Abstract
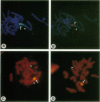
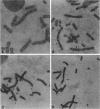
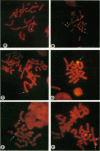
Recent studies of several drug-resistant Chinese hamster cell lines suggested that a breakage-fusion-bridge mechanism is frequently involved in the amplification of drug resistance genes. These observations underscore the importance of chromosome breakage in the initiation of DNA amplification in mammalian cells. However, the mechanism of this breakage is unknown. Here, we propose that the site of chromosome breakage consistent with the initial event of P-glycoprotein (P-gp) gene amplification via the breakage-fusion-bridge cycle in three independently established multidrug-resistant CHO cells was located at 1q31. This site is a major chromosome fragile site that can be induced by methotrexate and aphidicolin treatments. Pretreatments of CHO cells with methotrexate or aphidicolin enhanced the frequencies of resistance to vinca alkaloid and amplification of the P-gp gene. These observations suggest that chromosome fragile sites play a pivotal role in DNA amplification in mammalian cells. Our data are also consistent with the hypothesis that gene amplification can be initiated by stress-induced chromosome breakage that is independent of modes of action of cytotoxic agents. Drug-resistant variants may arise by their growth advantage due to overproduction of cellular target molecules via gene amplification.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amler L. C., Shibasaki Y., Savelyeva L., Schwab M. Amplification of the N-myc gene in human neuroblastomas: tandemly repeated amplicons within homogeneously staining regions on different chromosomes with the retention of single copy gene at the resident site. Mutat Res. 1992 May;276(3):291–297. doi: 10.1016/0165-1110(92)90015-2. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Scotto K. W., Melera P. W., Spengler B. A. Chromosomal organization of amplified genes in multidrug-resistant Chinese hamster cells. Cancer Res. 1988 Jun 1;48(11):3179–3187. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J Natl Cancer Inst. 1976 Sep;57(3):683–695. doi: 10.1093/jnci/57.3.683. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bradley G., Naik M., Ling V. P-glycoprotein expression in multidrug-resistant human ovarian carcinoma cell lines. Cancer Res. 1989 May 15;49(10):2790–2796. [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Elder F. F., Robinson T. J. Rodent common fragile sites: are they conserved? Evidence from mouse and rat. Chromosoma. 1989 May;97(6):459–464. doi: 10.1007/BF00295030. [DOI] [PubMed] [Google Scholar]
- Hagemeijer A., Lafage M., Mattei M. G., Simonetti J., Smit E., de Lapeyriere O., Birnbaum D. Localization of the HST/FGFK gene with regard to 11q13 chromosomal breakpoint and fragile site. Genes Chromosomes Cancer. 1991 May;3(3):210–214. doi: 10.1002/gcc.2870030307. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Leu T. H., Vaughn J. P., Ma C., Dijkwel P. A. Amplification of DNA sequences in mammalian cells. Prog Nucleic Acid Res Mol Biol. 1991;41:203–239. doi: 10.1016/s0079-6603(08)60010-0. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Rice G. C., Kovacs M., Schimke R. T. Over-replication of DNA in S phase Chinese hamster ovary cells after DNA synthesis inhibition. J Biol Chem. 1987 Sep 5;262(25):11927–11934. [PubMed] [Google Scholar]
- Johnston R. N., Feder J., Hill A. B., Sherwood S. W., Schimke R. T. Transient inhibition of DNA synthesis results in increased dihydrofolate reductase synthesis and subsequent increased DNA content per cell. Mol Cell Biol. 1986 Oct;6(10):3373–3381. doi: 10.1128/mcb.6.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Sudar D., Rutovitz D., Gray J. W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A., Latt S. A. Evolution of chromosomal regions containing transfected and amplified dihydrofolate reductase sequences. Mol Cell Biol. 1983 Apr;3(4):699–711. doi: 10.1128/mcb.3.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammie G. A., Peters G. Chromosome 11q13 abnormalities in human cancer. Cancer Cells. 1991 Nov;3(11):413–420. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Ma C., Martin S., Trask B., Hamlin J. L. Sister chromatid fusion initiates amplification of the dihydrofolate reductase gene in Chinese hamster cells. Genes Dev. 1993 Apr;7(4):605–620. doi: 10.1101/gad.7.4.605. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum R. L., Ledbetter D. H. Fragile X syndrome: a unique mutation in man. Annu Rev Genet. 1986;20:109–145. doi: 10.1146/annurev.ge.20.120186.000545. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M., Mohandas T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus). Cytogenet Cell Genet. 1976;16(1-5):83–91. doi: 10.1159/000130559. [DOI] [PubMed] [Google Scholar]
