Abstract
Visceral leishmaniasis (VL) is endemic in the tropical, sub-tropical regions of Asia, Africa, the Mediterranean, Southern Europe and South and Central America. Approximately 500,000 new cases are reported annually. Classically the diagnosis of VL is confirmed by the demonstration of the parasite in aspirates of spleen, bone marrow or liver which can yield false negative results, also these methods are invasive. In this study, we aimed to evaluate the serodiagnostic potential of two heat shock proteins (Hsps) i.e. Hsp70 and Hsp83 in combination by ELISA and western blotting in mouse model. Parasite proteins were separated by SDS-PAGE and transferred to PVDF membranes which were further incubated with serum samples of infected mice for visualizing different bands. Both the bands i.e. Hsp70 and Hsp83 were simultaneously visualized in all infected groups on different post infection days. The presence of both the antigens was also detected by sandwich ELISA. The results suggest that the simultaneous occurrence of both the antigens Hsp70 and Hsp83 may be useful in serodiagnostic assay of VL as it may reduce the need for traumatic bone marrow sampling and risky spleen aspiration.
Keywords: Hsp70, Hsp83, ELISA, Western blotting
Introduction
Since its resurgence, the increasing incidence of kala-azar remains unabated despite aggressive treatment and control measures. Routine diagnosis of the acute disease relies on classical microbiological methods like splenic/bone marrow aspirates which are cumbersome, invasive and painful (Sundar and Rai 2002). These classical methods are also limited by low sensitivity, requiring repeated tissue sampling and trained laboratory staff (Badaro et al. 1996). Frequently, false positive results are obtained when the sera from the chagas disease, leprosy, tuberculosis and malaria patients are assayed (Zijlstra et al. 1992). Therefore, there is a need to develop a more rapid, inexpensive and simple assay for diagnosis of visceral leishmaniasis (VL). Since high titres of antileishmanial antibodies are detected in the sera of VL patients, serodiagnosis may be a better alternative to the parasite detection in biopsy samples. Current serologic tests such as DAT, IFAT and ELISA use crude antigen preparations and are limited in terms of specificity as well as assay reproducibility (Kar 1995). In order to improve the specificity of the serodiagnostic tests a search for defined Leishmania-specific antigens have been undertaken and various candidates have been proposed (Ashford et al. 1992; Kar 1995; Jaffe et al. 1990). Two heat shock proteins, Hsp70 and Hsp83 have previously been reported to induce a strong cell mediated and humoral immune responses during leishmanial infections (Kaur et al. 2011a). Hsp70 is a major target of immune responses to a wide variety of pathogens including bacteria, fungi, helminths (worms) and protozoan parasites (Young and Elliot 1989; Kaufmann 1990; Young et al. 1990; Young 1990). It has been reported that parasite-derived Hsp70 plays an important role in the host-parasite interactions (Polla 1991). Hsp70 of Trypanosoma cruzi, the causative agent of chagas disease, has been reported to be a major target of humoral immunity in human infections. Despite its high degree of evolutionary conservation, antibodies are highly specific for the parasite Hsp70 and do not have cross-reactivity to the human Hsp70 (Engman et al. 1990). A sero-specific epitope has been identified in rHsp70 of Leishmania donovani (Arora et al. 2000). Hsp70 has also been found to enhance the immunogenicity of gp63 based protein against Leishmania donovani in mice (Kaur et al. 2011b). Hsp83 also has been shown to be an immunodominant antigen recognized by sera from diffuse cutaneous leishmaniasis patients (Skeiky et al. 1997). In an earlier study, Hsp83 of L. infantum has been found to be useful for serodiagnostic assays for canine leishmaniasis (Angel et al. 1996). Therefore, Hsp70 and Hsp83 might be good candidates as diagnostic antigens. Although various serologic techniques can detect active leishmaniasis when a high level of specific antibodies is present, they do not distinguish between various phases of the disease when levels of antibodies are at or near the cut-off level. Western blot analysis of whole parasite antigens is considered to be sensitive when low serum antibody titers are present (Riera et al. 2004). Western blotting is not preferred in case of cutaneous leishmaniasis because antileishmanial antibodies are present in low titers. Therefore most of the work concerning the diagnosis of leishmaniasis has been done on visceral leishmaniasis. In a study by Talmi-Frank et al. (2006)12, 14, 24, 29, 48, and 68 kDa bands were found to be immunodominant during experimental canine visceral leishmaniasis. Keeping in view the above background the present study was designed to evaluate the serodiagnostic potential of two defined Leishmania-specific antigens i.e. Hsp70 and Hsp83 in combination using sandwich ELISA and western blotting.
