. [Google Scholar]
- Cameron TWM. Parasites and parasitism. London: Methuen; 1956. [Google Scholar]
- Choe G, Lee HS, Seo JK, Chai JY, Lee SH, Eom KS, Chi JG. Hepatic capillariasis: first case report in the republic of Korea. Am J Trop Med Hyg. 1993;48:610–625. doi: 10.4269/ajtmh.1993.48.610. [DOI] [PubMed] [Google Scholar]
- Claveria FG, Causapin J, de Guzman MA, Toledo MG, Salibay C. Parasite biodiversity in Rattus spp. caught in wet markets. South Asian J Trop Public Health. 2005;36:146–148. [PubMed] [Google Scholar]
- Farhang-Azad A. Ecology of Capillaria hepatica (Bancroft 1893) (Nematoda). II. Egg-releasing mechanisms and transmission. J Parasitol. 1977;63:701–716. doi: 10.2307/3279576. [DOI] [PubMed] [Google Scholar]
- Gotardo BM, Andrade RG, Andrade ZA. Hepatic pathology in Capillaria hepatica infected mice. Rev Soc Bras Med Trop. 2000;33:341–346. doi: 10.1590/S0037-86822000000400002. [DOI] [PubMed] [Google Scholar]
- Humason GL. Animal tissue techniques. 4. San Francisco: W H Freeman & Co; 1979. [Google Scholar]
- Kelly WR. The liver and biliary system. In: Jubb KVF, Kennedy PC, Palmer N, editors. Pathology of domestic animals. 4. London: Academic Press; 1992. [Google Scholar]
- Krauss H, Schiefer HG, Weber A, Slenczka W, Appel M, von Graevenitz A, Enders B, Zahner H, Isenberg HD, editors. Zoonoses: infectious diseases transmissible from animals to humans. 3. Washington DC: ASM Press; 2003. pp. 216–217. [Google Scholar]
- Levine ND. Nematode parasites of domestic animals and man. Minneapolis: Burgess Publishers; 1968. [Google Scholar]
- Li CD, Yang HL, Wang Y. Capillaria hepatica in China. World J Gastroenterol. 2010;16:698–702. doi: 10.3748/wjg.v16.i6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Elwood CM, Smith KC. Capillaria hepatica (Calodium hepaticum) infection in a British dog. Vet Rec. 2002;151:419–420. doi: 10.1136/vr.151.14.419. [DOI] [PubMed] [Google Scholar]
- Mowat V, Turton J, Stewart J, Lui KC, Pilling AM. Histopathological features of Capillaria hepatica infection in laboratory rabbits. Toxicol Pathol. 2009;37:661–666. doi: 10.1177/0192623309339501. [DOI] [PubMed] [Google Scholar]
- Munroe GA. Pyloric stenosis in a yearling with an incidental finding of Capillaria hepatica in the liver. Equine Vet J. 1984;16:221–222. doi: 10.1111/j.2042-3306.1984.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Raut CG, Gengaje BB, Nipunge SV, Rajarshi MP, Vaidya SR, Rane SR, Pol SS, Kohale KN. Capillaria hepatica infection in a bandicoot rat (Bandicota indica) J Vet Parsitol. 2003;17:71–72. [Google Scholar]
- Singla LD, Singla N, Parshad VR, Sandhu B, Singh J. Occurrence and pathomorphological observations on Cysticercus fasciolaris in bandicoot rats in India. In: Singleton GR, Hinds LA, Krebs CJ, Spratt DM, editors. Rats, mice and people: rodent biology and management. Australia: ACIAR; 2003. pp. 57–59. [Google Scholar]
- Singla LD, Singla N, Parshad VR, Juyal PD, Sood NK. Rodents as reservoirs of parasites in India. Integr Zool. 2008;3:21–26. doi: 10.1111/j.1749-4877.2008.00071.x. [DOI] [PubMed] [Google Scholar]
- Slais J. Notes on the differentiation of Capillaria hepatica and visceral larva migrans. Folia Parasitol. 1974;21:95. [PubMed] [Google Scholar]
- Soulsby EJL. Helminthes, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. p. 116. [Google Scholar]
- Spratt DM, Singleton GR. Hepatic capilaariasis. In: Davis JW, Samuel WM, Pybus MJ, Alan Kocan A, editors. Parasitic diseases of wild animals. 2. Ames: Lowa State University Press; 2001. pp. 365–379. [Google Scholar]
- Sun T. Parasitic disorders: pathology, diagnosis and management. 2. Baltimore: Williams and Wilkins; 1999. [Google Scholar]
- Tyagi AP, Mishra SD. Occurrence of Taenia taeniaeformis cyst in wild rats. Rattus rattus. Indian J Parasitol. 1978;2:169. [Google Scholar]
- Wright KA. Observations on the life cycle of Capillaria hepatica (Bancroft, 1893) with a description of the adult. Can J Zool. 1961;38:167–182. doi: 10.1139/z61-022. [DOI] [Google Scholar]