- Raymond M., Rose E., Housman D. E., Gros P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol. 1990 Apr;10(4):1642–1651. doi: 10.1128/mcb.10.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Ling V., Schimke R. T. Frequencies of independent and simultaneous selection of Chinese hamster cells for methotrexate and doxorubicin (adriamycin) resistance. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9261–9264. doi: 10.1073/pnas.84.24.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. J., Elder F. F. Multiple common fragile sites are expressed in the genome of the laboratory rat. Chromosoma. 1987;96(1):45–49. doi: 10.1007/BF00285882. [DOI] [PubMed] [Google Scholar]
- Sager R., Gadi I. K., Stephens L., Grabowy C. T. Gene amplification: an example of accelerated evolution in tumorigenic cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7015–7019. doi: 10.1073/pnas.82.20.7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Schwab M. Oncogene amplification in neoplastic development and progression of human cancers. Crit Rev Oncog. 1990;2(1):35–51. [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sen S., Teeter L. D., Kuo T. Specific gene amplification associated with consistent chromosomal abnormality in independently established multidrug-resistant Chinese hamster ovary cells. Chromosoma. 1987;95(2):117–125. doi: 10.1007/BF00332184. [DOI] [PubMed] [Google Scholar]
- Sharma R. C., Schimke R. T. Enhancement of the frequency of methotrexate resistance by gamma-radiation in Chinese hamster ovary and mouse 3T6 cells. Cancer Res. 1989 Jul 15;49(14):3861–3866. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gorman P. A., Stark M. B., Groves R. P., Stark G. R. Distinctive chromosomal structures are formed very early in the amplification of CAD genes in Syrian hamster cells. Cell. 1990 Dec 21;63(6):1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Stark M. B., Gorman P. A., Stark G. R. Fusions near telomeres occur very early in the amplification of CAD genes in Syrian hamster cells. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5427–5431. doi: 10.1073/pnas.89.12.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R. Regulation and mechanisms of mammalian gene amplification. Adv Cancer Res. 1993;61:87–113. doi: 10.1016/s0065-230x(08)60956-2. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R. Chromosomal fragile sites. Genet Anal Tech Appl. 1991 Sep;8(6):161–166. doi: 10.1016/1050-3862(91)90056-w. [DOI] [PubMed] [Google Scholar]
- Teeter L. D., Atsumi S., Sen S., Kuo T. DNA amplification in multidrug, cross-resistant Chinese hamster ovary cells: molecular characterization and cytogenetic localization of the amplified DNA. J Cell Biol. 1986 Oct;103(4):1159–1166. doi: 10.1083/jcb.103.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D. Normal diploid human and rodent cells lack a detectable frequency of gene amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F., Le Roscouet D., Buttin G., Debatisse M. Co-amplified markers alternate in megabase long chromosomal inverted repeats and cluster independently in interphase nuclei at early steps of mammalian gene amplification. EMBO J. 1992 Jul;11(7):2665–2673. doi: 10.1002/j.1460-2075.1992.tb05332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F., Smith K. A., Buttin G., Debatisse M. The evolution of the amplified adenylate deaminase 2 domains in Chinese hamster cells suggests the sequential operation of different mechanisms of DNA amplification. Mutat Res. 1992 May;276(3):261–273. doi: 10.1016/0165-1110(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Hamlin J. L. Early dihydrofolate reductase gene amplification events in CHO cells usually occur on the same chromosome arm as the original locus. Genes Dev. 1989 Dec;3(12A):1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- Windle B. E., Wahl G. M. Molecular dissection of mammalian gene amplification: new mechanistic insights revealed by analyses of very early events. Mutat Res. 1992 May;276(3):199–224. doi: 10.1016/0165-1110(92)90009-x. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Smith H. S., Watt F. M., Hancock M. C., Hudson D. L., Stark G. R. DNA amplification is rare in normal human cells. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1791–1795. doi: 10.1073/pnas.87.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L., Bowe A. E. Fragile sites are targets of diverse mutagens and carcinogens. Oncogene. 1987 Mar;1(1):59–69. [PubMed] [Google Scholar]
- de Bruijn M. H., Van der Bliek A. M., Biedler J. L., Borst P. Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Mol Cell Biol. 1986 Dec;6(12):4717–4722. doi: 10.1128/mcb.6.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver M. J. Molecular genetic changes in human breast cancer. Adv Cancer Res. 1993;61:25–56. doi: 10.1016/s0065-230x(08)60954-9. [DOI] [PubMed] [Google Scholar]