Materials and methods
Parasite
The Indian strain of Leishmania donovani, viz; MHOM/IN/80/Dd8, originally obtained from the London School of Hygiene and Tropical Medicine, U.K, was used for doing the present experimental work.
In vitro culture of parasites
The promastigotes of L. donovani were grown at 22 ± 1 °C in RPMI-1640 supplemented with 10 % FCS. The promastigotes were examined by wet mount preparation and the promastigotes were seen as motile, spindle shaped, fast swimming organisms with long anterior flagellum. The strain was maintained by serial subculture after every 48–72 h. The amastigotes were grown in the same medium at a temperature of 37 °C and a pH of 5.5 with 5 % CO2 and 90–95 % relative humidity.
Animals
5–6 weeks old inbred BALB/c mice of either sex, weighing 20–25 g were used for this work. These were obtained from the central animal facility NIPER Mohali, Punjab. They were fed with water and mouse feed ad libitum in the Central Animal House, Panjab University Chandigarh. The ethical clearance for conducting the experiments was obtained from the Institutional Animal Ethics Committee, Panjab University, Chandigarh, India.
Identification of promastigote antigen by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to the method of Laemmli (1970). Parasites were harvested from the culture, dissolved in sample buffer and boiled for two minutes in boiling water bath. After loading the antigen the electrophoresis was carried out at 90 V from cathode to anode till the bromophenol blue tracer reached the bottom of the running gel. In one lane, molecular weight markers were also run. The gels were stained in Coomassie blue for 4 h and then destained in order to visualize the protein bands.
Electroelution of antigen band from SDS-PAGE gels
The proteins with a molecular mass of Hsp70 and Hsp83 were visualized, localized in gels and eluted by electrophoresis in running buffer (0.025 M Tris, 0.192 M glycine, 1 % SDS) at 10 mA for 5 h. After elution the proteins were dialyzed, lyophilized, suspended in PBS and filtered for further utilization. The proteins were quantified by Lowry method (1951).
Raising of antisera in rabbit against specific antigens
20 μg of purified antigen was injected into the New Zealand white rabbit along with complete Freund’s adjuvant subcutaneously. Two booster injections of same amount of antigen in Freund’s incomplete antigen were given at an interval of 2 weeks. Western blotting was carried out to check the antisera. It was found to be positive and the rabbits were given a booster dose and bled after 1 week. The blood was kept at 4 °C for 12–14 h and then centrifuged at 5,000×g for 15 min for collection of antisera (Azazy et al. 2008).
Detection of antigens in serum samples by ELISA
ELISA for detection of antigen was done by double antibody sandwich method (Voller et al. 1976). Wells of plates were coated with antisera raised against specific antigen made up to 5–10 mg/ml in coating buffer. Coating was done at 37 °C for 5 h. Plates were washed with PBS Tween-20. Test serum diluted serially with PBS Tween-20 was added to each well. Incubation was done at 4 °C overnight. Plates were washed again. HRP conjugated antimouse immunoglobulins were then added and incubated at 37 °C for 3 h. After washing, substrate and chromogen were added and absorbance was read on an ELISA plate reader at 450 nm.
Detection of Hsp70 and Hsp83 in serum samples by western blotting
Immunoblot assay was performed according to the method of Rolland-Burger et al. (1991)with slight modifications. For analysis the leishmanial antigen run on SDS-PAGE was transferred to a PVDF membrane in transfer buffer (25 mM Tris, 192 mM glycine, 20 % methanol and 0.038 % SDS) for 90 min at 250 mA. The blots were then incubated with blocking buffer (4 % BSA 100 mM NaCl, 25 mM Tris pH 7.6, 0.1 % Tween-20) overnight at room temperature. Now the blots were incubated with sera from different groups of mice. The blots were then washed and incubated with anti-mouse IgG–HRP conjugated in blocking buffer for 1 hour at room temperature. Enzymatic activity was revealed with 3,3′-diaminobenzidine tetrahydrochloride in tris buffered saline (TBS) containing 30 % H2O2.
Results and observations
Identification and purification of proteins
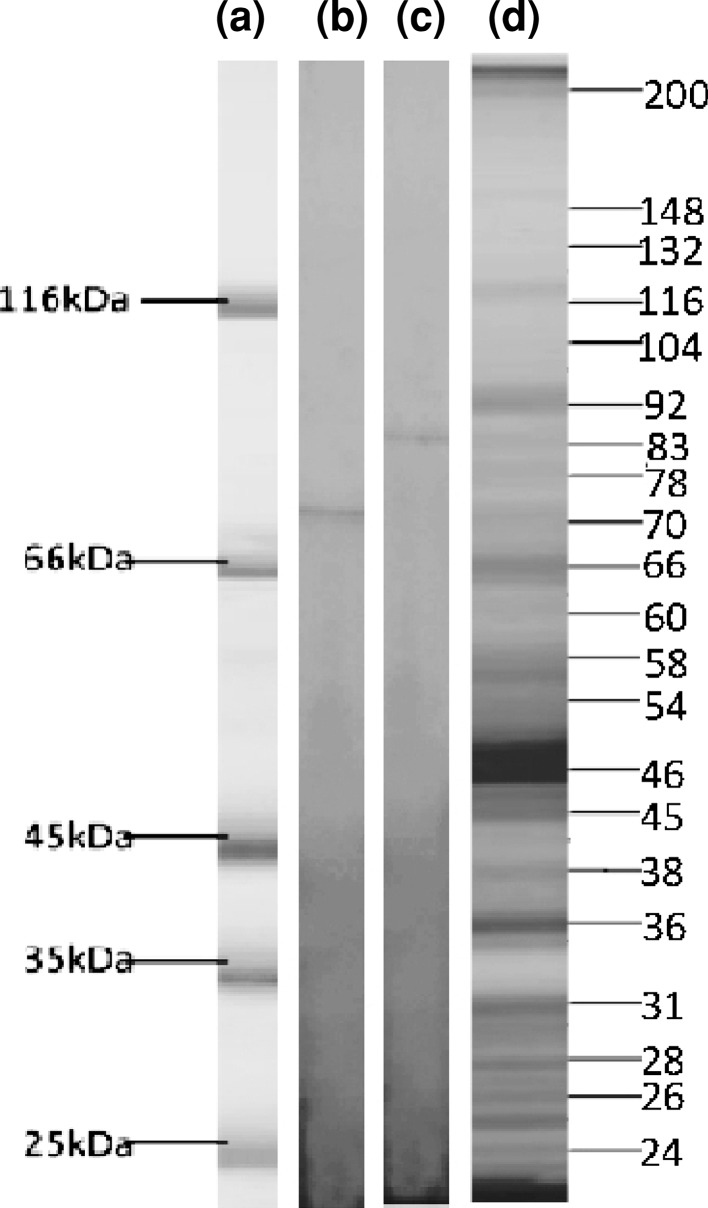
The parasite proteins were identified by running them in SDS-PAGE. After electrophoresis, the protein bands of 70 and 83 kDa were visualized (Fig. 1d). The bands were excised from the gel and submitted to electroelution (Fig. 1b, c). During electroelution a constant voltage of 50 V for 5–6 min was applied through the gel.
Fig. 1.
SDS-PAGE profile of L. donovani and electroeluted proteins. Lane (a) Molecular weight markers; Lane (b) Hsp70; Lane (c) Hsp83 and Lane (d): Parasite antigens
Sandwich ELISA
Antibodies against Hsp70 and Hsp83 were raised in rabbit by subcutaneous administration of these antigens. Two booster doses were given and blood was collected after 1 week of second booster dose and then serum was separated. The specificity of antigen was checked by western blotting. Two bands (83 and 70 kDa) were observed as depicted in Fig. 1b, c. For ELISA 96 well plate was coated with antiserum (overnight at 4 °C) raised in rabbit. Antiserum was decanted and plate was incubated with serum samples of infected groups of animals collected at different days post infection. Thereafter the plate was incubated with secondary antibody conjugated with horseradish peroxidase (HRP). After adding substrate and stop solution the absorbance was read at 450 nm.
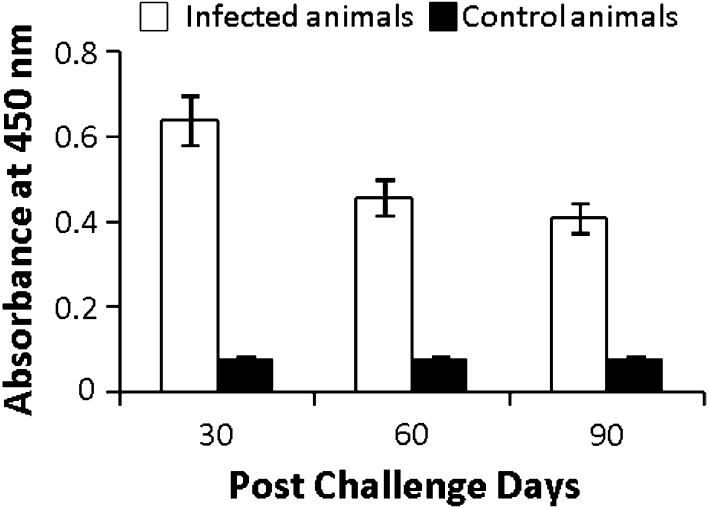
The absorbance values observed were 0.638 ± 0.059, 0.456 ± 0.042 and 0.408 ± 0.035 on 30, 60 and 90 days of post infection. The absorbance value for normal uninfected mice was 0.078 ± 0.004. The difference in absorbance values of infected and normal uninfected groups was significant (p < 0.001). The difference in absorbance values was also significant from 30 to 60 days.p.i. (p < 0.005). However it was not significant from 60 to 90 days.p.i. (Fig. 2).
Fig. 2.
Detection of Hsp83 and Hsp70 antigens in serum samples of infected mice on different days post infection by sandwich ELISA. p value: Infected mice versus normal mice *p < 0.001
Identification of Hsp83 and Hsp70 in infected mice and expression of other antigens by western blotting
The parasite proteins were separated electrophoretically using SDS-PAGE. Then they were transferred to PVDF membrane. The membrane was cut into strips. The strips were incubated with serum samples of different groups of animals. The bands appeared after incubation with secondary antibody and substrate. The antigen profiles in different groups were analyzed.
In the sera of infected control group many antigens were identified. Their molecular weight ranged from 36 to 104 kDa. The antigens which showed reactivity with secondary IgG antibody were 36, 46, 54, 58, 66, 70, 78, 83, 100 and 104 kDa. The antigenic profile was similar on different days post infection (Fig. 3).
Fig. 3.
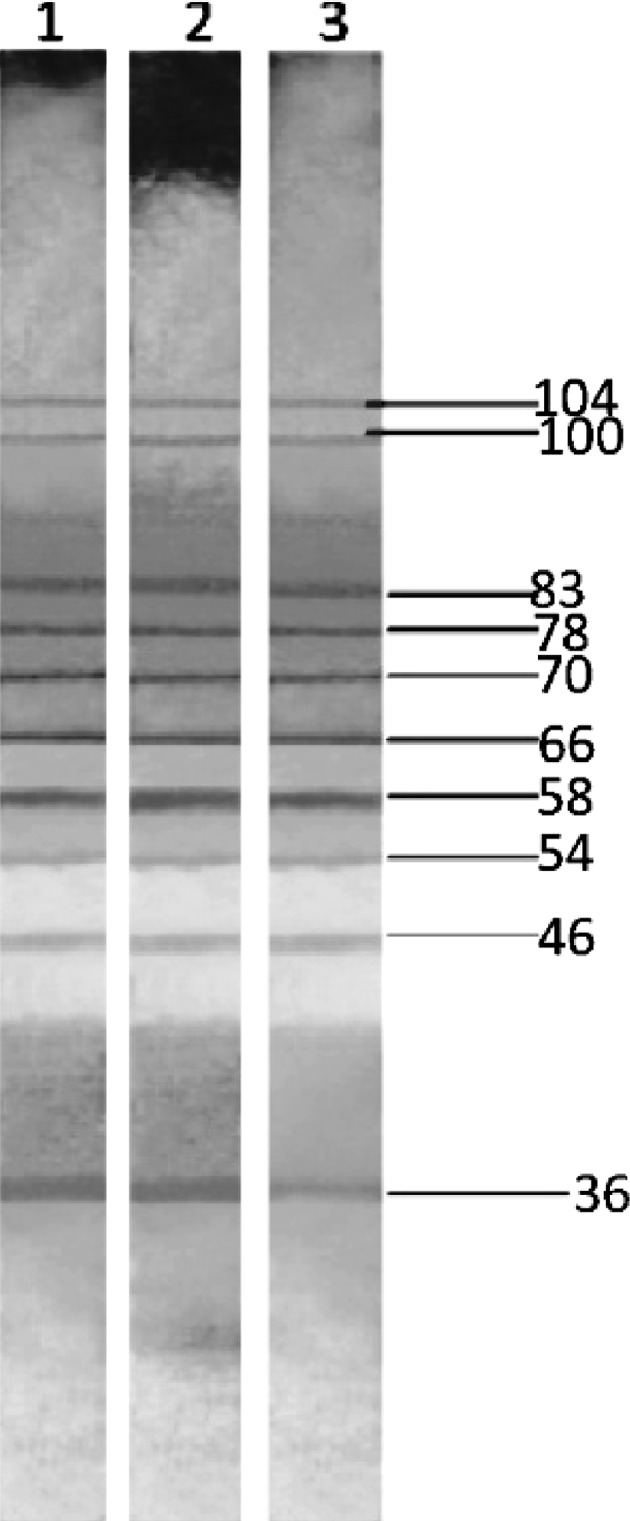
Detection of Hsp70 and Hsp83 antigens in the serum samples of infected mice on different post infection days by Western blotting. Lane 1, 2 and 3 shows blotting analysis with sera of mice on 30, 60 and 90 days post infection
Discussion
There are many challenges in the diagnosis of the leishmaniasis. Early diagnosis of the disease is difficult because the clinical and epidemiological findings in various forms of leishmaniasis resemble several other diseases. Untreated VL patients also act as a reservoir for parasites and therefore contribute to disease transmission in anthroponotic VL areas. Therefore, early case-finding is considered an essential component of VL management.
ELISA has shown high diagnostic accuracy in several studies (Iqbal et al. 2002; Sinha and Sehgal 1994; Sreenivas et al. 2002). In order to develop specific assays for the serodiagnosis of leishmaniasis, several promastigote and amastigote antigens, purified antigens such as FML, defined, synthetic peptides or recombinant antigens have been characterized and evaluated. Among the many antigens used in ELISA for the diagnosis of leishmaniasis are total soluble antigen (Hommel et al. 1978), dp72 (Jaffe and Zalis 1988), gp 63 (Okong’o-Odera et al. 1993), 78 kDa (Ravindran et al. 2004) and rK39, a recombinant and a kinesin-related protein (Burns et al. 1993). Some other antigens from L. infantum such as the acidic ribosomal proteins P2a and P2b, the ribosomal protein P0, the histones H2A (Soto et al. 1995) and H3 (Soto et al. 1996) were isolated and characterized for diagnosing canine VL. Similarly, the heat shock proteins Hsp70 and Hsp83 have shown the serodiagnostic potential against experimental murine visceral leishmaniasis.
An antigen detection test would, in principle, provide better means of diagnosis since antigen levels are expected to broadly correlate with the parasite load. Antigen detection systems are also an ideal alternative to the antibody detection systems in immunocompromised patients and more particularly with the growing number of HIV co-infected cases, especially in advanced cases where the immune response is impaired (Rosenthal et al. 1995). A latex agglutination test detecting a heat-stable, low-molecular-weight carbohydrate antigen in the urine of VL patients has shown promising results (Attar et al. 2001; Sarkari et al. 2002). Several studies conducted in East Africa and the Indian subcontinent showed good specificity but only low to moderate (48–87 %) sensitivity (Rijal et al. 2004; Sundar et al. 2005; Sundar et al. 2007). Apart from its low sensitivity, there are two practical limitations: the urine must be boiled to avoid false-positive reactions and it is difficult to distinguish weakly positive from negative results, which affects the reproducibility of the test (Chappuis et al. 2006; Rijal et al. 2004). Therefore, no satisfactory antigen detection test is currently commercially available. In the present work, an effort was made to develop an antigen detection test. For this the simultaneous occurrence of two heat shock proteins Hsp70 and Hsp83 was analyzed. A sensitivity of 100 % was observed in L. donovani model of experimental murine leishmaniasis. The absorbance value was 8.18-fold on 30 p.i.d in infected controls as compared to normal uninfected controls. However it decreased on 60 (5.850-fold) and 90 (5.23-fold) days post infection. Several other investigators have also reported heat shock proteins (Hsp) from Leishmania belonging to the 60, 70, 83, and 90 families that have been tested for the serodiagnosis of leishmaniasis. L. major Hsp 60 was tested with cutaneous leishmaniasis sera (Rey-Ladino et al. 1997) and L. braziliensis Hsp83 and Hsp70 with cutaneous, mucocutaneous and diffuse cutaneous leishmaniasis sera (Skeiky et al. 1997). In another study, two polypeptide fractions of 72–75 kDa were detected in the urine of 14 of 15 patients with visceral leishmaniasis (VL) and another fraction of 123 kDa was found in 10 of the 15 patients with 96 % sensitivity and 100 % specificity (De Colmenares et al. 1995).
Western blotting was used to identify the parasite antigens recognized by serum samples from the experimentally L. donovani infected BALB/c mice. The main advantage of western blotting over other serologic techniques that use whole Leishmania antigen is its capacity to discriminate early infections. The results showed that bands of both heat shock proteins (Hsp70 and Hsp83) were simultaneously present in all the infected animals on all post infection days. Other antigens frequently recognized were those with molecular weights of 36, 46, 54, 58, 78, 100 and 104 kDa. Therefore, the results suggest that Hsp70 and Hsp83 in combination may be considered good candidates for diagnosis of visceral leishmaniasis. Therefore, these studies must be further followed for diagnostic purposes in human patient’s sera.
Acknowledgments
The authors are thankful to University Grants Commission for providing financial assistance.
Contributor Information
Jaspreet Kaur, Email: kaurjaspreet37@gmail.com.
Sukhbir Kaur, Phone: +91-172-2541942, FAX: +91-172-2541409, Email: puzoology@yahoo.com.
References
- Angel SO, Requena JM, Soto M, Criado D, Alonso C. During canine leishmaniasis a protein belonging to the 83-kDa heat-shock protein family elicits a strong humoral response. Acta Trop. 1996;62:45–56. doi: 10.1016/S0001-706X(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Arora SK, Kaur D, Sehgal S, Datta U. Identification of sero-specific epitope of recombinant heat shock protein (HSP70) of Leishmania donovani. J Parasit Dis. 2000;24(1):21–26. [Google Scholar]
- Ashford RW, Desjeux P, Deraadt P. Estimation of population at risk of infection and number of cases of Leishmaniasis. Parasitol Today. 1992;8(3):104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Attar AJ, Chance ML, ElSafi S, Carney J, Azazy A, El Hadi M, Duorado C, Hommel M. Latex test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78:11–16. doi: 10.1016/S0001-706X(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Azazy AA, Al-Shibani LA, Mohammad Ael-S. Anti-leishmanial polyclonal antibodies to assess the performance characteristics of leishmanial antigen detection ELISA. J Egypt Soc Parasitol. 2008;38(3):1027–1036. [PubMed] [Google Scholar]
- Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR, Reed SG. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723–727. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Colmenares M, Portus M, Riera C, Gallego M, Aisa MJ, Torras S, Munoz C. Detection of 72–75 and 123 kDa fractions of Leishmania antigen in urine of patients with visceral leishmaniasis. Am J Trop Med Hyg. 1995;52:427–428. doi: 10.4269/ajtmh.1995.52.427. [DOI] [PubMed] [Google Scholar]
- Engman DM, Dragon EA, Donelson JE. Human humoral immunity to hsp70 duringTrypanosoma cruziinfection. J Immunol. 1990;144:3987–3991. [PubMed] [Google Scholar]
- Hommel M, Peters W, Ranque J, Quilici M, Lanotte G. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann Trop Med Parasitol. 1978;72:213–218. doi: 10.1080/00034983.1978.11719308. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Hira PR, Saroj G, Philip R, Al-Ali F, Madda PJ, Sher A. Imported visceral leishmaniasis: diagnostic dilemmas and comparative analysis of three assays. J Clin Microbiol. 2002;40:475–479. doi: 10.1128/JCM.40.3.475-479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe CL, Zalis M. Use of purified parasite proteins from Leishmania donovani for the rapid sero- diagnosis of visceral leishmaniasis. J Infect Dis. 1988;157:1212–1220. doi: 10.1093/infdis/157.6.1212. [DOI] [PubMed] [Google Scholar]
- Jaffe CL, Rachamim N, Sarfstein R. Characterization of two proteins from Leishmania donovani and their use for vaccination against visceral leishmaniasis. J Immunol. 1990;144:699–706. [PubMed] [Google Scholar]
- Kar K. Serodiagnosis of leishmaniasis. Crit Rev Microbiol. 1995;21:123–152. doi: 10.3109/10408419509113537. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Heat shock protein and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-J. [DOI] [PubMed] [Google Scholar]
- Kaur J, Kaur T, Kaur S. Studies on the protective efficacy and immunogenicity of Hsp70 and Hsp83 based vaccine formulations in Leishmania donovani infected BALB/c mice. Acta Trop. 2011;119:50–56. doi: 10.1016/j.actatropica.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Kaur T, Sobti RC, Kaur S. Cocktail of gp63 and Hsp70 induces protection against L. donovani in BALB/c mice. Parasite Immunol. 2011;33:95–103. doi: 10.1111/j.1365-3024.2010.01253.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Okong’o-Odera EA, Kurtzhals JA, Hey AS, Kharazmi A. Measurement of serum antibodies against native Leishmania gp63 distinguishes between ongoing and previous L. donovani infection. APMIS. 1993;101:642–646. doi: 10.1111/j.1699-0463.1993.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Polla BS. Heat shock proteins in host-parasite interactions. Immunol Today. 1991;12:A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Anam K, Bairagi BC, Saha B, Pramanik N, Guha SK, Goswami RP, Banerjee D, Ali N. Characterization of immunoglobulin G and its subclass response to Indian kala-azar infection before and after chemotherapy. Infect Immun. 2004;72:863–870. doi: 10.1128/IAI.72.2.863-870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Ladino JA, Joshi PB, Singh B, Gupta R, Reiner NE. Leishmania major-like: molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxy terminal peptide sequences. Exp Parasitol. 1997;85:249–263. doi: 10.1006/expr.1996.4137. [DOI] [PubMed] [Google Scholar]
- Riera C, Fisa R, Udina M, Gallego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102–110. doi: 10.1016/S0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Rijal S, Boelaert M, Regmi M, Karki BM, Jacguet D, Singh R, Chance ML, Chappuis F, Hommel M, Desjeux P, Van der Stuyft P, Le Ray D, Koirala S. Evaluation of a urinary antigen-based latex agglutination test in the diagnosis of kala-azar in eastern Nepal. Trop Med Int Health. 2004;9:724–729. doi: 10.1111/j.1365-3156.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- Rolland-Burger L, Rolland X, Grieve CW, Monjour L. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J Clin Microbiol. 1991;29(7):1429–1435. doi: 10.1128/jcm.29.7.1429-1435.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E, Marty P, Poizot-Martin I, Reynes J, Pratlong F, Lafeuillade A, Jaubert D, Boulat O, Dereure J, Gambarelli F, Gastaut JA, Dujardin P, Dellamonica P, Cassuto JP. Visceral leishmaniasis and HIV-1 co-infection in southern France. Trans R Soc Trop Med Hyg. 1995;89:159–162. doi: 10.1016/0035-9203(95)90476-X. [DOI] [PubMed] [Google Scholar]
- Sarkari B, Chance M, Hommel M. Antigenuria in visceral leishmaniasis: detection and partial characterisation of a carbohydrate antigen. Acta Trop. 2002;82:339–348. doi: 10.1016/S0001-706X(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Sehgal S. Comparative evaluation of serological tests in Indian kala-azar. J Trop Med Hyg. 1994;97:333–340. [PubMed] [Google Scholar]
- Skeiky YA, Benson DR, Costa JL, Badaro R, Reed SG. Association of Leishmania heat-shock protein 83 antigen and immunoglobulin G4 antibody titers in Brazilian patients with diffuse cutaneous leishmaniasis. Infect Immun. 1997;65:5368–5370. doi: 10.1128/iai.65.12.5368-5370.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, Requena JM, Quijada L, Garcia M, Guzman F, Patarroyo ME, Alonso C. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol Lett. 1995;48:209–214. doi: 10.1016/0165-2478(95)02473-5. [DOI] [PubMed] [Google Scholar]
- Soto M, Requena JM, Quijada L, Gomez LC, Guzman F, Patarroyo ME, Alonso C. Characterization of the antigenic determinants of the Leishmania infantum histone H3 recognized by antibodies elicited during canine visceral leishmaniasis. Clin Exp Immunol. 1996;106:454–461. doi: 10.1046/j.1365-2249.1996.d01-865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas G, Ansari NA, Singh R, Subba Raju BV, Bhatheja R, Negi NS, Salotra R. Diagnosis of visceral leishmaniasis: comparative potential of amastigote antigen, recombinant antigen and PCR. Br J Biomed Sci. 2002;59:218–222. [PubMed] [Google Scholar]
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Agrawal S, Pai K, Chance M, Hommel M. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. Am J Trop Med Hyg. 2005;73:269–271. [PubMed] [Google Scholar]
- Sundar S, Singh RK, Bimal SK, Gidwani K, Mishra A, Maurya R, Singh SK, Manandhar KD, Boelaert M, Rai M. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop Med Int Health. 2007;12:284–289. doi: 10.1111/j.1365-3156.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- Talmi-Frank D, Strauss-Ayali D, Jaffe CL, Baneth G. Kinetics and diagnostic and prognostic potential of quantitative western blot analysis and antigen-specific enzyme-linked immunosorbent assay in experimental canine leishmaniasis. Clin Vaccine Immunol. 2006;13:2271–2276. doi: 10.1128/CVI.13.2.271-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A, Bartlett A, Bidwell DE, Clark MF, Adams AN. The detection of viruses by enzyme-linked immunosorbent assay (ELISA) J Gen Virol. 1976;33:165–167. doi: 10.1099/0022-1317-33-1-165. [DOI] [PubMed] [Google Scholar]
- Young RA. Stress proteins and immunology. Ann Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- Young RA, Elliot TJ. Stress proteins, infection, and immune surveillance. Cell. 1989;59:5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Young DB, Mehlert A, Smith DF. Stress proteins and infectious diseases. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. Stress proteins in biology and medicine. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1990. pp. 131–165. [Google Scholar]
- Zijlstra EE, Ali MS, El-Hassan AM, El-Toum IA, Satti M, Ghalib HW, Kager PA. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86:505–507. doi: 10.1016/0035-9203(92)90086-R. [DOI] [PubMed] [Google Scholar]